Abstract
Sinonasal mucosal melanoma is a rare disease with poor survival. These tumors may have associated intraepithelial melanocytic proliferations, which are not extensively characterized. This retrospective analysis of 32 patients with sinonasal mucosal melanoma examined associated intraepithelial melanocytic proliferations in the context of diagnostic and prognostic features. Patient age ranged from 30 to 90 years (median 71) with a male to female ratio of approximately 3:2. Follow up for 31 patients ranged from 5 to 211 months (mean 42 months). Most patients died from melanoma-associated causes (18/31, 58 %), six (19 %) died from unknown causes, two (6 %) were alive with metastatic disease, and only five patients (16 %) remained alive without melanoma. The tumors were histopathologically heterogeneous, displaying epithelioid, spindled, and small cell cytomorphology. The presence of >2 mitoses/mm2 and necrosis correlated with tumor progression and overall survival, respectively (p = 0.04 for both). Melanoma in situ, defined as a confluent intraepithelial proliferation of cytologically atypical melanocytes, was identified in 20 of 30 evaluable cases (67 %) and confirmed with immunohistochemical staining for microphthalmia-associated transcription factor. Melanocytic hyperplasia, defined as intraepithelial melanocytic proliferation without confluent growth or marked atypia, was seen in five cases (16 %). This incidence of associated intraepithelial melanocytic proliferations (83 %) is higher than previously reported. Because of the locally aggressive nature of these tumors, an awareness of the high rate of associated intraepithelial melanocytic proliferations may inform future studies of therapeutic options.
Keywords: Mucosal melanoma, Sinonasal, Melanoma in situ, Aerodigestive tract, MITF
Introduction
Primary mucosal melanoma of the sinonasal tract is a rare, usually fatal disease with high rates of local recurrence and metastasis [1–3]. Primary mucosal melanoma may arise in the nasal cavity, paranasal sinuses, and oral cavity, and has an overall 5-year survival of approximately 20 % [1–4]. Many published series group oral mucosal melanoma with sinonasal melanoma. Pathologic staging of disease and clinical factors including patient age and tumor location offer some prognostic information [2, 3, 5, 6]. However, standard staging schemas used for cutaneous melanomas do not apply to these tumors. Histopathologic features associated with poor prognosis in cutaneous melanoma, including ulceration and increased tumor thickness are commonly observed in sinonasal melanoma [7]. Histologic prognostic factors, such as Breslow thickness in cutaneous melanoma, have not been definitively established in sinonasal melanoma, although some studies have correlated tumor thickness with poor outcome [8–10]. A large series of 115 sinonasal melanomas described undifferentiated tumor cell morphology and high mitotic index as important prognostic factors; these authors did not focus on the presence or absence of an associated intraepithelial component [5]. Smaller studies of sinonasal melanoma have not found consistent, prognostically significant histopathologic features and have variably described the intraepithelial melanocytic component [8–11].
As noted above, robust evaluations of the presence and significance of intraepithelial melanocytic proliferations associated with sinonasal melanoma are scarce. The vast majority of melanomas arising from the squamous mucosa of the head and neck have an in situ component [12, 13]. However, series of sinonasal melanoma have typically reported a much lower incidence of intraepithelial melanocytic proliferations [5, 10], although one study noted junctional change (defined as atypical melanocytes in the overlying epithelium) in 5 of 9 cases with intact epithelium [14]. Previous immunohistochemical studies have demonstrated the utility of S-100 protein, HMB-45, tyrosinase, Melan-A, and microphthalmia transcription factor (MITF) in the diagnosis of mucosal melanoma of the head and neck [1, 5, 14–18]. Other studies have correlated Ki-67 index and matrix metalloproteinase expression with prognosis [19, 20]. Immunohistochemistry may be particularly helpful in defining the extent of possible associated intraepithelial melanocytic proliferations.
The goal of this study was to perform a detailed clinicopathologic examination of sinonasal melanoma, investigate possible markers of prognosis, and better characterize the intraepithelial melanocytic proliferations associated with sinonasal melanoma through histopathologic and immunohistochemical analysis.
Materials and Methods
The study was approved by the Partners Human Research Committee (IRB# 2012-P-001718). An electronic search of the surgical pathology files of the Massachusetts General Hospital and Massachusetts Eye and Ear Infirmary from 1990 to 2012 yielded 33 cases of sinonasal melanoma. Of these, glass slides and archived tissue samples were available from 32. The electronic medical record for each patient was reviewed to document patient age, tissue site, treatment, response to therapy, and status at the last clinical visit or date and cause of death. Additional follow up information was obtained through the Social Security Death Index (SSDI) [21]. Histopathological analysis included documentation of tumor cell morphology, mitogenicity (number of mitotic figures/mm2; counted at the mitotic “hot spot” with a 40 × objective), ulceration, regions of tumor cell necrosis, perineural invasion, lymphovascular invasion, involvement of deep structures (such as bone, skeletal muscle, cartilage), margin status, and the presence and extent of intraepithelial melanocytic proliferations. Mucosal melanoma in situ (MMIS) was defined as a confluent intraepithelial melanocytic proliferation of cytologically atypical melanocytes, while melanocytic hyperplasia was defined as an intraepithelial melanocytic proliferation without confluent growth or marked cytologic atypia.
Immunohistochemical Studies
Immunohistochemical detection of microphthalmia-associated transcription factor (MITF) was performed on sections of formalin-fixed, paraffin-embedded tissue samples using standard peroxidase immunohistochemical techniques (Clone D5, DAKO, Carpentaria, CA). When the intraepithelial melanocytes were visible on hematoxylin and eosin-stained tissue sections (H&E), selected blocks from each case with the most robust appearing intraepithelial melanocytic proliferation were stained for MITF to confirm the presence of melanocytes. In some cases without apparent intraepithelial melanocytes, MITF was performed on the tissue blocks with melanoma and the most abundant epithelium. A case of MITF positive cutaneous melanoma was used as the positive control, while five cases of benign sinonasal tissue removed for rhinosinusitis were used as negative controls. Intraepithelial melanocytes were not detected in the normal control tissues. While MITF is not a melanocyte-specific stain and may mark histiocytes, the combination of H&E stained sections with MITF stains allowed for identification of intraepithelial melanocytes.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism software (Version 6.03; GraphPad Software, San Diego, California). The Kaplan–Meier method was utilized to construct survival curves, and univariate survival analysis was performed using the log-rank test. Overall survival was defined as the time from the date of the initial diagnostic biopsy to the date of death or last clinical follow-up. Fisher’s exact test was used to test mitogenicity vs. tumor progression.
Results
Clinical Characteristics
This study investigated 32 patients with mucosal melanoma of the sinonasal tract. Clinical data including follow up information are shown in Table 1. The median patient age at diagnosis was 71 years of age (range 30–90) and the male to female ratio was approximately 3:2. 23 patients were Caucasian, 1 was Hispanic, and 8 were of undocumented race. Follow up was available for 31 patients (range 5–211 months; mean 42 months; median 16 months). Of the 31 tumors with clinical follow up, 11 were intranasal, 3 in the maxillary sinus, 1 in both maxillary and ethmoid sinuses, and 16 were sinonasal without detailed information on location. Of the 25 patients with detailed social histories, 12 had a history of cigarette smoking and one had a history of radiation therapy for acne. None had a documented history of immunosuppression or exposure to formaldehyde, volatile solvents, or other industrial chemicals. At last available follow up, five were alive with no evidence of disease (ANED), two were alive with metastatic melanoma (AWD), 18 died of melanoma associated causes (DOD), and 6 were dead from unknown cause (DOC). The median overall survival was 26 months, and the observed 5-year overall survival rate was 22 %. Of the 19 patients with documented recurrence or metastases, 6 developed local recurrences from 1 to 18 months after diagnosis (median 7 months), while 14 developed metastatic disease to the lung (5), liver (3), brain (2), spine (2), subcutaneous tissue (2), lymph nodes (2), adrenal gland (1), and breast (1) from 3 to 37 months after diagnosis (median 6 months). One patient had both a local recurrence and eventual metastasis to the brain and another had metastases to the lung; both of these patients were alive at last follow up.
Table 1.
Clinical data of patients with primary sinonasal mucosal melanoma
| Characteristic | Number of patients (%) |
|---|---|
| Total patients | 32 |
| Age, y, median (range) | 71 (30–90) |
| Male | 19 (59) |
| Follow up, mo, mean (range) | 42 (5–211) |
| Documented local recurrence | 6 (19)a |
| Timeb, median (range) | 7 (1–18) |
| Documented metastasis | 14 (45)a |
| Timeb, median (range) | 6 (3–37) |
| At end of follow up: | |
| Dead of disease | 18 (58)a |
| Dead from uncertain cause | 6 (19)a |
| Alive with disease | 2 (6)a |
| Alive with NED | 5 (16)a |
aPercentage out of 31 patients with follow up
bTime in months after diagnosis when event occurred
Histopathologic and Immunohistochemical Characteristics
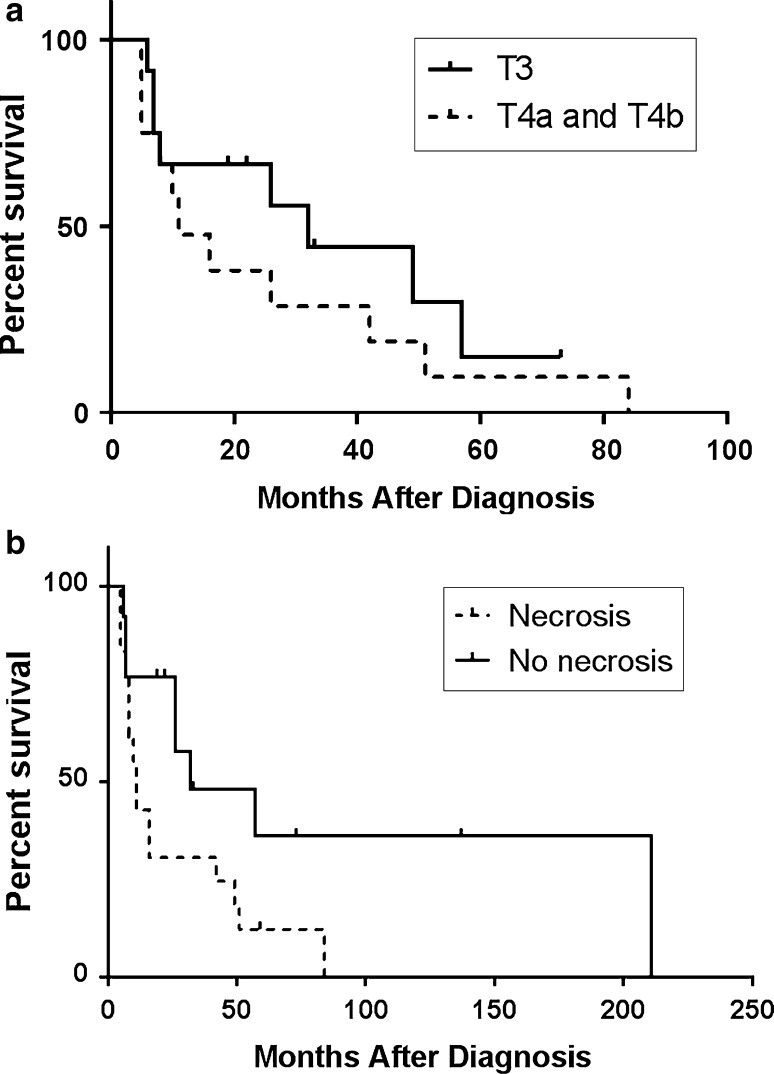
Thirty-nine specimens of sinonasal melanoma were examined from 32 patients. Pathologic features and correlations with survival data are shown in Table 2. While excisional specimens (and some biopsies) were reviewed for 28 patients, only representative biopsy material was available for four patients. Determination of tumor thickness was not possible in the vast majority of cases due to specimen fragmentation. Of the seven specimens with adequate orientation, the thickness from the surface of the epithelium to the deepest melanoma cell ranged from 0.3 to 15.0 mm (median 2.0 mm). By American Joint Committee on Cancer staging [22], there were twelve T3 tumors, eight T4a tumors, five T4b tumors, and seven with unknown staging where sampling did not permit evaluation of tumor invasion. T3 tumors appeared to have a slightly better prognosis than T4a and T4b tumors (Fig. 1a), but this difference was not statistically significant by log rank analysis. Regarding N and M stage at the time of diagnosis, three patients had positive lymph nodes, all of whom died of disease (at 10, 16, and 42 months) consistent with an aggressive course for node positive patients, and none had distant metastases.
Table 2.
Pathologic data of patients with primary sinonasal mucosal melanoma
| Characteristic | Number of patients (%) | Correlation with overall survival p |
|---|---|---|
| Ulceration | 15 (47) | 0.42 |
| Necrosis | 19 (59) | 0.04 |
| Morphology of invasive component | 0.72 | |
| Epithelioid | 25 (78) | |
| Mixed spindled and epithelioid | 4 (13) | |
| Small round cell | 3 (9) | |
| Intraepithelial melanocytic proliferation | 5 (83a) | 0.97 |
| Melanoma in situ | 20 (67) | |
| Melanocytic hyperplasia | 5 (17) | |
| Mitotic figures >3 per 10 HPF (>2 per mm2) | 21 (70b) | 0.23c |
| Perineural invasion | 1 (3) | NC |
| Lymphovascular invasion | 0 (0) | NC |
| T stage at diagnosis | 0.33d | |
| T3 | 12 (38) | |
| T4a | 8 (25) | |
| T4b | 5 (16) | |
| Unknown | 7 (22) | |
| Node positive at diagnosis (N1) | 3 (9) | 0.47 |
| Distant metastasis at diagnosis (M1) | 0 (0) | NC |
Bold value indicate statistical significance
NC Not calculated
aPercentage out of 30 cases with available epithelium
bPercentage out of 30 cases with at least 10 high power fields of invasive tumor
c p = 0.04 for correlation with risk of disease progression (metastasis or local recurrence)
dLog-rank p value for survival difference between T3 and both T4a and T4b tumors
Fig. 1.
a Overall survival of sinonasal melanoma by T-stage (log-rank p = 0.33). b Overall survival of sinonasal melanoma with respect to necrosis (log-rank p = 0.043)
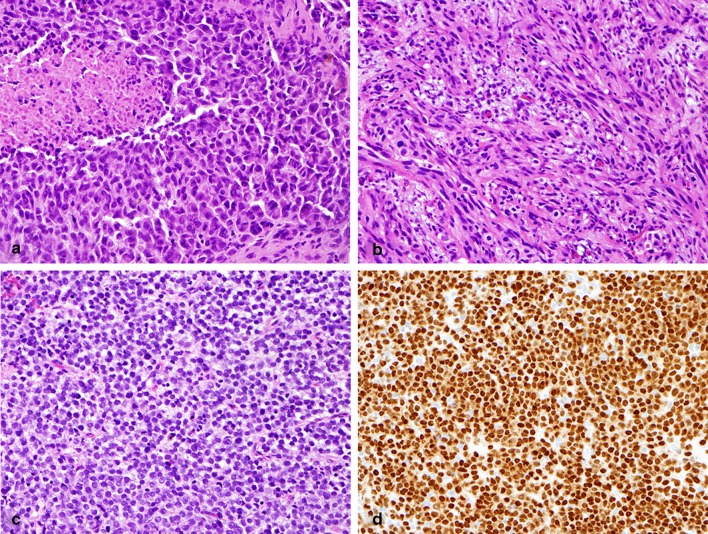
Histologic examination revealed intratumoral heterogeneity, including epithelioid, spindled, and small cell cytomorphology (Fig. 2). The sinonasal melanomas were predominately epithelioid (25 cases; Fig. 2a). Two of these epithelioid tumors also displayed a focally prominent plasmacytoid appearance. Four tumors had a mixed epithelioid and spindled appearance with only one of these tumors showing predominately spindled morphology (Fig. 2b). Three cases showed small round cell morphology (Fig. 2c). Invasive melanoma in all cases was at least focally strongly positive for MITF (Fig. 2d). Ulceration was present in 15 cases (47 %) and necrosis in 19 cases (59 %). The median number of mitotic figures was four mitoses per mm2 (range 0–27). While tumor cell morphology showed no significant association with outcome, the presence of tumor cell necrosis correlated with shorter survival (p = 0.04) as demonstrated in Fig. 1b. Patients with tumors bearing more than two mitoses per mm2 demonstrated a greater risk of developing progressive local disease and metastasis than patients with zero to two mitoses per mm2 (p = 0.04); however, mitotic count was not significantly correlated with overall survival. Ulceration was not associated with survival, although most patients (4/5) without metastases did not have ulcerated tumors. Perineural invasion was identified in one case and was associated with a very aggressive course with death from disease within 5 months of diagnosis. Definitive lymphovascular invasion was not identified in any of the cases.
Fig. 2.
Cytomorphology and MITF positivity of the invasive component of sinonasal melanoma. a Epithelioid morphology was seen in the majority of cases. Necrosis was seen in 59 % (H&E, ×400). b Spindle cell morphology (H&E, ×400). c Small round cell morphology (H&E, ×400). d Strong nuclear MITF positivity in invasive melanoma with small round cell morphology (×400)
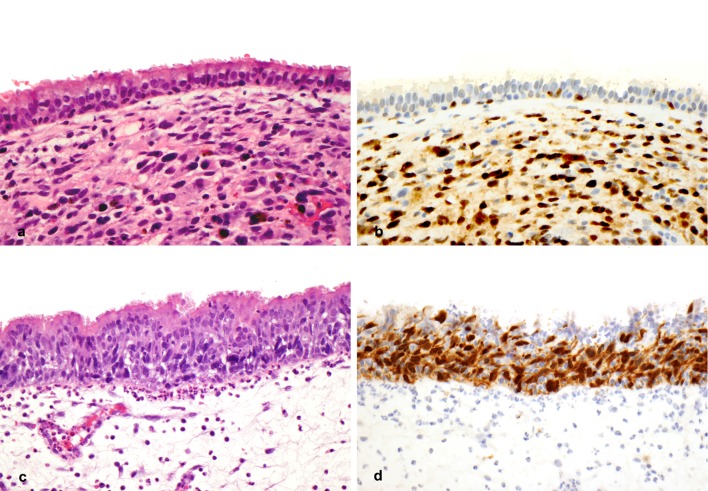
Intraepithelial melanocytic proliferations were seen in 25/30 (83 %) of cases and showed a range of morphologic appearances; epithelium was not present in specimens from two patients (Fig. 3). Normal sinonasal mucosa from five patients with chronic rhinosinusitis showed only rare intraepithelial MITF-positive cells, along with weak cytoplasmic staining in rare stromal histiocytes (data not shown). Melanocytic hyperplasia, defined as intraepithelial melanocytic proliferation without confluent growth or marked cytologic atypia, was observed in 5 cases (16 %) (Fig. 3a, b). Mucosal melanoma in situ (MMIS), defined as confluent intraepithelial melanocytic proliferation of cytologically atypical melanocytes, was identified in 20 (67 %) cases (Fig. 3c, d). MMIS was associated with areas of melanocytic hyperplasia in the majority of cases. MMIS within respiratory epithelium or sinonasal glands was often subtle and difficult to identify on H&E stained tissue sections. In all cases of MMIS, MITF staining confirmed the presence of an intraepithelial proliferation of cytologically atypical and confluent melanocytes. The presence of MMIS did not correlate significantly with overall survival (p = 0.91).
Fig. 3.
Intraepithelial melanocytic proliferations associated with sinonasal melanoma. a, b Melanocytic hyperplasia overlying invasive melanoma (a H&E, ×400; b MITF ×400). c, d Malignant melanoma in situ (c H&E, ×400; d MITF ×400)
Margin status of the excisional specimen was evaluable in 12 patients: margins were negative for in situ or invasive melanoma in seven patients, positive for the presence of intraepithelial atypical melanocytes in two, positive for in situ melanoma in one, and positive for invasive melanoma in two patients. Margins were undeterminable in 16 patients due to specimen fragmentation. While margin status was not significantly associated with survival (p = 0.23), the two patients with invasive melanoma at the margin died at 5 and 16 months.
Molecular testing was performed for clinical purposes on tumors from five patients. Four cases were negative for both BRAF and KIT mutations. A KIT exon 11 L576P mutation was detected in one case.
Discussion
In this study of 32 patients with sinonasal mucosal melanoma, most patients (83 %) were found to have intraepithelial melanocytic proliferations, including MMIS and melanocytic hyperplasia, associated with their invasive melanomas. This incidence of intraepithelial melanocytic proliferations is greater than previously reported. In a large previously published series of sinonasal melanoma, surface derivation or junctional melanocytic activity was reported in only 20 % of cases [5]. Pagetoid spread, defined as the intraepithelial spread of tumor cells beyond the basilar epithelial component, was found in 16 % of cases [5]. In another study, an in situ component was found in 7/7 (100 %) of oral melanomas, but only 1/22 (5 %) of sinonasal melanomas [10]. Franquemont and Mills found atypical intraepithelial melanocytes in 5/9 (56 %) of sinonasal melanomas with intact epithelium [14]. Another series of five cases noted lentiginous melanocytes within mucosal epithelium in three cases of sinonasal melanoma (60 %), two of which were only observed after immunohistochemistry [18].
The finding in this study of MMIS in 67 % and melanocytic hyperplasia in 16 % of sinonasal melanomas suggests that intraepithelial melanocytic proliferations associated with these tumors are more common than previously thought. While the reported lower frequency of intraepithelial melanocytic proliferations has led to speculations that melanoma can arise from subepithelial or gland-based melanocytes, this study supports the hypothesis that precursor melanocytic lesions more likely develop in the mucosal epithelium of these tumors. As noted in this study, specimens of sinonasal melanoma often contain only scant or fragmented epithelium. Additionally, when the epithelium is present the identification of intraepithelial melanocytes may be difficult on H&E stained tissue sections alone. While older studies were performed prior to the availability of sensitive immunohistochemical markers such as MITF and Melan-A, more recent studies have focused on using these markers for invasive tumor diagnosis rather than evaluation of the intraepithelial component [1, 5, 14–18].
In this study, no significant melanocytic proliferation was seen in the mucosal epithelium or submucosal glands in control sinonasal specimens. The identification of intraepithelial melanocytic proliferations in association with over 80 % of sinonasal melanomas suggests the possibility that these intraepithelial proliferations represent precursor lesions. Importantly, melanocytic hyperplasias (occasionally termed “melanosis”) in the absence of melanoma may be identified rarely in head and neck mucosal sites, presenting a clinical management challenge, given the uncertain clinical behavior of these lesions and typically extensive and diffuse involvement of mucosal surfaces [23–25]. Indeed, this type of melanocytic proliferation has been previously proposed as a precursor of melanoma [23, 24] and has been reported in a patient with two subsequent foci of nasal melanoma [26]. In a detailed investigation of a single case, Hofbauer et al. [25] demonstrated that the atypical intraepithelial melanocytes in “melanosis” associated with a nasal melanoma demonstrated loss of heterozygosity of a marker flanking the p16 gene; the component of invasive melanoma showed loss of both alleles, indicating a possible precursor role of the intraepithelial melanocytic proliferation.
While MITF staining of invasive mucosal melanoma has been extensively described, the use of this marker in the evaluation of the intraepithelial component is not well established. In this study, staining for MITF offered high sensitivity for the detection of intraepithelial melanocytic proliferations, which may be challenging to identify in this setting. Similarly, MITF is a useful adjunct to H&E in the evaluation of intraepidermal melanocytic proliferations in chronically sun-damaged skin [27]. MITF is a particularly useful marker in evaluating intraepithelial melanocytes because it is a sensitive and specific nuclear marker [28]. Although MITF may also stain histiocytes, this is not a common confounder in mucosal epithelium. Melan-A, MART-1 and HMB-45 stain cytoplasmic proteins that are associated with pigmentation and have been found in some cases to stain epithelium [29, 30]. S-100 protein is the most sensitive stain for melanocytes; however, S-100 protein lacks specificity in that it also will stain intraepithelial dendritic cells and myoepithelial cells within the submucosal glands. In this study MITF revealed the frequent presence of MMIS and sinonasal gland extension which was often extremely subtle on H&E.
MITF immunohistochemistry could be considered in the assessment of surgical margins. However, this study did not find a significant correlation of margin status with prognosis, although data were limited due to tissue fragmentation at excision. In addition, the clinical significance of intraepithelial melanocytic proliferations at the margins of sinonasal melanoma excisions has not been determined in previous studies. Thus, additional studies would first be required to further investigate if examination of margins for intraepithelial disease, similar to cutaneous melanoma, results in improved outcome.
Previous publications describe sinonasal melanoma as a rare disease with a poor prognosis; the median survival (26 months) and 5-year survival rate (22 %) observed in this study support those prior findings [1–3, 5]. The rate of distant metastasis (45 %) was at the lower end of the approximately 40–70 % range found in previous studies of sinonasal melanoma [1, 7, 8, 11]. The association of necrosis with poorer survival in this study is, to the authors’ knowledge, a novel finding in sinonasal melanoma, and analogous to a previous study that correlated poorer survival with necrosis in the context of non-sinonasal head and neck melanomas of squamous mucosa [12]. Previous studies of sinonasal melanoma have found no correlation with necrosis [5, 9, 11], although comparisons are difficult since in most studies mucosal melanomas arising at various head and neck sites were grouped together, except for one large study on sinonasal melanoma [5]. Mitogenicity, specifically the presence of more than 2 mitoses per mm2 was associated with an increased risk of disease progression, supporting an earlier study that correlated mitotic rate with survival [5].
The limitations of this study include the retrospective nature of the analysis and the small number of cases. Nevertheless, this represents a relatively large clinicopathologic review of this rare entity. The identification of necrosis and mitogenicity as potential prognostic factors in sinonasal melanoma warrants further investigation.
This study reported the mutational analysis performed as part of clinical care in these patients. The absence of BRAF mutations and the presence of a rare KIT mutation support prior reports showing a relatively low BRAF and KIT mutation frequency in sinonasal melanomas.
The advent of targeted therapy has coincided with several studies of oncogenic mutations in head and neck mucosal melanoma. KIT mutations have been reported in <2 % of cutaneous melanoma but are found in 13–27 % of head and neck mucosal melanoma [31]. A recent study detected a KIT mutation in 1 of 12 sinonasal melanomas [32]. An additional study of 32 cases of sinonasal mucosal melanoma found KIT mutations in 13 %, NRAS mutations in 22 %, and a BRAF mutation in 1 case [33]. These authors also demonstrated activation of the PI3/Akt and MAPK pathways through protein expression studies [33]. These molecular findings may have important implications for both the pathogenesis of sinonasal melanoma as well as targeted therapies [34].
In summary, this study demonstrates a high rate of associated intraepithelial melanocytic proliferations in sinonasal melanoma. This is a malignancy with poor survival with local aggressiveness and high metastatic potential. This study also found that necrosis and mitogenicity correlated with survival and tumor progression in sinonasal melanoma. Based on these results, careful examination of sinonasal melanoma including the use of immunohistochemistry may be useful to pathologists in the diagnosis of primary mucosal melanoma and in the assessment of margins. Additional work to identify meaningful tumor prognostic factors in these rare but often fatal tumors may include evaluation of tumor necrosis and mitogenicity.
Conflict of interest
The authors have no conflicts of interest or funding to disclose.
References
- 1.Dauer EH, Lewis JE, Rohlinger AL, Weaver AL, Olsen KD. Sinonasal melanoma: a clinicopathologic review of 61 cases. Otolaryngol Head Neck Surg. 2008;138:347–352. doi: 10.1016/j.otohns.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 2.Gal TJ, Silver N, Huang B. Demographics and treatment trends in sinonasal mucosal melanoma. Laryngoscope. 2011;121:2026–2033. doi: 10.1002/lary.21925. [DOI] [PubMed] [Google Scholar]
- 3.Jethanamest D, Vila PM, Sikora AG, Morris LG. Predictors of survival in mucosal melanoma of the head and neck. Ann Surg Oncol. 2011;18:2748–2756. doi: 10.1245/s10434-011-1685-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manolidis S, Donald PJ. Malignant mucosal melanoma of the head and neck: review of the literature and report of 14 patients. Cancer. 1997;80:1373–1376. doi: 10.1002/(SICI)1097-0142(19971015)80:8<1373::AID-CNCR3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 5.Thompson LD, Wieneke JA, Miettinen M. Sinonasal tract and nasopharyngeal melanomas: a clinicopathologic study of 115 cases with a proposed staging system. Am J Surg Pathol. 2003;27:594–601. doi: 10.1097/00000478-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Shuman AG, Light E, Olsen SH, et al. Mucosal melanoma of the head and neck: predictors of prognosis. Arch Otolaryngol Head Neck Surg. 2011;137:331–337. doi: 10.1001/archoto.2011.46. [DOI] [PubMed] [Google Scholar]
- 7.Prasad ML, Busam KJ, Patel SG, Hoshaw-Woodard S, Shah JP, Huvos AG. Clinicopathologic differences in malignant melanoma arising in oral squamous and sinonasal respiratory mucosa of the upper aerodigestive tract. Arch Pathol Lab Med. 2003;127:997–1002. doi: 10.5858/2003-127-997-CDIMMA. [DOI] [PubMed] [Google Scholar]
- 8.Patel SG, Prasad ML, Escrig M, et al. Primary mucosal malignant melanoma of the head and neck. Head Neck. 2002;24:247–257. doi: 10.1002/hed.10019. [DOI] [PubMed] [Google Scholar]
- 9.Prasad ML, Patel SG, Huvos AG, Shah JP, Busam KJ. Primary mucosal melanoma of the head and neck: a proposal for microstaging localized, Stage I (lymph node-negative) tumors. Cancer. 2004;100:1657–1664. doi: 10.1002/cncr.20201. [DOI] [PubMed] [Google Scholar]
- 10.McLean N, Tighiouart M, Muller S. Primary mucosal melanoma of the head and neck. Comparison of clinical presentation and histopathologic features of oral and sinonasal melanoma. Oral Oncol. 2008;44:1039–1046. doi: 10.1016/j.oraloncology.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Kerr EH, Hameed O, Lewis JS, Jr, Bartolucci AA, Wang D, Said-Al-Naief N. Head and neck mucosal malignant melanoma: clinicopathologic correlation with contemporary review of prognostic indicators. Int J Surg Pathol. 2012;20:37–46. doi: 10.1177/1066896911417970. [DOI] [PubMed] [Google Scholar]
- 12.Prasad ML, Patel S, Hoshaw-Woodard S, et al. Prognostic factors for malignant melanoma of the squamous mucosa of the head and neck. Am J Surg Pathol. 2002;26:883–892. doi: 10.1097/00000478-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Umeda M, Komatsubara H, Shibuya Y, Yokoo S, Komori T. Premalignant melanocytic dysplasia and malignant melanoma of the oral mucosa. Oral Oncol. 2002;38:714–722. doi: 10.1016/S1368-8375(02)00008-8. [DOI] [PubMed] [Google Scholar]
- 14.Franquemont DW, Mills SE. Sinonasal malignant melanoma. A clinicopathologic and immunohistochemical study of 14 cases. Am J Clin Pathol. 1991;96:689–697. doi: 10.1093/ajcp/96.6.689. [DOI] [PubMed] [Google Scholar]
- 15.Wick MR, Stanley SJ, Swanson PE. Immunohistochemical diagnosis of sinonasal melanoma, carcinoma, and neuroblastoma with monoclonal antibodies HMB-45 and anti-synaptophysin. Arch Pathol Lab Med. 1988;112:616–620. [PubMed] [Google Scholar]
- 16.Fitzgibbons PL, Chaurushiya PS, Nichols PW, Chandrasoma PT, Martin SE. Primary mucosal malignant melanoma: an immunohistochemical study of 12 cases with comparison to cutaneous and metastatic melanomas. Hum Pathol. 1989;20:269–272. doi: 10.1016/0046-8177(89)90135-4. [DOI] [PubMed] [Google Scholar]
- 17.Prasad ML, Jungbluth AA, Iversen K, Huvos AG, Busam KJ. Expression of melanocytic differentiation markers in malignant melanomas of the oral and sinonasal mucosa. Am J Surg Pathol. 2001;25:782–787. doi: 10.1097/00000478-200106000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Lee H, Torres FX, McLean SA, Chen R, Lee MW. Immunophenotypic heterogeneity of primary sinonasal melanoma with aberrant expression of neuroendocrine markers and calponin. Appl Immunohistochem Mol Morphol. 2011;19:48–53. doi: 10.1097/PAI.0b013e3181ee8dcb. [DOI] [PubMed] [Google Scholar]
- 19.Kim DK, Kim DW, Kim SW, Kim DY, Lee CH, Rhee CS. Ki67 antigen as a predictive factor for prognosis of sinonasal mucosal melanoma. Clin Exp Otorhinolaryngol. 2008;1:206–210. doi: 10.3342/ceo.2008.1.4.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kondratiev S, Gnepp DR, Yakirevich E, et al. Expression and prognostic role of MMP2, MMP9, MMP13, and MMP14 matrix metalloproteinases in sinonasal and oral malignant melanomas. Hum Pathol. 2008;39:337–343. doi: 10.1016/j.humpath.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Social Security Death Index. Ancestry.com, 2013. http://www.ancestry.com/ps_ssdi. Accessed 1 Dec 2013.
- 22.Edge SB, Byrd DR, Compton CC, eds. Mucosal melanoma of the head and neck. American joint committee on cancer. 7th ed. Chicago, IL: Springer; 2010. p 97–100.
- 23.Lewis MG, Martin JA. Malignant melanoma of the nasal cavity in Ugandan Africans. Relationship of ectopic pigmentation. Cancer. 1967;20:1699–1705. doi: 10.1002/1097-0142(196710)20:10<1699::aid-cncr2820201020>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 24.Cove H. Melanosis, melanocytic hyperplasia, and primary malignant melanoma of the nasal cavity. Cancer. 1979;44:1424–1433. doi: 10.1002/1097-0142(197910)44:4<1424::AID-CNCR2820440438>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 25.Hofbauer GF, Böni R, Simmen D, et al. Histological, immunological and molecular features of a nasal mucosa primary melanoma associated with nasal melanosis. Melanoma Res. 2002;12:77–82. doi: 10.1097/00008390-200202000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Köppl H, Köppl R, Maier W, Freudenberg N. Recurrent, primary multifocal malignant melanoma of the mucous membrane of the upper respiratory tract. Peculiarities Situ Compon Pathol. 1999;20:195–199. doi: 10.1007/s002920050344. [DOI] [PubMed] [Google Scholar]
- 27.Kim J, Taube JM, McCalmont TH, Glusac EJ. Quantitative comparison of MITF, Melan-A, HMB-45, and Mel-5 in solar lentigines and melanoma in situ. J Cutan Pathol. 2011;38:775–779. doi: 10.1111/j.1600-0560.2011.01717.x. [DOI] [PubMed] [Google Scholar]
- 28.King R, Weilbaecher KN, McGill G, et al. Microphthalmia transcription factor. A sensitive and specific melanocyte marker for melanoma diagnosis. Am J Pathol. 1999;155:731–738. doi: 10.1016/S0002-9440(10)65172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhawan J. Mel-5: a novel antibody for differential diagnosis of epidermal pigmented lesions of the skin in paraffin-embedded sections. Melanoma Res. 1997;7:43–48. doi: 10.1097/00008390-199702000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Mangini JL, Li N, Bhawan J. Immunohistochemical markers of melanocytic lesions: a review of their diagnostic usefulness. Am J Dermatopathol. 2002;24:270–281. doi: 10.1097/00000372-200206000-00016. [DOI] [PubMed] [Google Scholar]
- 31.Beadling C, Jacobson-Dunlop E, Hodi FS, et al. KIT gene mutations and copy number in melanoma subtypes. Clin Cancer Res. 2008;14:6821–6828. doi: 10.1158/1078-0432.CCR-08-0575. [DOI] [PubMed] [Google Scholar]
- 32.Schoenewolf NL, Bull C, Belloni B, et al. Sinonasal, genital and acrolentiginous melanomas show distinct characteristics of KIT expression and mutations. Eur J Cancer. 2012;48:1842–1852. doi: 10.1016/j.ejca.2012.02.049. [DOI] [PubMed] [Google Scholar]
- 33.Turri-Zanoni M, Medicina D, Lombardi D, et al. Sinonasal mucosal melanoma: molecular profile and therapeutic implications from a series of 32 cases. Head Neck. 2013;35:1066–1077. doi: 10.1002/hed.23079. [DOI] [PubMed] [Google Scholar]
- 34.Papaspyrou G, Garbe C, Schadendorf D, Werner JA, Hauschild A, Egberts F. Mucosal melanomas of the head and neck: new aspects of the clinical outcome, molecular pathology, and treatment with c-kit inhibitors. Melanoma Res. 2011;21:475–482. doi: 10.1097/CMR.0b013e32834b58cf. [DOI] [PubMed] [Google Scholar]