Abstract
Glucocorticoids are pleiotropic regulators of multiple cell types with critical roles in physiological systems that change across the life-course. Although glucocorticoids have been associated with aging, available data on the aging trajectory in basal circulating glucocorticoids are conflicting. A literature search reveals sparse life-course data. We evaluated (1) the profile of basal circulating corticosterone across the life-course from weaning (postnatal day—PND 21), young adult PND 110, adult PND 450, mature adult PND 650 to aged phase PND 850 in a well-characterized homogeneous rat colony to determine existence of significant changes in trajectory in the second half of life; (2) sex differences; and (3) whether developmental programming of offspring by exposure to maternal obesity during development alters the later-life circulating corticosterone trajectory. We identified (1) a fall in corticosterone between PND 450 and 650 in both males and females (p < 0.05) and (2) higher female than male concentrations (p < 0.05). (3) Using our five life-course time-point data set, corticosterone fell at a similar age but from higher levels in male and female offspring of obese mothers. In all four groups studied, there was a second half of life fall in corticosterone. Higher corticosterone levels in offspring of obese mothers may play a role in their shorter life-span, but the age-associated fall occurs at a similar time to control offspring. Although even more life-course time-points would be useful, a five life-course time-point analysis provides important new information on normative and programmed aging of circulating corticosterone.
Keywords: Aging, Glucocorticoids, Developmental programming, Maternal obesity
Introduction
Glucocorticoids are pleiotropic regulators of multiple cell types with critical regulatory roles in many physiological systems that change across the life-course. Exposure to both high (Cushing’s disease) and low glucocorticoid concentrations (Addison’s disease) produces weakness and frailty. Data in the literature are conflicting as to whether aging is accompanied by an increase or decrease in basal circulating glucocorticoid concentrations. Our search of the literature indicated that there is a lack of measurements across the complete life-course in any dataset available. In the light of the key roles that glucocorticoids play at different stages of development (Zambrano et al. 2014), we propose that the first step in understanding glucocorticoids’ role in aging is to establish the timing of life-course changes in basal circulating glucocorticoids with normal aging. To set a baseline, there is a need for data from as many life-course stages as practically possible. Measurements are needed even before a clear aged phenotype emerges. There is also a need to determine any differences between males and females.
Differences in conclusions about timing and extent of aging related basal glucocorticoid function are likely due to several factors. Importantly, heterogeneity in prior life-course history of subjects significantly affects the aging trajectory. This is especially true for developmental programming by early life factors whose outcomes may lay dormant to emerge later. Existing reviews discuss glucocorticoids’ as well as adrenal dehydroepiandrosterone sulfate’s role in programming lifetime health and suggest a potential key role in aging (Langie et al. 2012; Maestripieri et al. 2009) life-course glucocorticoid profiles.
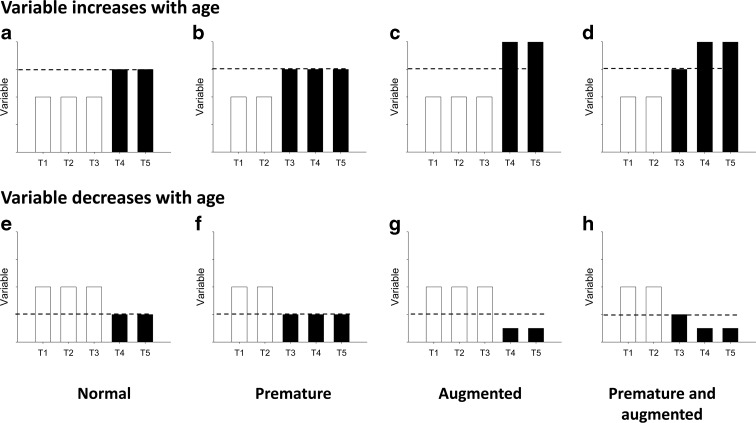
Normative basal values are required to determine the critical time windows at which to seek mechanisms involved in normal, premature, or augmented aging. One problem in establishing the normative aging trajectory is the life-course time-range of available data. Plasma glucocorticoid concentrations are often only obtained at one or two life-course time-points without the baseline data needed at early ages, making the timing of progression through the aging process difficult to analyze and interpret (Bowman et al. 2006; Ferrari et al. 2001; Peeters et al. 2007). While measures from one or two stages of the life-course are valuable, obviously, the greater the number of time-points determined, the firmer the conclusions that can be drawn. We propose that studies assessing life-course changes in aging should preferably cover at least five life-course time-points (Fig. 1).
Fig. 1.
Proposed strategy to determine at five life-course time-points. The timing of an age-related change represented by dash line in a variable that increases (a–d) or decreases (e–h) with age. a, e Represent a normal; b, f a premature; c, g an augmented; and d, h a premature and augmented aging process
Human (Kral et al. 2006; Villamor and Cnattingius 2006) and experimental animal (Nivoit et al. 2009; Samuelsson et al. 2008) studies have shown that offspring obesity is one of the adverse outcomes of maternal obesity. Many different mechanisms are involved in this predisposition. Glucocorticoids play a critical role during gestation in maturing a variety of fetal organs by stimulating differentiation and inhibiting growth and proliferation. We have previously published (Nathanielsz et al. 2013; Rodriguez et al. 2012; Vega et al. 2015; Zambrano and Nathanielsz 2013) that maternal serum corticosterone concentrations in maternal obesity (MO) were higher than controls (C) at the time of breeding, end of gestation, 19 days gestation (dG), and end of lactation, postnatal day (PND) 21. Prenatal exposure to increased levels of glucocorticoids changes hypothalamic pituitary adrenal axis function (Braun et al. 2013).
We determined (1) the profile of basal circulating corticosterone in the rat across the complete life-course from weaning (PND 21) to old age (PND 850) in a well-characterized homogeneous colony to provide evidence for the existence of any significant change in trajectory in the second half of life; (2) differences between absolute corticosterone concentrations in males and females; and (3) whether developmental programming of offspring by exposure to maternal obesity during development alters the later-life circulating corticosterone trajectory.
Materials and methods
Care and maintenance of animals
All procedures were approved by the Animal Experimentation Ethics Committee of the Instituto Nacional de Ciencias Médicas y Nutrición, Salvador Zubirán (INNSZ), Mexico, and in accordance with the guidelines of Mexican law on animal protection (NOM-062-ZOO-1999). General procedures relating to maternal diet, breeding, and management of control and obese mothers have been previously described in detail (Zambrano et al. 2010). Briefly, at 4 months of age, 28 female Wistar rats weighing between 220 and 260 g were obtained from the INNSZ animal colony and maintained on normal laboratory chow (Zeigler Rodent RQ 22–5, USA) containing 22.0 % protein, 5.0 % fat, 31.0 % polysaccharide, 31.0 % simple sugars, 4.0 % fiber, 6.0 % minerals and 1.0 % vitamins (w/w), energy 4.0 kcal g−1, under controlled lighting (lights-on from 7:00 a.m. to 7:00 p.m. at 22–23 °C). Female rats were mated overnight with proven male breeders. The day on which spermatozoa were detected in a vaginal smear was designated as conception day 0. Only rats that were pregnant within 5 days of mating with males were studied. All rats were delivered vaginally. To ensure homogeneity, only litters between 12 and 15 pups were studied. Litters were adjusted to 12 pups for each mother while maintaining as close to a 1:1 sex ratio as possible. After birth, all mother were fed with C diet. The females in these litters constituted the founder generation (F0) mothers. At weaning, one F0 female from each litter was placed on chow diet (controls C) (n = 14) and one on the high fat high energy obesogenic diet containing 23.5 % protein, 20.0 % animal lard, 5.0 % fat, 20.2 % polysaccharide, 20.2 % simple sugars, 5.0 % fiber, 5.0 % mineral mix, 1.0 % vitamin mix (w/w), energy 4.9 kcal g−1 diet from weaning until they were bred at PND 120 (Zambrano et al. 2010) when obese F0 mothers were 17 % heavier than control F0 mothers. High-fat diet continued to be fed to the obese F0 mothers in pregnancy and lactation. F0 control mothers ate normal laboratory chow throughout. The high-fat diet increased obese F0 insulin, glucose, HOMA, leptin, triglycerides, and retroperitoneal fat before breeding (Vega et al. 2015). Control and obese F0 mothers were sisters and thus F1 (the offspring of F0) were cousins, helping to homogenize genetic factors. F1 of both control and obese mothers ate standard laboratory diet after weaning. Food and water were available ad libitum.
Postnatal maintenance
After weaning (PND 21), both F1 male and female pups were separated into groups of three to four per cage and fed standard rodent chow diet ad libitum throughout the study. At PND 21 (weaning), 110 (young adult), 450 (adult), 650 (mature adult), and 850 (aged phase), after 6 h of fasting, rats were euthanized between 12:00 p.m. and 2:00 p.m. by decapitation using a rodent guillotine (Thomas Scientific, NJ) by trained and experienced personnel. For each age group, trunk blood was collected and serum separated. F1 evaluated at each of the five ages were siblings as far as possible.
Corticosterone measurements
For each age group, blood was collected, centrifuged, and serum frozen until assayed for corticosterone. Corticosterone was measured in fasting serum by radioimmunoassay (Rodriguez et al. 2012).
Statistical analysis
Data are expressed as means ± SEM. Data were in-transformed. Male and female data for each point were analyzed by t test and were different, therefore sexes were analyzed separately. For the timing of the corticosterone fall in each group, we used the approach shown in Fig. 1 to analyze data from five points across the life-course to set baselines and determine the time at which changes occur related to aging. The strategy uses the unpaired t test to compare data from the oldest age available (T5) to determine a significant difference from the preceding time-point (T4). If there is no difference, T4 and T3 are then compared, repeating the analysis to determine all points at which the later of the two points compared differs from the one before. For C vs. obese F1 at the three ages in each sex, two-way ANOVA and Sidak’s multiple comparison tests were used.
Results
Normative fall in corticosterone with aging
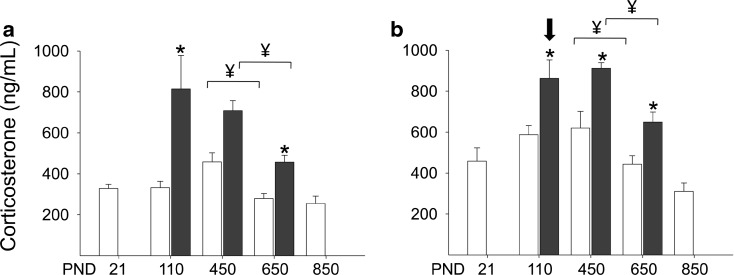
Corticosterone concentrations in the control animals were higher in females than in males (Fig. 2). Using the criteria established in Fig. 1, corticosterone fell between PND 450 and 650 in both male and female from control and F1 of obese mothers (Fig. 2a, b).
Fig. 2.
Plasma corticosterone in a male and b female control rats (open histograms) at five life-course stages (PND postnatal day). The yen sign indicates the first significant change when comparing each age with the one prior starting with the oldest age as described for the analysis used in Fig. 1. Data are also presented (solid histogram) for F1 of obese mothers a male and b female. Data are mean ± SEM; group n = 5–14. Data were in-transformed, and sexes were analyzed separately. Female corticosterone values were higher than male in all groups at all ages except in offspring of obese group at PND 110 (downward-pointing arrow). For the timing of the corticosterone fall in each group analysis was as described in relation to Fig. 1. For C vs. obese at the three ages in each sex, two-way ANOVA with Sidak’s multiple comparison tests. p < 0.05 (asterisk) vs. C
Having established the timing of the normative corticosterone fall in both males and females, we examined the same stage of life in the developmental programming paradigm of F1 offspring of obese F0 mothers. F1 offspring of obese F0 mothers themselves become obese even on the same diet eaten by control F1 (Nathanielsz et al. 2013). Corticosterone in male and female F1 offspring of obese mothers fell between PND 450 and 650, a similar time window as control F1 (Fig. 2).
Discussion
One major confound in the interpretation of available human aging data on life-course profiles of glucocorticoids is the inclusion of data from subjects with chronic diseases such as Alzheimer’s, hypertension, or diabetes (Huang et al. 2009), which themselves can alter glucocorticoid production. In one study that included subjects with high blood pressure and heart disease, young subjects were all men to avoid ovarian cycle changes, while the older population was mixed male and female subjects (Zhao et al. 2003). Our data above show clearly the need to separate male and female data. One comprehensive human study on eight young (18–35 years) and eight elderly men (60–72 years) demonstrated a higher circadian cortisol rhythm mesor in aged males (Bergendahl et al. 2000). However, this study only included data at two time-points in the life-course. In one study in rhesus monkeys, values in older monkeys were higher than younger animals (Downs et al. 2008). In another study, plasma cortisol concentration was measured on day 2 after capture and transfer of 53 rhesus macaque mothers from a free-ranging situation to single cages (Maestripieri et al. 2009). Ages ranged between 15 and 25 years, and subjects had been pregnant and delivered at variable times before sampling. There was a negative relationship between cortisol and age that did not quite reach significance (p = 0.09). These conflicting results show the need for data from homogeneous well-controlled subjects that fulfill the criteria given in Fig. 1.
One might consider that this question is easier to resolve in rodents than other species for many practical reasons. However, even in rodents, there is a lack of data from longitudinal studies of glucocorticoid concentrations across the life-span such as we present here (Fig. 2). Beginning studies in early life is important because early events set a baseline as well as themselves potentially influencing future function. We standardized the study subjects to remove disease-related confounds of the aging process. All animals were from our well-characterized colony of healthy male and female rats that has been maintained for several generations in standard conditions (Rodriguez et al. 2012; Vega et al. 2015; Zambrano et al. 2010). Background patrilineal and matrilineal data were available from all subjects to ensure homogeneity and lack of siblings among F0 mothers in different studies. It is important to note that all study animals and their mothers are reared under very constant conditions with detailed observations on growth and general health throughout their life. Thus, study animals are not exposed to the variety of environmental confounds that inevitably occur in human studies.
The question needs to be posed “Is it possible to determine normal aging or should the aging process always be defined in the context of the life-course history of the individual.” In addition to clear evidence of a strong genetic component, the final trajectory of aging will be determined by gene-environment interactions (nature and nurture) that occur even before conception. For example, maternal obesity affects gametes and alters offspring phenotype throughout life in ways that potentially influencing the rate of aging (Igosheva et al. 2010). The aging trajectory is clearly modified by age-related diseases. However, it is necessary to reconcile two opposing points of view. One holds that aging produces age-related diseases, and the other, that age-related diseases such as diabetes are themselves mechanisms that modify the aging trajectory.
There is support for the view that glucocorticoids accelerate aging processes (Anderson et al. 2014) as well as for development of both glucocorticoid resistance and glucocorticoid enhanced aging in different metabolic pathways (Chen et al. 2013). We propose that circulating glucocorticoids follow an age-related trajectory, which can be affected by both the external environment and internal physiological events. Importantly, life-span effects are influenced by developmental programming by challenges such as altered F1 nutrition during their development. The mechanisms are likely multiple and include both programmed changes within the hypothalamo-pituitary adrenal axis (Braun et al. 2013; Zambrano et al. 2014) and altered peripheral production of glucocorticoids in adipose tissue that are influenced by maternal nutrition (Guo et al. 2013). The influence of developmental programming is clearly shown by the difference in the corticosterone concentrations in the F1 from control and obese mothers. This part of the study was terminated at PND 650 because F1 offspring of obese mothers begin to die around PND 650; thus, no data are available in this group at PND 850, which is equivalent to 75 years in human life (Quinn 2005), although making age comparisons between humans and rat life-span is not directly linear. Maximal rat life-span is dependent on the strain and—as this study indicates programming effects in the history of the animals under study—the probable maximum is around 3 years (Quinn 2005), but the differences in development must be taken into consideration when age is a crucial factor for comparison with human life.
The relationship of shorter life-span and high body mass index (BMI) in human populations is complicated by a wide variety of confounders and is not a linear relationship. However, human BMI levels in the obese range are correlated with shortened life-span (National Research Council 2011).
In conclusion, our goal was to address three precise and limited objectives for which no data exist in the literature. We conclude that, in rats, if samples cover a sufficient period of the life-course, corticosterone levels fall with aging in both normal controls and F1 of obese F0 mothers. The timing of the decrease in corticosterone concentrations appears similar in F1 females and males of control and obese F0 mothers, but the fall occurs from higher levels in F1 from obese F0 mothers. Corticosterone levels were higher in control and obese females than in males at all ages except PND 110 F1 of obese mothers. Further studies are needed to determine whether this fall from a higher level and plays a role in the earlier death of F1 from obese mothers that we have observed. Further, our observations are compatible with the view that the elevated corticosterone levels, of themselves, contribute to this aging process. Future studies need to evaluate a wider range of glucocorticoid endpoints such as the parameters of the cosinor analysis of circadian rhythms.
Acknowledgments
LARC is a graduate student from Doctorado en Ciencias Biomédicas, Facultad de Medicina, Universidad Nacional Autónoma de México and is a recipient of the CONACyT fellowship; this work was supported by CONACyT 155166 México. We are grateful to Guadalupe L. Rodríguez-González for assistance with the manuscript.
Conflict of interest
The authors have nothing to disclose and have no conflict of interest.
Abbreviations
- BMI
Body mass index
- C
Control
- dG
Days gestation
- PND
Postnatal day
References
- Anderson RM, Birnie AK, Koblesky NK, Romig-Martin SA, Radley JJ. Adrenocortical status predicts the degree of age-related deficits in prefrontal structural plasticity and working memory. J Neurosci. 2014;34:8387–8397. doi: 10.1523/JNEUROSCI.1385-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergendahl M, Iranmanesh A, Mulligan T, Veldhuis JD. Impact of age on cortisol secretory dynamics basally and as driven by nutrient-withdrawal stress. J Clin Endocrinol Metab. 2000;85:2203–2214. doi: 10.1210/jcem.85.6.6628. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Maclusky NJ, Diaz SE, Zrull MC, Luine VN. Aged rats: sex differences and responses to chronic stress. Brain Res. 2006;1126:156–166. doi: 10.1016/j.brainres.2006.07.047. [DOI] [PubMed] [Google Scholar]
- Braun T, Challis JR, Newnham JP, Sloboda DM. Early-life glucocorticoid exposure: the hypothalamic-pituitary-adrenal axis, placental function, and long-term disease risk. Endocr Rev. 2013;34:885–916. doi: 10.1210/er.2013-1012. [DOI] [PubMed] [Google Scholar]
- Chen KC, et al. Glucocorticoid-dependent hippocampal transcriptome in male rats: pathway-specific alterations with aging. Endocrinology. 2013;154:2807–2820. doi: 10.1210/en.2013-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs JL, Mattison JA, Ingram DK, Urbanski HF. Effect of age and caloric restriction on circadian adrenal steroid rhythms in rhesus macaques. Neurobiol Aging. 2008;29:1412–1422. doi: 10.1016/j.neurobiolaging.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari E, et al. Age-related changes of the hypothalamic-pituitary-adrenal axis: pathophysiological correlates. Eur J Endocrinol. 2001;144:319–329. doi: 10.1530/eje.0.1440319. [DOI] [PubMed] [Google Scholar]
- Guo C, Li C, Myatt L, Nathanielsz PW, Sun K. Sexually dimorphic effects of maternal nutrient reduction on expression of genes regulating cortisol metabolism in fetal baboon adipose and liver tissues. Diabetes. 2013;62:1175–1185. doi: 10.2337/db12-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CW, Lui CC, Chang WN, Lu CH, Wang YL, Chang CC. Elevated basal cortisol level predicts lower hippocampal volume and cognitive decline in Alzheimer’s disease. J Clin Neurosci. 2009;16:1283–1286. doi: 10.1016/j.jocn.2008.12.026. [DOI] [PubMed] [Google Scholar]
- Igosheva N, Abramov AY, Poston L, Eckert JJ, Fleming TP, Duchen MR, McConnell J. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral JG, Biron S, Simard S, Hould FS, Lebel S, Marceau S, Marceau P. Large maternal weight loss from obesity surgery prevents transmission of obesity to children who were followed for 2 to 18 years. Pediatrics. 2006;118:e1644–e1649. doi: 10.1542/peds.2006-1379. [DOI] [PubMed] [Google Scholar]
- Langie SA, Lara J, Mathers JC. Early determinants of the ageing trajectory. Best Pract Res Clin Endocrinol Metab. 2012;26:613–626. doi: 10.1016/j.beem.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Hoffman CL, Anderson GM, Carter CS, Higley JD. Mother-infant interactions in free-ranging rhesus macaques: relationships between physiological and behavioral variables. Physiol Behav. 2009;96:613–619. doi: 10.1016/j.physbeh.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanielsz PW, Ford SP, Long NM, Vega CC, Reyes-Castro LA, Zambrano E. Interventions to prevent adverse fetal programming due to maternal obesity during pregnancy. Nutr Rev. 2013;71(Suppl 1):S78–S87. doi: 10.1111/nure.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . Explaining divergent levels of longevity in high-income countries. In: Crimmins EM, Preston SH, Cohen B, editors. Panel on understanding divergent trends in longevity in high-income countries. Committee on population, division of behavioral and social sciences and education. Washington DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- Nivoit P, Morens C, Van Assche FA, Jansen E, Poston L, Remacle C, Reusens B. Established diet-induced obesity in female rats leads to offspring hyperphagia, adiposity and insulin resistance. Diabetologia. 2009;52:1133–1142. doi: 10.1007/s00125-009-1316-9. [DOI] [PubMed] [Google Scholar]
- Peeters GM, van Schoor NM, Visser M, Knol DL, Eekhoff EM, de Ronde W, Lips P. Relationship between cortisol and physical performance in older persons. Clin Endocrinol. 2007;67:398–406. doi: 10.1111/j.1365-2265.2007.02900.x. [DOI] [PubMed] [Google Scholar]
- Quinn R. Comparing rat’s to human’s age: how old is my rat in people years? Nutrition. 2005;21:775–777. doi: 10.1016/j.nut.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Rodriguez JS, et al. Maternal obesity in the rat programs male offspring exploratory, learning and motivation behavior: prevention by dietary intervention pre-gestation or in gestation. Int J Dev Neurosci. 2012;30:75–81. doi: 10.1016/j.ijdevneu.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Samuelsson AM, et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension. 2008;51:383–392. doi: 10.1161/HYPERTENSIONAHA.107.101477. [DOI] [PubMed] [Google Scholar]
- Vega CC, Reyes-Castro LA, Bautista CJ, Larrea F, Nathanielsz PW, Zambrano E (2015) Exercise in obese female rats has beneficial effects on maternal and male and female offspring metabolism. Int J Obes (Lond) 39(4):712–719. doi:10.1038/ijo.2013.150 [DOI] [PMC free article] [PubMed]
- Villamor E, Cnattingius S. Interpregnancy weight change and risk of adverse pregnancy outcomes: a population-based study. Lancet. 2006;368:1164–1170. doi: 10.1016/S0140-6736(06)69473-7. [DOI] [PubMed] [Google Scholar]
- Zambrano E, Nathanielsz PW. Mechanisms by which maternal obesity programs offspring for obesity: evidence from animal studies. Nutr Rev. 2013;71(Suppl 1):S42–S54. doi: 10.1111/nure.12068. [DOI] [PubMed] [Google Scholar]
- Zambrano E, Martinez-Samayoa PM, Rodriguez-Gonzalez GL, Nathanielsz PW. Dietary intervention prior to pregnancy reverses metabolic programming in male offspring of obese rats. J Physiol. 2010;588:1791–1799. doi: 10.1113/jphysiol.2010.190033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano E et al (2014) Increased central and peripheral glucocorticoid synthesis act as an orchestrator of developmental programming. In: stress and developmental programming of health and disease: beyond phenomenology nova Science Publishers, Inc
- Zhao ZY, Lu FH, Xie Y, Fu YR, Bogdan A, Touitou Y. Cortisol secretion in the elderly. Influence of age, sex and cardiovascular disease in a Chinese population. Steroids. 2003;68:551–555. doi: 10.1016/S0039-128X(03)00083-7. [DOI] [PubMed] [Google Scholar]