Abstract
Merkel cell carcinoma is a neuroendocrine tumor that occurs predominantly on the sun-exposed skin, with rare cases in the extracutaneous sites. It represents one of the extremely rare malignant neuroendocrine tumors of the salivary glands. We report a case of primary Merkel cell carcinoma of the right submandibular gland. The preoperative diagnosis was doubtful and the definitive histological diagnosis proved to be very difficult considering the extreme rarity of this tumor. The intraoperative evaluation of the macroscopic characteristics of the lesion led to an elective lymph node dissection. The extreme aggressiveness of the disease has resulted in the necessity of a new post-operative staging and in a multimodal treatment. This is the first primary submandibular gland Merkel cell carcinoma described in the literature. Differential diagnosis may be challenging and proper hematoxylin-eosin staining and immunohistochemical studies are mandatory.
Keywords: Merkel cell carcinoma, Neuroendocrine carcinoma, Salivary gland, Submandibular gland
Introduction
Merkel cell carcinoma (MCC) is a rare neuroendocrine cancer first described by Toker as “trabecular carcinoma of the skin” [1], that was initially believed to originate only from the Merkel corpuscles of the epidermis [2]. However, MCC probably arises from stem cells, which are able to differentiate into different cell lines [3–5]. Indeed, MCC can occur at many extracutaneous sites [6–10]. MCC is generally considered to be an aggressive neoplasm with a high metastatic potential [11, 12], and is one of the salivary gland neuroendocrine carcinomas. We report an exceedingly rare case of primary MCC of the submandibular gland with some uncommon immunophenotypical aspects.
Case Report
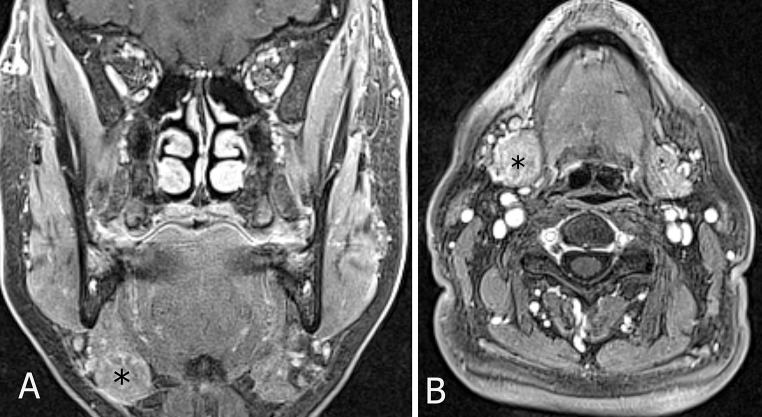
A 67-year-old woman was referred to the Department of Otorhinolaryngology at the University of Brescia for a right submandibular swelling that appeared 3 months before. Her clinical history included breast cancer treated with surgery and radiotherapy 2 years earlier. Clinical examination revealed a hard mass, about 2.0 cm in diameter, in the right submandibular triangle. The patient underwent ultrasound guided fine needle aspiration cytology (FNAC) that revealed the presence of a neoplastic lesion, not otherwise defined, even though some cellular atypia could be identified. At immunocytochemical evaluation, positivity for pancytokeratin was observed, whereas S-100, chromogranin, TTF-1, and estrogen-progesterone markers were all negative. A breast metastasis was therefore ruled out. Endoscopic evaluation of the upper aereo-digestive tract was negative for mucosal lesions. The patient underwent MRI of the neck that showed a submandibular lesion, confined to the gland, 2.2 cm in diameter, with sharp margins and non-homogeneous enhancement (Fig. 1). No lymph node suspicious for metastasis was identified.
Fig. 1.
Preoperative contrast-enhanced MR (VIBE sequence) on coronal (a) and axial plane (b) showing a 22-mm nodule (asterisk) with sharp margins and non-homogeneous enhancement within the right submandibular gland
Even though no element clearly suggested a diagnosis of malignancy, the equivocal cytologic and imaging features lead us to plan removal of the submandibular gland and selective neck dissection (levels I–III). At intraoperative evaluation, the submandibular gland was slightly adherent to the platysma. An enlarged level IB lymph node was sent for frozen sections, but no malignant cells were identified; no other lymph node suspicious for metastasis was clearly visible. Even in the absence of a diagnosis of malignancy, hard consistence and adherence to platysma reinforced our preoperative impression of an aggressive lesion and selective neck dissection was therefore performed.
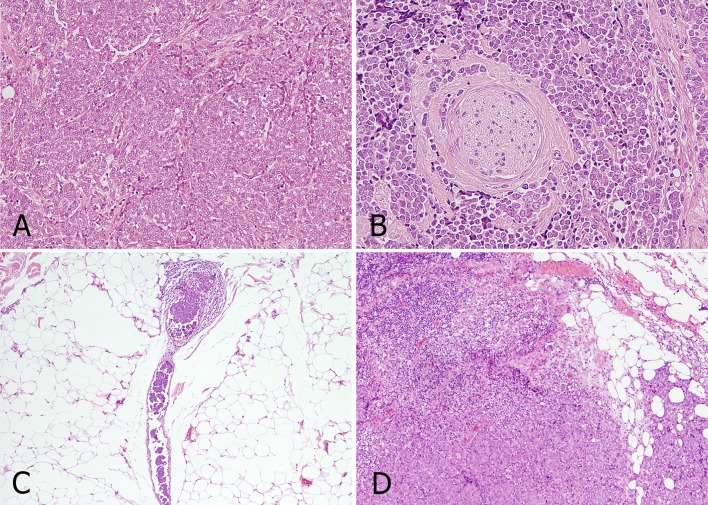
At histology, gross examination revealed a hard, gray mass 2.0 × 2.2 cm diameter within normal salivary parenchyma. Microscopically, the tumor was characterized by a solid growth of epithelioid cells that was not demarcated by a capsule; cells were arranged in nests with fibrous strands interposed and presented round ovular nuclei with finely scattered chromatin and scarce cytoplasm (Fig. 2a). There were focal areas of necrosis and numerous mitoses. The tumor was characterized by an infiltrative pattern of growth with extraglandular extension. Both perineural spread and intravascular growth were identified (Fig. 2b, c). Three small metastatic lymph nodes at level IA (4 mm), IB (3 mm), and III (4 mm), all with extracapsular extension (Fig. 2d), were also identified.
Fig. 2.
Histologic features of the tumor (hematoxylin-eosin): epithelioid cells organized in nests with fibrous strands interposed, showing round ovular nuclei with finely scattered chromatin and scarce cytoplasm (a). Perineural spread: tumor cells invading the perineurium (b). Intravascular growth: clot of neoplastic cells inside the lumen of a small vessel (c). Nodal metastasis with extranodal extension: residual lymphoid parenchyma invaded by malignant cells; infiltrated fat tissue is also visible (d)
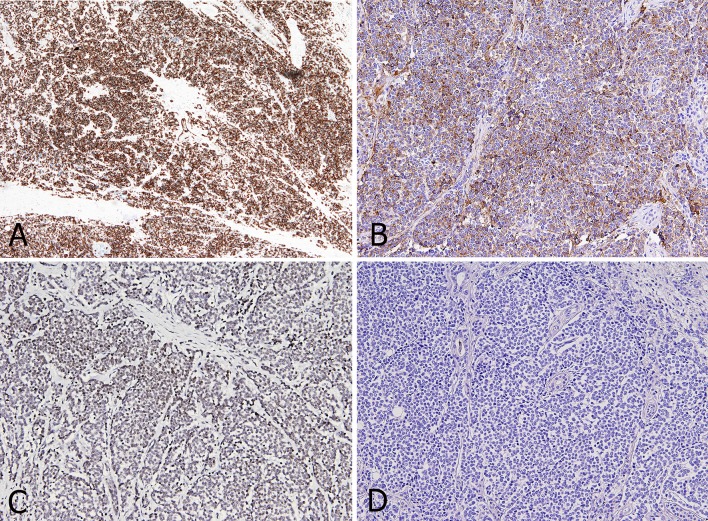
These features were consistent with an aggressive, poorly differentiated malignant tumor, possibly of neuroendocrine origin. The specimen was therefore analyzed with specific markers, accordingly. The tumor showed positivity for CD99, cytokeratin (CK) 14 (Fig. 3a), chromogranin (Fig. 3b), polyomavirus (Fig. 3c) and focal and weak positivity for synaptophysin. Negativity was demonstrated for CD-56, TTF-1, S-100, estrogen and progesterone receptors, CK7, and CK20 (Fig. 3d). The proliferative activity (positivity for MIB1/Ki-67) was 20 %.
Fig. 3.
Tumor cells show strong and diffuse positivity for CK14 staining (4x) (a) and cytoplasmic and peri-nuclear positivity for Chromogranin A staining (10x) (b). MCPyV staining (10x): granular nuclear positivity for specific Merkel Cell polyomavirus large T antigen (c). CK20 staining (10x): tumor cells are completely negative (d)
Definitive diagnosis was puzzling: nuclear dot-like positivity for Merkel cell polyomavirus large T antigen and negativity for TTF-1 ruled out a diagnosis of small cell lung carcinoma and seemed to be consistent with primary or metastatic MCC. Nevertheless, negativity for CK20, which is considered to be almost invariably associated with MCC, was unexpected.
Dermatologic evaluation and gallium scintigraphy excluded the presence of a primary skin lesion and distant metastases, respectively. A definitive diagnosis of primary submandibular gland MCC was therefore rendered. The tumor was staged as pT3 N2b ecs + M0 G3-4. The patient underwent adjuvant concomitant radiotherapy (IMRT, 60 Gy [2 Gy x30]) and chemotherapy (cisplatin 50 mg/m2 every 21 days); the treatment was well tolerated and no major complications were reported. Post-treatment PET-CT, US, and clinical evaluations were negative, and 1 year after completion of treatment the patient is free of disease.
Discussion
Neuroendocrine carcinomas of the salivary glands are aggressive malignancies, most commonly arising within the parotid gland,13 with rare cases involving the submandibular gland [14, 15]. They account for <1 % of all carcinomas of the parotid gland and 3.5 % of all malignant tumors of minor salivary glands [15]. The presence of overlap in morphologic and immunohistochemical (IHC) findings between salivary gland and cutaneous MCC may cause considerable diagnostic problems. Cutaneous MCCs arise on sun-exposed skin; the cutaneous type represents 97.6 % of all MCCs [7]. Cutaneous MCCs of head and neck commonly metastasize to lymph nodes contained within or adjacent to major salivary glands, thus making difficult the differentiation with a primary salivary gland tumor [13]. This is particularly problematic in the parotid gland, which is rich in intraparenchymal lymph nodes. Moreover, histologic evidence of parotid intraparenchymal lymph node involvement is not always consistent with metastasis, because primary salivary tumors may spread into lymph nodes by contiguity.
Neuroendocrine salivary carcinomas can be primarily classified based on their immunophenotype. Those positive for CK20 are defined as “Merkel cell type”, since this protein is typically expressed by histologically-similar high-grade neuroendocrine MCCs of the skin [13]. CK20-negative cases are referred to as “pulmonary type”, since CK20 is not expressed by neuroendocrine small cell carcinomas of pulmonary origin, which are TTF1 positive in 90 % of cases [16, 17]. Thus, positivity for CK20 is an important diagnostic marker for MCC of salivary gland, but it can be negative or scarcely expressed, especially in poorly differentiated lesions, in about 13 % of patients [17–20]. Consequently, the diagnosis of MCC can not be excluded only on the basis of CK20 negativity [19] and therefore the IHC panel should entail some other factors.
Feng et al. [21] indeed sequenced a novel polyomavirus named Merkel cell polyomavirus (MCPyV) which is found in most cases of MCC; afterwards, other independent groups confirmed the presence of MCPyV DNA in a large proportion (40–100 %) of MCCs cases [22–25]. The virus is clonally integrated in the tumor cell genome, suggesting that viral infection occurs early in tumorigenesis and thus probably plays a role in pathogenesis [21, 24]. It has been demonstrated that MCPyV DNA is not present in non-MCC cutaneous lesions or other visceral neuroendocrine tumors [26]. MCPyV encodes for large and small T antigens that force the host cell DNA replication; in particular the large T antigen binds to and inactivates tumor suppressor proteins p53 and Rb, playing a crucial role in tumorigenesis [27]. The recently developed mouse monoclonal anti-MCPyV large T antigen antibody CM2B4 (sc136172, 1:100, Santa Cruz Inc, California) is highly sensitive and specific for the presence of a high viral copy number in cells [27]. Therefore, the determination of the MCPyV status by simple IHC staining is a reliable, additional diagnostic tool applicable to most pathology laboratories, useful in particular in cases when Merkel cell carcinomas lack CK20 [27]. Moreover, there is a 100 % concordance of IHC positivity for MCPyV between primary MCC and corresponding metastatic cells [26]. Using quantitative PCR, multiple investigators have shown a substantial variability in the copy number of MCV DNA among various MCCs. This may explain the non-uniform, and sometimes weak, IHC expression of MCV large T antigen among different cases [26].
Regarding the present case, the definitive diagnosis was very challenging and obtained in a stepwise manner. At first, cell morphology and positivity for synaptophysin and chromogranin led us to the diagnosis of a neuroendocrine tumor; on the other hand, negativity for TTF1 excluded the possibility of a metastasis from a small cell lung cancer. As already stated, the positivity for CK20 is considered typical, but not exclusive of MCCs [19]. In our case, CK20 negativity along with the concomitant positivity for MCPyV, the absence of primary mucosal or skin primary at physical and imaging evaluation lead to the diagnosis of primary MCC of the submandibular gland [27, 28]. To the best of our knowledge, this is the first case of primary submandibular gland MCC. We identified in the literature 9 cases of primary neuroendocrine carcinomas of the submandibular gland (Table 1), none of them defined as MCC; the most relevant IHC features of each case have been reported [15, 20, 29–33]. Other cases of small cell carcinomas lacking details about the IHC profile and/or displaying negativity for neuroendocrine markers (synaptophysin and chromogranin) were not included in the table [15, 34, 35]. In the literature, one case of primary parotid gland MCC was tested and was MCPyV + [28]. MCPyV was not tested in any of the nine cases listed in Table 1, and therefore the diagnosis of MCC was not rendered. However, on the basis of IHC, the diagnosis of primary MCC is not excluded in the CK20 + case of Nagao et al. (Table 1) [20].
Table 1.
Primary submandibular gland neuroendocrine carcinomas
| Author | Year | Synaptophysin | Chromogranin | TTF-1 | CK20 | MCPyV |
|---|---|---|---|---|---|---|
| Gnepp et al. [15] | 1990 | − + |
+ (rare cell) − |
n.a. n.a. |
n.a. n.a. |
n.a. n.a. |
| De Vicente Rodiguez et al. [29] | 2004 | + | + | n.a | n.a | n.a. |
| Nagao et al. [20] | 2004 | + + |
+ + |
+ − |
− + |
n.a. n.a. |
| Sowerby et al. [30] | 2007 | + | − | − | − | n.a. |
| Kawaratani et al. [31] | 2013 | + | + | n.a. | n.a. | n.a. |
| Petrone et al.[32] | 2014 | + | + | n.a. | − | n.a. |
| Yamamoto et al.[33] | 2014 | + | − | n.a. | n.a. | n.a. |
| Present case | 2014 | + | + | − | − | + |
n.a. not available
The prognosis of these tumors is poor, and they require an aggressive, possibly multimodal treatment. Many authors have suggested a combination of surgery, radiotherapy and/or chemotherapy to prevent the high risk of loco-regional and/or metastatic recurrence [15, 36, 37].
In the present case, due to the presence of extraparenchymal extension and multiple metastatic lymph nodes, all with extracapsular spread, adjuvant concomitant chemo-radiation was scheduled. One of the most controversial issues regarding our treatment strategy is the choice to perform planned selective neck dissection and not only submandibular gland excision. In the literature there is no general consensus on the role, if any, of elective neck dissection in salivary gland cancer. Most authors consider prophylactic neck dissection appropriate for high-grade and/or high T-stage lesions only [38], while others advocate neck dissection with elective intent in all malignant neoplasms [39, 40]. In the present case, we planned a selective neck dissection due to the presence of clinical and cytological elements suspicious for malignancy, even in absence of preoperative and intraoperative findings clearly indicating a diagnosis of cancer. However, it is worth remembering that one of the most relevant concerns in salivary gland cancer is the relatively low diagnostic accuracy of FNAC and frozen sections in defining both histologic subtype and grading, two of the most important parameters, along with clinical presentation and T stage, predicting the risk of occult nodal metastases. Moreover, the malignancy rate in submandibular gland tumor is generally considered higher than in the parotid gland. Our strategy reinforces the concept that elective neck dissection should be planned whenever a salivary tumor displays an aggressive behavior, either clinically or cytologically; the decision should be confirmed intraoperatively, whenever signs suggesting aggressiveness (i.e. extraglandular extension, lymph node metastasis) are detected.
Conclusion
We present an exceedingly rare case of submandibular gland neuroendocrine carcinoma and, to the best of our knowledge, this is the first primary submandibular gland MCC described in the literature. Differential diagnosis may be challenging and proper hematoxylin-eosin staining and IHC studies are mandatory. Since it has been documented that extra-cutaneous MCC (including salivary gland MCC) can express MCPyV [28], we defined the present case as a MCC of the submandibular gland with an unusual cytokeratin profile.
References
- 1.Toker C. Trabecular carcinoma of the skin. Arch Dermatol. 1972;105:107–110. doi: 10.1001/archderm.1972.01620040075020. [DOI] [PubMed] [Google Scholar]
- 2.Tang CK, Toker C. Trabecular carcinoma of the skin: an ultrastructural study. Cancer. 1978;42:2311–2321. doi: 10.1002/1097-0142(197811)42:5<2311::AID-CNCR2820420531>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 3.Foschini MP, Eusebi V. Divergent differentiation in endocrine and non-endocrine tumors of the skin. Semin Diagn Pathol. 2000;17:162–168. [PubMed] [Google Scholar]
- 4.Walsh NM. Primary neuroendocrine (Merkel cell) carcinoma of the skin: morphologic diversity and implications thereof. Hum Pathol. 2001;32:680–689. doi: 10.1053/hupa.2001.25904. [DOI] [PubMed] [Google Scholar]
- 5.Saeb-Lima M. Montante-Montes de Oca D, Albores-Saavedra J. Merkel cell carcinoma with eccrine differentiation: a clinicopathologic study of 7 cases. Ann Diagn Pathol. 2008;12:410–414. doi: 10.1016/j.anndiagpath.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Mir R, Sciubba JJ, Bhuiya TA, et al. Merkel cell carcinoma arising in the oral mucosa. Oral Surg Oral Med Oral Pathol. 1988;65:71. doi: 10.1016/0030-4220(88)90195-8. [DOI] [PubMed] [Google Scholar]
- 7.Albores-Saavedra J, Batich K, Chable-Montero F, et al. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol. 2010;37:20–27. doi: 10.1111/j.1600-0560.2009.01370.x. [DOI] [PubMed] [Google Scholar]
- 8.Fornelli A, Eusebi V, Pasquinelli G, et al. Merkel cell carcinoma of the parotid gland associated with Warthin tumor: report of two cases. Histopathology. 2001;39:342–346. doi: 10.1046/j.1365-2559.2001.01240.x. [DOI] [PubMed] [Google Scholar]
- 9.Capella C, Marando A, Longhi E, et al. Primary gastric Merkel cell carcinoma harboring DNA polyomavirus: first description of an unusual high-grade neuroendocrine carcinoma. Hum Pathol. 2014;45:1310–1314. doi: 10.1016/j.humpath.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 10.Coleman NM, Smith-Zagone MJ, Tanyi J, et al. Primary neuroendocrine carcinoma of the vagina with Merkel cell carcinoma phenotype. Am J Surg Pathol. 2006;30:405–410. doi: 10.1097/01.pas.0000194737.95421.9d. [DOI] [PubMed] [Google Scholar]
- 11.Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49:832–841. doi: 10.1016/S0190-9622(03)02108-X. [DOI] [PubMed] [Google Scholar]
- 12.Agelli M, Clegg LX, Becker JC, et al. The etiology and epidemiology of Merkel cell carcinoma. Curr Probl Cancer. 2010;34:14–37. doi: 10.1016/j.currproblcancer.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Chernock RD, Duncavage EJ, Gnepp DR. Absence of Merkel cell polyomavirus in primary parotid high-grade neuroendocrine carcinomas regardless of cytokeratin 20 immunophenotype. Am J Surg Pathol. 2011;35:1806–1811. doi: 10.1097/PAS.0b013e318236a9b0. [DOI] [PubMed] [Google Scholar]
- 14.Yang GC, Schneck MJ, Hayden RE, et al. Merkel cell tumor-like neuroendocrine carcinoma associated with the submandibular gland. Report of a case with cytologic, immunohistochemical, electron microscopic and flow cytometric studies. Acta Cytol. 1994;38:742–746. [PubMed] [Google Scholar]
- 15.Gnepp DR, Corio RL, Brannon RB. Small cell carcinoma of the major salivary glands. Cancer. 1986;58:705–714. doi: 10.1002/1097-0142(19860801)58:3<705::AID-CNCR2820580318>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 16.Hanly AJ, Elgart GW, Jorda M, et al. Analysis of thyroid transcription factor-1 and cytokeratin 20 separates Merkel cell carcinoma from small cell carcinoma of lung. J Cutan Pathol. 2000;27:118–120. doi: 10.1034/j.1600-0560.2000.027003118.x. [DOI] [PubMed] [Google Scholar]
- 17.Bobos M, Hytiroglu P, Kostopoulos I, et al. Immunohistochemical distinction between Merkel cell carcinoma and small cell carcinoma of the lung. Am J Dermatopathol. 2006;28:99–104. doi: 10.1097/01.dad.0000183701.67366.c7. [DOI] [PubMed] [Google Scholar]
- 18.Pilloni L, Manieli C, Senes G, et al. Merkel cell carcinoma with an unusual immunohistochemical profile. Eur J Histochem. 2009;53:e33. doi: 10.4081/ejh.2009.e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calder KB, Coplowitz S, Schlauder S, et al. A case series and immunophenotypic analysis of CK20−/CK7+ primary neuroendocrine carcinoma of the skin. J CutanPathol. 2007;34:918–923. doi: 10.1111/j.1600-0560.2007.00759.x. [DOI] [PubMed] [Google Scholar]
- 20.Nagao T, Gaffey TA, Olsen KD, et al. Small cell carcinoma of the major salivary glands. Clinicopathological study with emphasis on cytokeratin 20 immunoreactivity and clinical outcome. Am J Surg Pathol. 2004;28:762–770. doi: 10.1097/01.pas.0000126776.65815.48. [DOI] [PubMed] [Google Scholar]
- 21.Feng H, Shuda M, Chang Y, et al. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Busam KJ, Jungbluth AA, Rekthman N. Merkel cell polyomavirus expression in Merkel cell carcinomas and its absence in combined tumors and pulmonary neuroendocrine carcinomas. Am J Surg Pathol. 2009;33:1378–1385. doi: 10.1097/PAS.0b013e3181aa30a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duncavage EJ, Zehnbauer BA, Pfeifer JD. Prevalence of Merkel cell polyomavirus in Merkel cell carcinoma. Mod Pathol. 2009;22:516–521. doi: 10.1038/modpathol.2009.3. [DOI] [PubMed] [Google Scholar]
- 24.Garneski KM, DeCaprio JA, Nghiem P. Does a new polyomavirus contribute to Merkel cell carcinoma? Genome Biol. 2008;9:228. doi: 10.1186/gb-2008-9-6-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kassem A, Schopflin A, Diaz C, et al. Frequent detection of Merkel cell polyomavirus in human Merkel cell carcinomas and identification of a unique deletion in the VP1 gene. Cancer Res. 2008;68:5009–5013. doi: 10.1158/0008-5472.CAN-08-0949. [DOI] [PubMed] [Google Scholar]
- 26.Ly TY, Walsh NM, Pasternak S. The spectrum of Merkel cell polyomavirus expression in Merkel cell carcinoma, in a variety of cutaneous neoplasms, and in neuroendocrine carcinomas from different anatomical sites. Hum Pathol. 2012;43:557–566. doi: 10.1016/j.humpath.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Erovic BM, Al Habeeb A, Harris L, et al. Significant overexpression of the Merkel cell polyomavirus (MCPyV) large T antigen in Merkel cell carcinoma. Head Neck. 2013;35:184–189. doi: 10.1002/hed.22942. [DOI] [PubMed] [Google Scholar]
- 28.de Biase D, Ragazzi M, Asioli S, et al. Extracutaneous Merkel cell carcinomas harbor polyomavirus DNA. Hum Pathol. 2012;43:980–985. doi: 10.1016/j.humpath.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 29.De Vincente Rodriguez JC, Fresno FMF, Junquera GLM, et al. Small cell undifferentiated carcinoma of the submandibular gland with neuroendocrine features. Ann Otol Rhinol Laryngol. 2004;113:55–59. doi: 10.1177/000348940411300113. [DOI] [PubMed] [Google Scholar]
- 30.Sowerby LJ, Matthews TW, Khalil M, et al. Primary large cell neuroendocrine carcinoma of the submandibular gland: unique presentation and surprising treatment response. J Otolaryngol. 2007;36:65–69. doi: 10.2310/7070.2007.E007. [DOI] [PubMed] [Google Scholar]
- 31.Kawaratani H, Tsujimoto T, Yoshikawa M, et al. Large cell neuroendocrine carcinoma presenting with neck swelling in the submandibular gland: a case report. J Med Case Rep. 2013;19:81. doi: 10.1186/1752-1947-7-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petrone G, Santoro A, Angrisani B, et al. Neuroendocrine tumors of the submandibular gland: literature review and report of a case. Int J Surg Pathol. 2013;21:85–88. doi: 10.1177/1066896912446747. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto N, Minami S, Kidoguchi M, et al. Large cell neuroendocrine carcinoma of the submandibular gland: case report and literature review. Auris Nasus Larynx. 2014;41:105–108. doi: 10.1016/j.anl.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 34.Toyosawa S, Ohnishi A, Ito R, et al. Small cell undifferentiated carcinoma of the submandibular gland: immunohistochemical evidence of myoepithelial, basal and luminal cell features. Pathol Int. 1999;49:887–892. doi: 10.1046/j.1440-1827.1999.00952.x. [DOI] [PubMed] [Google Scholar]
- 35.Dardick I. Electron microscopy. In: Gnepp DR, editor. Pathology of the head and neck. New York: Churchill Livingstone; 1988. pp. 101–190. [Google Scholar]
- 36.Hui KK, Luna MA, Batsakis JG, et al. Undifferentiated carcinomas of the major salivary glands. Oral Surg Oral Med Oral Pathol. 1990;69:76–83. doi: 10.1016/0030-4220(90)90271-S. [DOI] [PubMed] [Google Scholar]
- 37.Jorcano S, Casado A, Berenguer J. Primary neuroendocrine small cell undifferentiated carcinoma of the parotid gland. Clin Transl Oncol. 2008;10:303–306. doi: 10.1007/s12094-008-0203-z. [DOI] [PubMed] [Google Scholar]
- 38.Ettl T, Gosau M, Brockhoff G, et al. Predictors of cervical lymph node metastasis in salivary gland cancer. Head Neck. 2014;36:517–523. doi: 10.1002/hed.23332. [DOI] [PubMed] [Google Scholar]
- 39.Nobis CP, Rohleder NH, Wolff KD, et al. Head and neck salivary gland carcinomas-elective neck dissection, yes or no? J Oral Maxillofac Surg. 2014;72:205–210. doi: 10.1016/j.joms.2013.05.024. [DOI] [PubMed] [Google Scholar]
- 40.Zbaren P, Schupbach J, Nuyens M. Elective neck dissection versus observation in primary parotid carcinoma. Otolaryngol Head Neck Surg. 2005;132:387–391. doi: 10.1016/j.otohns.2004.09.029. [DOI] [PubMed] [Google Scholar]