Abstract
Introduction
Maximizing responses of malignant gliomas is hampered by resistance to temozolomide (TMZ). Increasing efficacy but not toxicity is a key issue when testing drug combinations. The antimyeloma agent bortezomib (BZ) has shown promising results in vitro and is currently being tested in glioblastoma (GBM) patients. In this study we investigate whether reduction of TMZ dosage is feasible without compromising the antitumor effect of TMZ-BZ combination.
Material and methods
U87 GBM cells were treated with increasing doses of TMZ (1, 10, 100, 1000 µM), BZ (0.001, 0.01, 0.1, 1) and the combination during a 48-hour period, and apoptotic or/and necrotic cell death was evaluated by flow cytometry.
Results
Bortezomib alone at a dose as low as 0.001 µM markedly induced cell death, particularly late apoptosis, to a level which was comparable with high TMZ dosage. For combination treatments, the dose of 0.1 µM BZ, which was more potent than the maximal dose of TMZ (1000 µM), was chosen to be added to increasing TMZ concentrations. The combination of 0.1 BZ µM BZ with low doses of TMZ (1, 10 µM) further increased the cell death rate in an additive manner, at levels higher than those induced by high doses of TMZ monotherapy (100, 1000 µM).
Conclusions
Efficacy of TMZ-BZ combination is feasible with low doses of TMZ in vitro.
Keywords: temozolomide, bortezomib, glioma, U87, flow cytometry
Introduction
Temozolomide (TMZ) with radiotherapy is the current standard of care for newly diagnosed glioblastoma multiforme (GBM), based on the randomized phase III EORTC-NCIC trial of Stupp et al. [1] However, improvement in overall survival of such patients was marginal and prognosis remains poor due to the high resistance of these tumors to treatment. Alternative dosing regimens have been tested for TMZ, in an effort to overcome repletion of O6-methylguanine methyltransferase (MGMT), which is considered to be the major mechanism of resistance to TMZ [2]. Prolonged administration of low doses of TMZ was shown to deplete MGMT activity in peripheral blood mononuclear cells, at the cost of increased hematologic toxicity in many cases [2]. Dose-limiting myelotoxicity was also observed when combinations of TMZ with MGMT inhibitors were tested with the aim of inducing chemo- and/or radio-sensitization [3].
Emerging evidence on the role of the proteasome in orchestration of DNA repair and on the antitumor activity of the proteasome inhibitor bortezomib (BZ) in GBM cell lines has led to the design of clinical trials evaluating the combination of this agent, already in clinical use for the treatment of myeloma, with TMZ [4–8]. However, existing preclinical data on the combination of proteasome inhibitors with TMZ in terms of chemosensitization and/or radiosensitization of gliomas are contradictory [9, 10].
In this work, we studied the cytotoxic effect of this combination in U87 GBM cells by flow cytometry, with the aim of deciphering whether BZ addition might magnify the cell death effect of lower doses of TMZ.
Material and methods
Cell culture and reagents
The human glioblastoma cell line U87 was purchased from the European Collection of Animal Cell Cultures (ECACC, UK) and all experiments were performed on cells between passage number 5 and 10, within 6 months of purchase. The cell line was cultured in Dulbecco's modified Eagle's medium (GIBCO, UK) supplemented with 10% heat-inactivated FBS (GIBCO, UK), 5% L-glutamine (GIBCO, UK) and 1% penicillin-streptomycin (Euroclone, UK) at 37°C in a humidified 5% CO2 atm. Temozolomide (TMZ) was purchased from Merck, UK. Bortezomib (BZ) was purchased from Janssen-Cilag Pharmaceuticals, Greece.
Flow cytometry
To study induction of apoptosis and/or necrosis, annexin V assays (TACS Annexin V-FITC Apoptosis Detection Kit, R&D Systems Europe, UK) were performed according to the manufacturer's instructions. Briefly, 106 cells were allowed to attach for 24 h at 37°C and were then either left untreated or were treated with TMZ for 48 h at ten-fold serial dilution concentrations between 1 and 103 µM, or were treated with BZ at ten-fold serial dilution concentrations between 10−9 and 10−6 M. Bortezomib at a dose of 10−7 M was chosen for co-administration with TMZ at the above-mentioned doses and time. After incubations, treated and untreated cells were trypsinized and washed with 1× PBS. For each sample of 1 × 105 cells, 100 µl of Annexin Incubation Reagent were prepared, containing 10 µl of 10X Binding Buffer, 10 µl of propidium iodide (PI), 1 µl of Annexin V-FITC and 79 µl of deionized H2O. Cells were resuspended in the Annexin Incubation Reagent and incubated in the dark for 15 min at room temperature. Then, 400 µl of 1× Binding Buffer were added and samples were analyzed by an EPICS XL-MCL Counter (Beckman Coulter, USA). Data were acquired from 50,000-100,000 cells (events) and were analyzed with Cell Quest software. Two independent experiments were carried out in duplicate and representative results are shown (p < 0.01).
Synergism quotient calculation
The synergism quotient was calculated by subtracting baseline values from all treatments and then dividing effects of combined treatments by the sum of individual treatments. A synergism quotient greater than 1.0 indicates that there is synergism for a given measured response [11].
Results
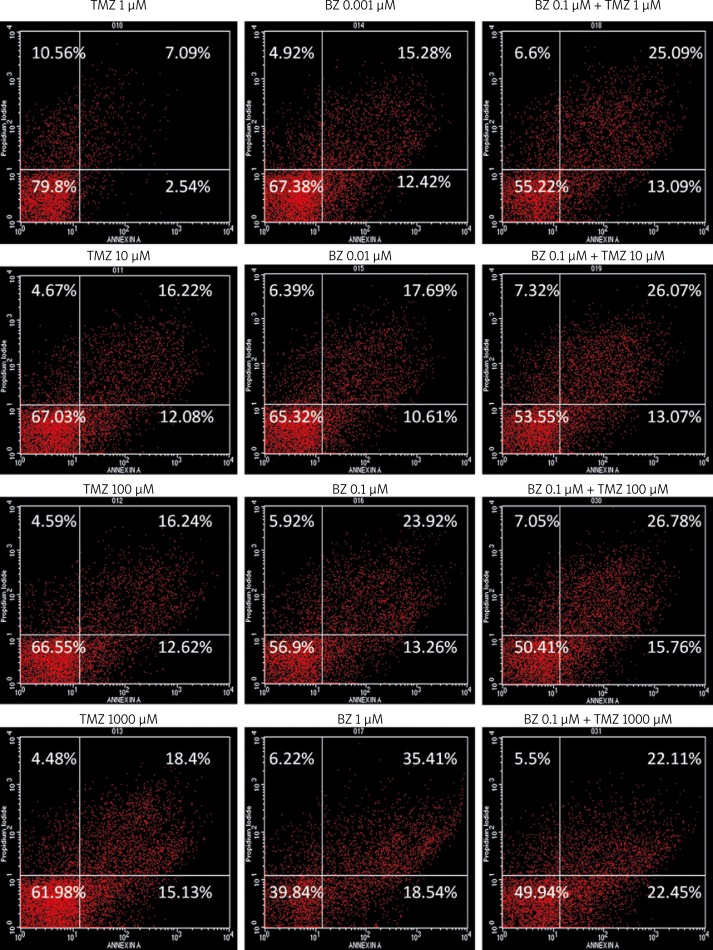
98.12 ±0.26% of untreated U87 cells were alive after 48 h. When 1 µM TMZ was administered, increased cell death began to occur, primarily through necrosis, whereas both early (Annexin+/PI−) and late apoptosis (Annexin+/PI+) were observed with higher doses of TMZ. Bortezomib alone at a dose as low as 0.001 µM markedly induced cell death, particularly late apoptosis, to a level which was comparable with high TMZ dosage. Thus, BZ is more cytotoxic compared to TMZ for U87 cells. Increasing doses of BZ induced even greater apoptosis. For combination treatments, the dose of 0.1 µM BZ, which was more potent than the maximal dose of TMZ (103 µM), was chosen to be added to increasing TMZ concentrations. The combination of BZ with low doses of TMZ further increased the cell death rate, particularly late apoptosis, leaving approximately 50% of the cell population alive (Figure 1). For the given doses and times, the interaction between the two drugs was considered to be additive, regarding apoptosis, necrosis or both (Table I).
Figure 1.
Representative Annexin V/propidium iodide (PI) intensity dot blots of U87MG cells treated for 48 h with temozolomide (TMZ) and/or bortezomib (BZ) at the indicated doses. Cell death was determined by Annexin V and PI staining (see Material and methods for details). Data are means from two independent experiments run in duplicate (p < 0.01)
Table I.
Synergism quotations of TMZ-BZ combinations in U87 cells
| Treatment (48 h) | Mean ± standard deviation | ||
|---|---|---|---|
| Apoptosis (Annexin+) | Necrosis (PI+) | Both (Annexin+, PI+, Annexin+/PI+) | |
| TMZ 1 µM+BZ 10−1 µM | 0.88 ±0.09 | 0.43 ±0.12 | 0.72 ±0.10 |
| TMZ 10 µM+BZ 10−1 µM | 0.52 ±0.25 | 0.69 ±0.14 | 0.61 ±0.18 |
| TMZ 102 µM+BZ 10−1 µM | 0.61 ±0.23 | 0.67 ±0.17 | 0.65 ±0.21 |
Discussion
The combination of concurrent TMZ and radiotherapy with BZ in high grade gliomas is feasible in the clinical setting and produces tolerable toxicity [8]. Preclinical data on the interaction of the two drugs and type of cell death they induce together are limited. Therefore, we performed a FACS analysis of each drug alone and of the combination, evaluating apoptotic and/or necrotic events. Given previous data by others and our group on the use of 0.1 µM BZ as an inducer of apoptosis through several mechanisms, including PARP, Bcl-2, Bcl-xl, JNK/c-jun, NF-κB, TRAIL, and MGMT, we specifically focused on this schedule [6, 7]. Indeed, we found an additive effect on induction of cell death, mostly involving increased late apoptosis compared to either drug alone, which was evident even with the lowest dose of TMZ used.
A previous preclinical study on the antitumor efficacy of the new generation of proteasome inhibitor NPI-0052 as a single agent and in combination with TMZ and radiation in different glioma lines, including U87, failed to show radiosensitization of p53 wild-type cells [10]. However, the short time of TMZ exposure (3 h) and the high sensitivity to NPI-0052 might have compromised the radiotoxic effect of the combinations. In contrast, the NPI-0052-TMZ combination inhibited survival of U87 cells, which is a reproducible effect of proteasome inhibitors when added to TMZ, in our and previous studies [9].
Given the previous isolation and characterization of cancer stem cells from the U87 cell line [12], increased activity of the BZ-TMZ combination might also result from the fact that these drugs target different cell sub-populations. Indeed, TMZ is more toxic for neural stem cells (NSCs) compared to glioma stem cells (GSCs), causing necrotic death at prolonged administration (7 days), whereas BZ induces apoptosis of GSCs, even at doses as low as 5 nM, yet produces minimal effects on NSCs [13].
In conclusion, BZ is a potent chemosensitizer in U87 GBM cells during co-administration with TMZ, enabling low doses of the latter to be pro-apoptotic. This could theoretically lead to a reduction of hematologic toxicity from protracted courses of TMZ treatment without compromising the anticancer effects of the drug, but needs to be tested in detail in vivo.
Acknowledgments
The authors would like to thank Professor Aspasia Tsezou, Departments of Cytogenetics and Molecular Genetics, and Biology, University of Thessaly, Faculty of Medicine, 41110 Larissa, and Department of Biomedical Research and Technology, Center for Research and Technology-Thessaly (CE.RE.TE.TH), Larissa 41222, Greece, for use of flow cytometry facilities. We also thank Dr. Athanasios Mavropoulos, Department of Biomedical Research and Technology, Center for Research and Technology-Thessaly (CE.RE.TE.TH), Larissa 41222, Greece, for assistance with flow cytometric analysis.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Wick W, Platten M, Weller M. New (alternative) temozolomide regimens for the treatment of glioma. Neuro Oncol. 2009;11:69–79. doi: 10.1215/15228517-2008-078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quinn JA, Jiang SX, Reardon DA, et al. Phase I trial of temozolomide plus O6-benzylguanine 5-day regimen with recurrent malignant glioma. Neuro Oncol. 2009;11:556–61. doi: 10.1215/15228517-2009-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vlachostergios PJ, Patrikidou A, Daliani DD, Papandreou CN. The ubiquitin-proteasome system in cancer, a major player in DNA repair. Part 1: post-translational regulation. J Cell Mol Med. 2009;13:3006–18. doi: 10.1111/j.1582-4934.2009.00824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vlachostergios PJ, Patrikidou A, Daliani DD, Papandreou CN. The ubiquitin-proteasome system in cancer, a major player in DNA repair. Part 2: transcriptional regulation. J Cell Mol Med. 2009;13:3019–31. doi: 10.1111/j.1582-4934.2009.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin D, Zhou H, Kumagai T, et al. Proteasome inhibitor PS-341 causes cell growth arrest and apoptosis in human glioblastoma multiforme (GBM) Oncogene. 2005;24:344–54. doi: 10.1038/sj.onc.1208225. [DOI] [PubMed] [Google Scholar]
- 7.Vlachostergios PJ, Hatzidaki E, Stathakis NE, Koukoulis GK, Papandreou CN. Bortezomib downregulates MGMT expression in T98G glioblastoma cells. Cell Mol Neurobiol. 2013;33:313–8. doi: 10.1007/s10571-013-9910-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kubicek GJ, Werner-Wasik M, Machtay M, et al. Phase I trial using proteasome inhibitor bortezomib and concurrent temozolomide and radiotherapy for central nervous system malignancies. Int J Radiat Oncol Biol Phys. 2009;74:433–9. doi: 10.1016/j.ijrobp.2008.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng K, Nitta M, Hu L, et al. A small interference RNA screen revealed proteasome inhibition as strategy for glioblastoma therapy. Clin Neurosurg. 2009;56:107–18. [PubMed] [Google Scholar]
- 10.Vlashi E, Mattes M, Lagadec C, et al. Differential effects of the proteasome inhibitor NPI-0052 against glioma cells. Transl Oncol. 2010;3:50–5. doi: 10.1593/tlo.09244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Guo J, Wu S, et al. Synergistic effects of nanosecond pulsed electric fields combined with low concentration of gemcitabine on human oral squamous cell carcinoma in vitro. PLoS One. 2012;7:e43213. doi: 10.1371/journal.pone.0043213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu SC, Ping YF, Yi L, et al. Isolation and characterization of cancer stem cells from a human glioblastoma cell line U87. Cancer Lett. 2008;265:124–34. doi: 10.1016/j.canlet.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Gong X, Schwartz PH, Linskey ME, Bota DA. Neural stem/progenitors and glioma stem-like cells have differential sensitivity to chemotherapy. Neurology. 2011;76:1126–34. doi: 10.1212/WNL.0b013e318212a89f. [DOI] [PMC free article] [PubMed] [Google Scholar]