Abstract
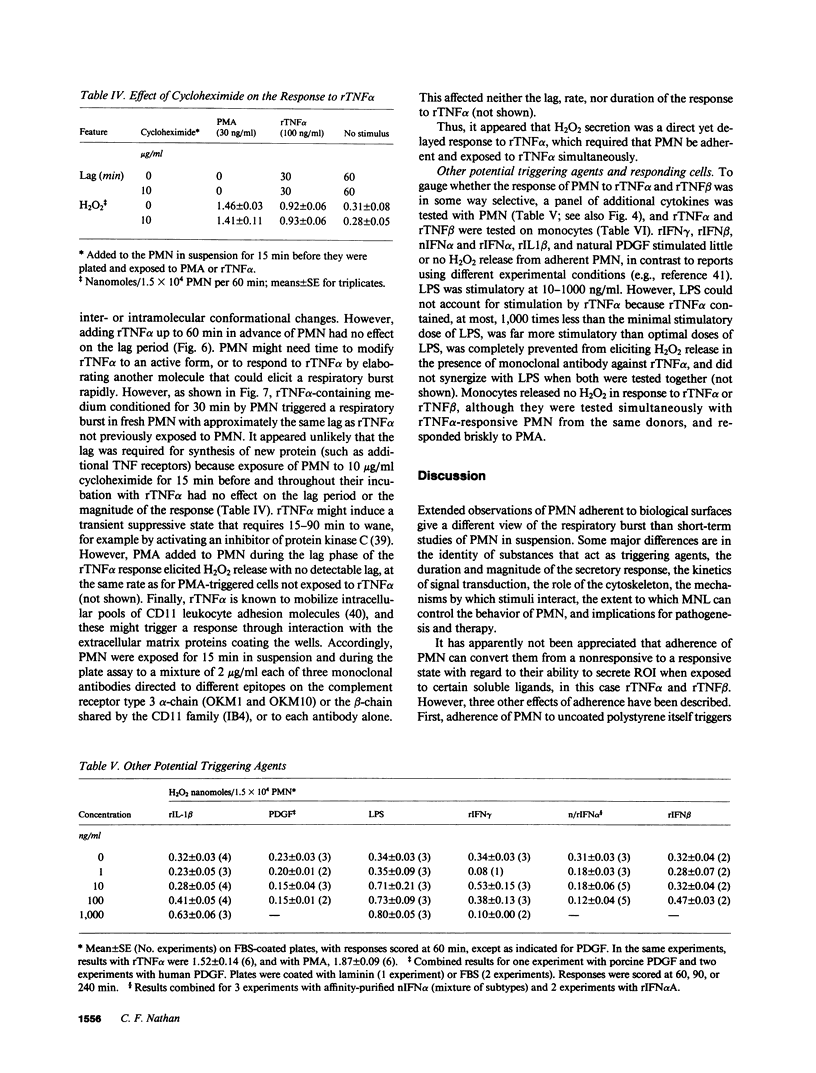
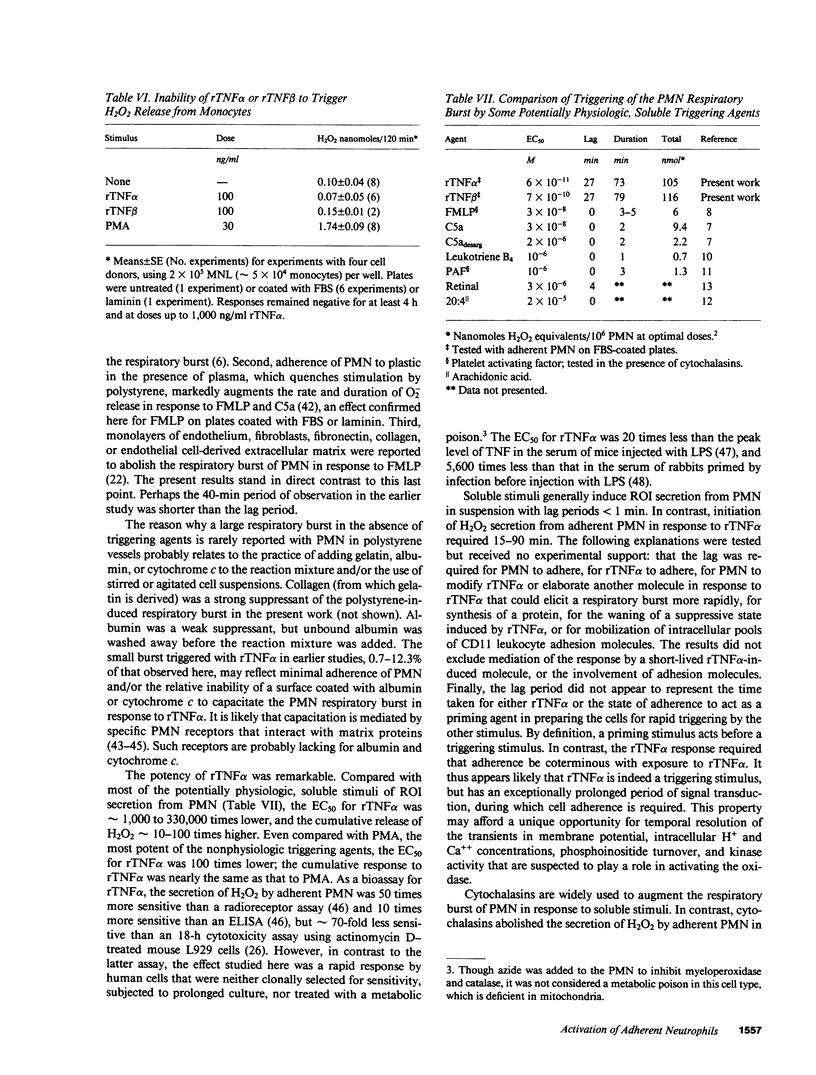
Recombinant tumor necrosis factor alpha (rTNF alpha) and beta (rTNF beta) did not trigger H2O2 release from PMN in suspension. However, when PMN were plated on polystyrene surfaces coated with serum, fibronectin, vitronectin, laminin, or human umbilical vein endothelial cells (HUVEC), rTNFs induced a massive, prolonged secretory response, similar to that elicited by phorbol myristate acetate (PMA) or bacteria. On serum-coated plates, the maximum sustained rate of H2O2 release in response to rTNF alpha was 2.6 +/- 0.2 nmol/min per 10(6) PMN, the same as that with PMA; release continued for 73 +/- 4 min. On laminin-coated surfaces or HUVEC, release of H2O2 in response to rTNFs was slower, but lasted approximately 3.5 h, reaching the same total (greater than 100 nmol/10(6) PMN). Not only was this response far longer and larger than for other soluble stimuli of the respiratory burst studied with PMN in suspension, but the concentration necessary to elicit a half-maximal response (EC50) for rTNF alpha was orders of magnitude lower (55 pM). Responses were similar with FMLP, but ranged from zero to small with recombinant IFN alpha, recombinant IFN beta, recombinant IFN gamma, platelet-derived growth factor, recombinant IL-1 beta, or bacterial lipopolysaccharide. Adherent monocytes did not secrete H2O2 in response to rTNFs. H2O2 secretion by adherent PMN was first detectable 15-90 min after addition of rTNFs or FMLP. This lag period was unaffected by prior exposure of PMN to rTNF alpha in suspension, by allowing PMN to adhere before adding rTNF alpha, or by incubating adherent PMN in medium conditioned by rTNF alpha-treated PMN. Cytochalasins abolished H2O2 secretion in response to rTNFs, but not FMLP, if added during, but not after, the lag period. Thus, H2O2 secretion from rTNF alpha-treated PMN appears to be a direct but delayed response that requires assembly of microfilaments during exposure to the cytokine. These results suggest that PMN adherent to intra- or extravascular surfaces may undergo a massive, prolonged respiratory burst at the command of macrophages and lymphocytes reacting to microbial products and antigens.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe S., Gatanaga T., Yamazaki M., Soma G., Mizuno D. Purification of rabbit tumor necrosis factor. FEBS Lett. 1985 Jan 28;180(2):203–206. doi: 10.1016/0014-5793(85)81071-1. [DOI] [PubMed] [Google Scholar]
- Aggarwal B. B., Henzel W. J., Moffat B., Kohr W. J., Harkins R. N. Primary structure of human lymphotoxin derived from 1788 lymphoblastoid cell line. J Biol Chem. 1985 Feb 25;260(4):2334–2344. [PubMed] [Google Scholar]
- Arrick B. A., Nathan C. F., Cohn Z. A. Inhibition of glutathione synthesis augments lysis of murine tumor cells by sulfhydryl-reactive antineoplastics. J Clin Invest. 1983 Feb;71(2):258–267. doi: 10.1172/JCI110766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrick B. A., Nathan C. F., Griffith O. W., Cohn Z. A. Glutathione depletion sensitizes tumor cells to oxidative cytolysis. J Biol Chem. 1982 Feb 10;257(3):1231–1237. [PubMed] [Google Scholar]
- Asch A. S., Kinoshita T., Jaffe E. A., Nussenzweig V. Decay-accelerating factor is present on cultured human umbilical vein endothelial cells. J Exp Med. 1986 Jan 1;163(1):221–226. doi: 10.1084/jem.163.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badwey J. A., Curnutte J. T., Karnovsky M. L. cis-Polyunsaturated fatty acids induce high levels of superoxide production by human neutrophils. J Biol Chem. 1981 Dec 25;256(24):12640–12643. [PubMed] [Google Scholar]
- Badwey J. A., Robinson J. M., Curnutte J. T., Karnovsky M. J., Karnovsky M. L. Retinoids stimulate the release of superoxide by neutrophils and change their morphology. J Cell Physiol. 1986 May;127(2):223–228. doi: 10.1002/jcp.1041270206. [DOI] [PubMed] [Google Scholar]
- Balazovich K. J., Smolen J. E., Boxer L. A. Endogenous inhibitor of protein kinase C: association with human peripheral blood neutrophils but not with specific granule-deficient neutrophils or cytoplasts. J Immunol. 1986 Sep 1;137(5):1665–1673. [PubMed] [Google Scholar]
- Becker E. L., Sigman M., Oliver J. M. Superoxide production induced in rabbit polymorphonuclear leukocytes by synthetic chemotactic peptides and A23187. Am J Pathol. 1979 Apr;95(1):81–97. [PMC free article] [PubMed] [Google Scholar]
- Berendt M. J., North R. J., Kirstein D. P. The immunological basis of endotoxin-induced tumor regression. Requirement for T-cell-mediated immunity. J Exp Med. 1978 Dec 1;148(6):1550–1559. doi: 10.1084/jem.148.6.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton G., Zeni L., Cassatella M. A., Rossi F. Gamma interferon is able to enhance the oxidative metabolism of human neutrophils. Biochem Biophys Res Commun. 1986 Aug 14;138(3):1276–1282. doi: 10.1016/s0006-291x(86)80421-1. [DOI] [PubMed] [Google Scholar]
- Beutler B. A., Milsark I. W., Cerami A. Cachectin/tumor necrosis factor: production, distribution, and metabolic fate in vivo. J Immunol. 1985 Dec;135(6):3972–3977. [PubMed] [Google Scholar]
- Carp H. Mitochondrial N-formylmethionyl proteins as chemoattractants for neutrophils. J Exp Med. 1982 Jan 1;155(1):264–275. doi: 10.1084/jem.155.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane C. G., Spragg R., Revak S. D. Pathogenesis of the adult respiratory distress syndrome. Evidence of oxidant activity in bronchoalveolar lavage fluid. J Clin Invest. 1983 Mar;71(3):754–761. doi: 10.1172/JCI110823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahinden C. A., Fehr J., Hugli T. E. Role of cell surface contact in the kinetics of superoxide production by granulocytes. J Clin Invest. 1983 Jul;72(1):113–121. doi: 10.1172/JCI110948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Harpe J., Nathan C. F. A semi-automated micro-assay for H2O2 release by human blood monocytes and mouse peritoneal macrophages. J Immunol Methods. 1985 Apr 22;78(2):323–336. doi: 10.1016/0022-1759(85)90089-4. [DOI] [PubMed] [Google Scholar]
- Fehr J., Moser R., Leppert D., Groscurth P. Antiadhesive properties of biological surfaces are protective against stimulated granulocytes. J Clin Invest. 1985 Aug;76(2):535–542. doi: 10.1172/JCI112003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante A., Thong Y. H. Optimal conditions for simultaneous purification of mononuclear and polymorphonuclear leucocytes from human blood by the Hypaque-Ficoll method. J Immunol Methods. 1980;36(2):109–117. doi: 10.1016/0022-1759(80)90036-8. [DOI] [PubMed] [Google Scholar]
- Gamble J. R., Harlan J. M., Klebanoff S. J., Vadas M. A. Stimulation of the adherence of neutrophils to umbilical vein endothelium by human recombinant tumor necrosis factor. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8667–8671. doi: 10.1073/pnas.82.24.8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie L. A., McPhail L. C., Henson P. M., Johnston R. B., Jr Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. J Exp Med. 1984 Dec 1;160(6):1656–1671. doi: 10.1084/jem.160.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M., Levine J. D., Callahan K. S., Schwartz B. R., Harker L. A. Glutathione redox cycle protects cultured endothelial cells against lysis by extracellularly generated hydrogen peroxide. J Clin Invest. 1984 Mar;73(3):706–713. doi: 10.1172/JCI111263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Johnston R. B., Jr Tissue injury in inflammation. Oxidants, proteinases, and cationic proteins. J Clin Invest. 1987 Mar;79(3):669–674. doi: 10.1172/JCI112869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai M., Okamura N., Terano Y., Tsujimoto M., Nakazato H. Production and characterization of monoclonal antibodies to human tumor necrosis factor. J Immunol Methods. 1987 Jan 26;96(1):57–62. doi: 10.1016/0022-1759(87)90367-x. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Jesaitis A. J., Tolley J. O., Allen R. A. Receptor-cytoskeleton interactions and membrane traffic may regulate chemoattractant-induced superoxide production in human granulocytes. J Biol Chem. 1986 Oct 15;261(29):13662–13669. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Lehmeyer J. E. Elaboration of toxic oxygen by-products by neutrophils in a model of immune complex disease. J Clin Invest. 1976 Apr;57(4):836–841. doi: 10.1172/JCI108359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., Vadas M. A., Harlan J. M., Sparks L. H., Gamble J. R., Agosti J. M., Waltersdorph A. M. Stimulation of neutrophils by tumor necrosis factor. J Immunol. 1986 Jun 1;136(11):4220–4225. [PubMed] [Google Scholar]
- Klempner M. S., Dinarello C. A., Henderson W. R., Gallin J. I. Stimulation of neutrophil oxygen-dependent metabolism by human leukocytic pyrogen. J Clin Invest. 1979 Oct;64(4):996–1002. doi: 10.1172/JCI109566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrick J. W., Graham D., Toy K., Lin L. S., Senyk G., Fendly B. M. Recombinant tumor necrosis factor causes activation of human granulocytes. Blood. 1987 Feb;69(2):640–644. [PubMed] [Google Scholar]
- Makino R., Tanaka T., Iizuka T., Ishimura Y., Kanegasaki S. Stoichiometric conversion of oxygen to superoxide anion during the respiratory burst in neutrophils. Direct evidence by a new method for measurement of superoxide anion with diacetyldeuteroheme-substituted horseradish peroxidase. J Biol Chem. 1986 Sep 5;261(25):11444–11447. [PubMed] [Google Scholar]
- Marasco W. A., Phan S. H., Krutzsch H., Showell H. J., Feltner D. E., Nairn R., Becker E. L., Ward P. A. Purification and identification of formyl-methionyl-leucyl-phenylalanine as the major peptide neutrophil chemotactic factor produced by Escherichia coli. J Biol Chem. 1984 May 10;259(9):5430–5439. [PubMed] [Google Scholar]
- Nathan C. F., Arrick B. A., Murray H. W., DeSantis N. M., Cohn Z. A. Tumor cell anti-oxidant defenses. Inhibition of the glutathione redox cycle enhances macrophage-mediated cytolysis. J Exp Med. 1981 Apr 1;153(4):766–782. doi: 10.1084/jem.153.4.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Prendergast T. J., Wiebe M. E., Stanley E. R., Platzer E., Remold H. G., Welte K., Rubin B. Y., Murray H. W. Activation of human macrophages. Comparison of other cytokines with interferon-gamma. J Exp Med. 1984 Aug 1;160(2):600–605. doi: 10.1084/jem.160.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perussia B., Kobayashi M., Rossi M. E., Anegon I., Trinchieri G. Immune interferon enhances functional properties of human granulocytes: role of Fc receptors and effect of lymphotoxin, tumor necrosis factor, and granulocyte-macrophage colony-stimulating factor. J Immunol. 1987 Feb 1;138(3):765–774. [PubMed] [Google Scholar]
- Platzer E., Welte K., Gabrilove J. L., Lu L., Harris P., Mertelsmann R., Moore M. A. Biological activities of a human pluripotent hemopoietic colony stimulating factor on normal and leukemic cells. J Exp Med. 1985 Dec 1;162(6):1788–1801. doi: 10.1084/jem.162.6.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier C. G., O'Shea J., Chused T., Yancey K., Frank M. M., Takahashi T., Brown E. J. Studies on the fibronectin receptors of human peripheral blood leukocytes. Morphologic and functional characterization. J Exp Med. 1984 Jan 1;159(1):137–151. doi: 10.1084/jem.159.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revak S. D., Rice C. L., Schraufstätter I. U., Halsey W. A., Jr, Bohl B. P., Clancy R. M., Cochrane C. G. Experimental pulmonary inflammatory injury in the monkey. J Clin Invest. 1985 Sep;76(3):1182–1192. doi: 10.1172/JCI112074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer-Pokora B., Kunkel L. M., Monaco A. P., Goff S. C., Newburger P. E., Baehner R. L., Cole F. S., Curnutte J. T., Orkin S. H. Cloning the gene for an inherited human disorder--chronic granulomatous disease--on the basis of its chromosomal location. Nature. 1986 Jul 3;322(6074):32–38. doi: 10.1038/322032a0. [DOI] [PubMed] [Google Scholar]
- STETSON C. A., Jr Studies on the mechanism of the Shwartzman phenomenon; certain factors involved in the production of the local hemorrhagic necrosis. J Exp Med. 1951 May;93(5):489–504. doi: 10.1084/jem.93.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleimer R. P., Rutledge B. K. Cultured human vascular endothelial cells acquire adhesiveness for neutrophils after stimulation with interleukin 1, endotoxin, and tumor-promoting phorbol diesters. J Immunol. 1986 Jan;136(2):649–654. [PubMed] [Google Scholar]
- Segal A. W. Absence of both cytochrome b-245 subunits from neutrophils in X-linked chronic granulomatous disease. Nature. 1987 Mar 5;326(6108):88–91. doi: 10.1038/326088a0. [DOI] [PubMed] [Google Scholar]
- Shalaby M. R., Palladino M. A., Jr, Hirabayashi S. E., Eessalu T. E., Lewis G. D., Shepard H. M., Aggarwal B. B. Receptor binding and activation of polymorphonuclear neutrophils by tumor necrosis factor-alpha. J Leukoc Biol. 1987 Mar;41(3):196–204. doi: 10.1002/jlb.41.3.196. [DOI] [PubMed] [Google Scholar]
- Shaw J. O., Pinckard R. N., Ferrigni K. S., McManus L. M., Hanahan D. J. Activation of human neutrophils with 1-O-hexadecyl/octadecyl-2-acetyl-sn-glycerol-3-phosphorylcholine (platelet activating factor). J Immunol. 1981 Sep;127(3):1250–1255. [PubMed] [Google Scholar]
- Simchowitz L., Spilberg I. Chemotactic factor-induced generation of superoxide radicals by human neutrophils: evidence for the role of sodium. J Immunol. 1979 Nov;123(5):2428–2435. [PubMed] [Google Scholar]
- Smedly L. A., Tonnesen M. G., Sandhaus R. A., Haslett C., Guthrie L. A., Johnston R. B., Jr, Henson P. M., Worthen G. S. Neutrophil-mediated injury to endothelial cells. Enhancement by endotoxin and essential role of neutrophil elastase. J Clin Invest. 1986 Apr;77(4):1233–1243. doi: 10.1172/JCI112426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumimoto H., Takeshige K., Minakami S. Superoxide production of human polymorphonuclear leukocytes stimulated by leukotriene B4. Biochim Biophys Acta. 1984 Apr 16;803(4):271–277. doi: 10.1016/0167-4889(84)90117-4. [DOI] [PubMed] [Google Scholar]
- Thorens B., Mermod J. J., Vassalli P. Phagocytosis and inflammatory stimuli induce GM-CSF mRNA in macrophages through posttranscriptional regulation. Cell. 1987 Feb 27;48(4):671–679. doi: 10.1016/0092-8674(87)90245-5. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Tsujimoto M., Yokota S., Vilcek J., Weissmann G. Tumor necrosis factor provokes superoxide anion generation from neutrophils. Biochem Biophys Res Commun. 1986 Jun 30;137(3):1094–1100. doi: 10.1016/0006-291x(86)90337-2. [DOI] [PubMed] [Google Scholar]
- Tvedten H. W., Till G. O., Ward P. A. Mediators of lung injury in mice following systemic activation of complement. Am J Pathol. 1985 Apr;119(1):92–100. [PMC free article] [PubMed] [Google Scholar]
- Tzeng D. Y., Deuel T. F., Huang J. S., Senior R. M., Boxer L. A., Baehner R. L. Platelet-derived growth factor promotes polymorphonuclear leukocyte activation. Blood. 1984 Nov;64(5):1123–1128. [PubMed] [Google Scholar]
- Webster R. O., Hong S. R., Johnston R. B., Jr, Henson P. M. Biologial effects of the human complement fragments C5a and C5ades Arg on neutrophil function. Immunopharmacology. 1980 Jun;2(3):201–219. doi: 10.1016/0162-3109(80)90050-8. [DOI] [PubMed] [Google Scholar]
- Weisbart R. H., Golde D. W., Gasson J. C. Biosynthetic human GM-CSF modulates the number and affinity of neutrophil f-Met-Leu-Phe receptors. J Immunol. 1986 Dec 1;137(11):3584–3587. [PubMed] [Google Scholar]
- Wright G. G., Mandell G. L. Anthrax toxin blocks priming of neutrophils by lipopolysaccharide and by muramyl dipeptide. J Exp Med. 1986 Nov 1;164(5):1700–1709. doi: 10.1084/jem.164.5.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Licht M. R., Craigmyle L. S., Silverstein S. C. Communication between receptors for different ligands on a single cell: ligation of fibronectin receptors induces a reversible alteration in the function of complement receptors on cultured human monocytes. J Cell Biol. 1984 Jul;99(1 Pt 1):336–339. doi: 10.1083/jcb.99.1.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon P. S., Boxer L. A., Mayo L. A., Yang A. Y., Wicha M. S. Human neutrophil laminin receptors: activation-dependent receptor expression. J Immunol. 1987 Jan 1;138(1):259–265. [PubMed] [Google Scholar]



