Abstract
Introduction
The aim of this study was to evaluate the potential biological activity of N-(6-oxo-5,6-dihydrophenanthridin-2-yl)-(N,N-dimethylamino)acetamide hydrochloride (PJ34) on the genotoxicity induced by melphalan in human multiple myeloma cells.
Material and methods
The inhibitory effects of the drugs on the growth of RPMI8226 cells were determined by Cell Counting Kit-8 (CCK-8) assay. The expression of Fanconi anemia/breast cancer (FA/BRCA) pathway related genes was determined by western blot analysis. Cell cycle phase and apoptosis were analyzed by flow cytometry. Coadministration of PJ34 and melphalan had additional effects on cell cycle distribution and enhanced apoptosis of RPMI8226 cells. PJ34 plus melphalan inhibited cell-cycle progression, as evidenced by the increased proportion of cells in the G2/M phase with the decreasing proportion of cells in the G0/1 and S phases.
Results
However, no significant synergistic effect of PJ34 and melphalan on cell proliferation was observed. These effects were accompanied by inhibition of the FA/BRCA pathway by downregulation of Fanconi D2 (FANCD2) protein expression. The results showed that treatment with 60 µmol/l of PJ34 previously to melphalan administration increased cell apoptosis. Pretreatment also caused cell cycle arrest.
Conclusions
This study suggests that enhancement of melphalan efficacy may be best achieved by the poly(ADP-ribose) polymerase-1 (PARP-1) inhibitor PJ34. The effects of PJ34 are associated with inhibition of the FA/BRCA pathway, increased apoptosis percentage, and G2/M cell cycle arrest. Administration of PJ34 has been shown to protect DNA from damage induced by melphalan. This corroborates the biological activities of PJ34 and points to the need for further studies.
Keywords: FA/BRCA (Fanconi anemia/BRCA) pathway, DNA damage, multiple myeloma
Introduction
Some targeted drugs, such as thalidomide, lenalidomide and bortezomib, can be effective in multiple myeloma (MM) treatment, but they do not always work. Some patients relapse after several courses of treatment or are even intrinsically drug resistant. Autologous hematopoietic stem cell transplantation (auto-HSCT) can improve survival rates of MM patients, but some patients cannot accept auto-HSCT due to old age, bad performance status or renal failure. For those patients, combined chemotherapy is indispensable. In this context it is important to pay more attention to conventional chemotherapy drugs, such as alkylating agents (namely cross-linking agents). Alkylating agents include cyclophosphamide and melphalan, which affect DNA integrity either directly or indirectly. They act on DNA and form interstrand cross-link (ICL) damage, resulting in single or double stranded breaks (DSBs) [1]. If these breaks cannot be repaired, the persistent DNA damage will give rise to mutations and genomic instability leading to cell death [2].
The FA/BRCA pathway may play an important role in the process of DNA damage repair [3–5]. The proteins encoded by various FA genes maintain cellular genome stability through the FA/BRCA pathway. At least eight FA proteins (A, B, C, E, F, G, L and M) interact with each other to form an FA core protein complex, which activates FANCD2 by promoting FANCD2 monoubiquitination. BRCA2 (also named FANCD1) and BRIP1 (also named FANCJ) play roles downstream independently or synergistically with FANCD2. Alkylating agents exert their genotoxic effects on tumor cells through the FA/BRCA pathway, so the suppression of this pathway can improve the sensitivity of tumor cells to alkylating agents [6–15].
Poly (ADP-ribose) polymerases (PARP) exhibit various functions ranging from DNA repair to cell death regulation; thus they have become interesting targets in cancer treatment [16]. Indeed, PARP inhibitors appear to be important tools in cancer therapy. Various PARP-1 inhibitors, as monotherapy or in combination with other compounds, are currently being investigated in clinical trials [17]. Several studies have confirmed that PARP-1 inhibitors can obviously improve the effects of alkylating agents including cisplatin, cyclophosphamide and oxaliplatin used for chemotherapy [18–21]. However, the molecular mechanisms of PARP-1 inhibitors increasing tumor cell sensitivity to alkylating agents remain to be elucidated.
Most members of the FA/BRCA gene family participate in several DNA repair pathways, including base excision repair (BER), homologous recombination repair and non-homologous end joining [3–5]. Poly (ADP-ribose) polymerase-1 inhibitors can inhibit BER after ICLs caused by alkylating agents, and improve the efficacy of alkylating agents such as cisplatin and cyclophosphamide. So we speculated that the effects of PARP-1 inhibitors might be associated with FA/BRCA pathway inhibition. In this study, we compared the sensitivities of RPMI-8226 cells to melphalan before and after treatment with PJ34, a PARP-1 inhibitor. We attempted to demonstrate the influence of PJ34 on DNA damage repair and the possible relationship with the FA/BRCA pathway.
Material and methods
Materials
Human multiple myeloma cell line RPMI8226 was kindly gifted by Professor Zhou (Department of Hematology, Tongji Hospital of Huazhong University of Science and Technology). The original cell line was purchased from the American Type Culture Collection (ATCC, Manassas, VA). PJ34, melphalan and DMSO were purchased from Sigma Chemical Company (St Louis, Missouri, USA). CCK-8 was purchased from Dojindo Laboratories, Japan. RPMI 1640 and fetal calf serum were purchased from Invitrogen Life Technologies. The primary and secondary antibodies of FANCD2, BRCA2, RAD51, γH2AX and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell culture
RPMI8226 was cultured in RPMI 1640, supplemented with 10% heat-inactivated fetal calf serum. The environment of the culture flask was kept at 37°C, with saturated humidity and 5% CO2.
Cytotoxicity assay
The CCK-8 assay was chosen to detect the effect of PJ34 on cell growth. RPMI8226 cells in the exponential proliferation period were seeded to a 96-well plate at a density of 5 × 104 cells per well. Twenty-four hours later, PJ34, melphalan, and melphalan plus PJ34 were added to 3 test groups with concentrations ordinarily diluted. The reagent of the control group was replaced by RMPI 1640. Every group included four parallel samples. The total medium volume of each well was 100 µl. Three days after drug addition, 10 µl of CCK-8 solution was added 4 h before detection. Then the absorbency (A450 value) was measured, and the growth rates of cells were computed.
Expression of FANCD2, BRCA2, RAD51 and γH2AX
Cell lysates were prepared by suspending cell pellets in lysis buffer. After quantification, the protein samples were separated by SDS-PAGE. Western blotting was used to detect the expression of FANCD2, BRCA2, RAD51 and γH2AX proteins. Each lane was loaded with 50 µg of protein from the cell lysates. β-actin was used for the loading control.
Cell cycle analysis
To determine the effect of PJ34 on the cell cycle, the cells of five groups (control, PJ34 10 µM, PJ34 30 µM, PJ34 60 µM and PJ34 90 µM) were treated for 48 h, washed, and fixed with 70% ethanol. After incubation overnight at 4°C, cells were washed with PBS prior to staining with propidium iodide (PI), and then suspended in staining buffer (10 µg/ml PI; 0.5% Tween-20; 0.1% RNase in PBS). The cells were analyzed by a FACScan flow cytometer (Becton Dickinson, San Jose, CA) using CellQuest software version 3.1.
Measurement of apoptosis
To determine the apoptosis, cells of seven groups (blank control, PJ34 60 µM, melphalan, PJ34 10 µM + melphalan, PJ34 30 µM + melphalan, PJ34 60 µM + melphalan and PJ34 90 µM + melphalan) were washed in PBS, resuspended in 500 µl binding buffer containing Annexin V-FITC/PI and analyzed by flow cytometry.
Statistical analysis
SPSS 13.0 was used to perform the statistical analysis. Data were presented as mean ± SD, and analyzed by Student's t test. Values of p below 0.05 were considered statistically significant.
Results
Growth inhibition induced by PJ34
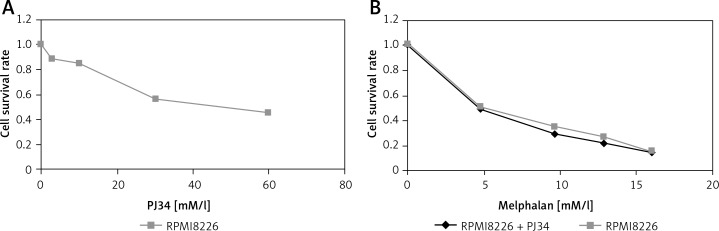
Concentration-dependent growth inhibition of PJ34 alone was detected in the RPMI8226 cell line (Figure 1A). We also investigated the effect of a combination of PJ34 and melphalan on cell growth. We found that PJ34 at 60 µM concentration enhanced the toxicity of melphalan in RPMI8226 cells (Figure 1B, Table I), but there was no statistical significance (p > 0.05). After pretreatment with 60 µM PJ34 for 24 h, the susceptibility of RPMI8226 cells to melphalan was maximum.
Figure 1.
The effects of PJ34 on cell growth of RPMI8226. A – The effects of PJ34 alone on cell growth. B – The effects of PJ34 on cell growth inhibited by melphalan
Table I.
The effect of PJ34 on RPMI8226 cell growth inhibited by melphalan
| Melphalan [µM] | Cell growth (%) | ||
|---|---|---|---|
| PJ34 group | Blank control group | Value of p | |
| 4.8 | 48.56 ±1.10 | 50.08 ±1.33 | 0.28 |
| 9.6 | 28.65 ±2.30 | 34.41 ±2.05 | 0.053 |
| 12.8 | 21.57 ±2.02 | 26.12 ±2.50 | 0.061 |
| 16 | 14.24 ±1.40 | 14.84 ±0.75 | 0.094 |
Expression of FA/BRCA pathway related factors regulated by PJ34
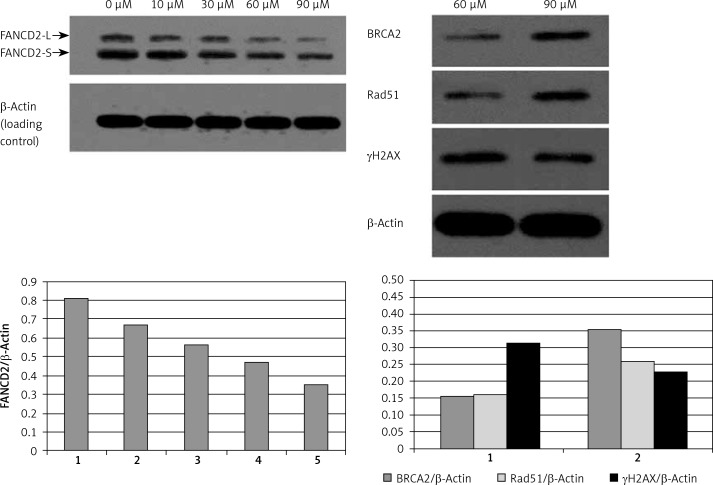
The expression of FA/BRCA pathway related factors in RPMI8226 cells was detected by Western blotting (Figure 2). PJ34 inhibited FANCD2 expression significantly in a dose-dependent manner, whereas it worked oppositely on γH2AX expression. Representing DNA damage, the expression of γH2AX protein was increased. Meanwhile, the expression of BRCA2 and Rad51 proteins was significantly decreased (Table II). The above results showed that the activity of the FA/BRCA pathway was suppressed in the RPMI8226 cell line by PJ34, which would result in reduced DNA repair of the damage induced by melphalan.
Figure 2.
The effects of PJ34 treatment at different concentration on FA/BRCA pathway related factors expression in RPMI8226 cells
Table II.
Relative expression levels of FA/BRCA pathway related factors in RPMI8226 cells
| PJ34 | FANCD2/β-actin | BRCA2/β-actin | Rad51/β-actin | γH2AX/β-actin |
|---|---|---|---|---|
| 0 µM | 0.812 ±0.01 | 0.353 ±0.05 | 0.259 ±0.05 | 0.228 ±0.09 |
| 60 µM | 0.472 ±0.01 | 0.158 ±0.02 | 0.162 ±0.12 | 0.314 ±0.04 |
| Value of p | 0.022 | 0.004 | 0.023 | 0.012 |
PJ34 enhanced cell cycle arrest induced by melphalan
Because FANCD2 activation mainly happens in the G1/S phase, BRCA2 and Rad51 play roles downstream partly depending on activated FANCD2. We investigated the effect of PJ34 on cell cycle distribution in the RPMI8226 cell line to explain the possible reason for the functional regulation of the FA/BRCA pathway. PJ34 plus melphalan inhibited cell-cycle progression, as evidenced by the increased proportion of cells in the G2/M phase with the decreasing proportion of cells in the G0/1 and S phases. PJ34 enhanced cell cycle arrest induced by melphalan in a dose-dependent manner. However, the difference was not significant when the concentration exceeded the IC50 value of 60 µM (Table III).
Table III.
Effects of PJ34 on RPMI8226 cell cycle arrested by melphalan
| Group | G0/1 | S | G2/M |
|---|---|---|---|
| 1) Control | 39.70 ±0.28 | 50.52 ±0.33 | 9.80 ±0.60 |
| 2) PJ-34 10 µM | 37.48 ±0.31 | 49.67 ±0.70 | 12.85 ±0.36 |
| 3) PJ-34 30 µM | 34.30 ±0.52 | 46.56 ±0.55 | 17.14 ±0.34 |
| 4) PJ-34 60 µM | 31.65 ±0.62 | 45.28 ±0.52 | 23.07 ±0.92 |
| 5) PJ-34 90 µM | 30.31 ±0.48 | 43.55 ±0.48 | 26.14 ±0.41 |
| P 1) vs. 2) | 0.066 | 0.742 | 0.013 |
| P 1) vs. 3) | 0.002 | 0.043 | 0.001 |
| P 1) vs. 4) | 0.001 | 0.02 | 0.000 |
| P 1) vs. 5) | 0.000 | 0.000 | 0.000 |
| P 2) vs. 3) | 0.012 | 0.031 | 0.010 |
| P 3) vs. 4) | 0.014 | 0.026 | 0.000 |
| P 4) vs. 5) | 0.135 | 0.083 | 0.05 |
Apoptosis induced by PJ34
When RPMI8226 cells were treated with PJ34 alone, there was no significant difference between the PJ34 group and the blank control group in the apoptosis percentage (p = 0.471) (Table IV). In contrast, the apoptosis percentage of the melphalan plus PJ34 group was significantly higher than that of the melphalan alone group in a dose-dependent manner. With the increasing concentration of PJ34, the apoptosis percentage also increased. But the difference was not significant when the concentration exceeds the IC50 value of 60 µM either (Table IV). Therefore, PJ34 significantly improved the apoptosis of RPMI8226 cells with melphalan.
Table IV.
Effects of PJ34 on RPMI8226 cell apoptosis induced by melphalan
| Group | Cell apoptosis (%) |
|---|---|
| 1) Control | 15.28 ±2.84 |
| 2) PJ-34 60 µM | 22.70 ±2.16 |
| 3) Melphalan | 31.08 ±0.47 |
| 4) PJ-34 10 µM + melphalan | 32.73 ±0.60 |
| 5) PJ-34 30 µM + melphalan | 37.58 ±0.73 |
| 6) PJ-34 60 µM + melphalan | 45.38 ±4.53 |
| 7) PJ-34 90 µM + melphalan | 48.30 ±0.91 |
1) vs. 2), p = 0.471; 1) vs. 3), p = 0.039; 2) vs. 3), p = 0.038; 1) vs. 6), p = 0.004; 2) vs. 6), p = 0.009; 3) vs. 6), p = 0.045; 1) vs. 4), p = 0.005; 2) vs. 4), p = 0.019; 3) vs. 4), p = 0.206; 1) vs. 5), p = 0.002; 2) vs. 5), p = 0.004; 3) vs. 5), p = 0.007; 1) vs. 7), p = 0.000; 2) vs. 7), p = 0.001; 3) vs. 7), p = 0.001; 4) vs. 5), p = 0.011; 5) vs. 6), p = 0.009; 6) vs. 7), p = 0.144.
Discussion
Here, the effects of the PARP-1 inhibitor PJ34 were investigated in the MM cell line RPMI8226. We demonstrated that PJ34 could induce negative regulation of the FA/BRCA pathway. This study showed that inhibition of PARP-1 had the potential to improve the efficacy of melphalan in combination therapy by increasing chemosensitivity. Taking into account the responses observed in vitro, our results suggested that the enhancement of melphalan efficacy might be best achieved by the PARP-1 inhibitor PJ34. The effects of PJ34 were associated with inhibition of the FA/BRCA pathway, increased apoptosis percentage, and G2/M cell cycle arrest. Elucidation of the basis for sensitization after pretreatment with PJ34 may still allow for further refinement and be worthy of investigation.
The FA/BRCA pathway plays an important role in the regulation of DNA damage repair. It can adjust intracellular reactions to cisplatin and other alkylating agents [6–15]. Our results showed that PJ34 could inhibit proliferation and induce apoptosis of RPMI8226 cells. The mechanism may be related to suppression of the FA/BRCA pathway through down-regulating the expression of related factors; thereby DNA damage repair is weakened, resulting in improved sensitivity to alkylating agents. Histone H2AX is a sensor of DNA damage. One of the early occurring events after DNA DSBs is the formation of phosphorylated histone H2AX (γH2AX), so γH2AX is a gold standard for detecting DSBs [22]. Therefore, the level of γH2AX can reflect the DNA damage caused by chemotherapy drugs in tumor cells, and it can also be an index of chemotherapy sensitivity. This study also found that the expression level of γH2AX increased with increasing concentration of PJ34 in RPMI8226 cells, which meant increasing DSBs and enhanced drug sensitivity. At the same time, PJ34 stimulated apoptosis of RPMI8226 cells and induced cell cycle arrest. The ability of PJ34 to regulate chemosensitivity was dependent on the FA/BRCA pathway. DNA damage mediated by the FA/BRCA pathway enhanced the sensitivity of melphalan, suggesting that the reduced capacity to repair DSBs may be contributory. These data have clinical implications, which may influence the selection of chemotherapy sensitizing agents based on DNA damage repair.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 81001053) and the Fundamental Research Funds for the Central Universities (Grant No. 4101041).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Wesierska-Gadek J, Skladanowski A. Therapeutic intervention by the simultaneous inhibition of DNA repair and type I or type II DNA topoisomerases: one strategy, many outcomes. Future Med Chem. 2012;4:51–72. doi: 10.4155/fmc.11.175. [DOI] [PubMed] [Google Scholar]
- 2.Fulda S. Targeting apoptosis signaling pathways for anticancer therapy. Front Oncol. 2011;1:23. doi: 10.3389/fonc.2011.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodríguez A, Sosa D, Torres L, et al. A Boolean network model of the FA/BRCA pathway. Bioinformatics. 2012;28:858–66. doi: 10.1093/bioinformatics/bts036. [DOI] [PubMed] [Google Scholar]
- 4.Karanja KK, Cox SW, Duxin JP, et al. DNA2 and EXO1 in replication-coupled, homology-directed repair and in the interplay between HDR and the FA/BRCA network. Cell Cycle. 2012;11:3983–96. doi: 10.4161/cc.22215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stecklein SR, Jensen RA. Identifying and exploiting defects in the Fanconi anemia/BRCA pathway in oncology. Transl Res. 2012;160:178–97. doi: 10.1016/j.trsl.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Jacquemont C, Simon JA, D'Andrea AD, et al. Non-specific chemical inhibition of the Fanconi anemia pathway sensitizes cancer cells to cisplatin. Mol Cancer. 2012;11:26. doi: 10.1186/1476-4598-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chirnomas D, Taniguchi T, de la Vega M, et al. Chemosensitization to cisplatin by inhibitors of the Fanconi anemia/BRCA pathway. Mol Cancer Ther. 2006;5:952–61. doi: 10.1158/1535-7163.MCT-05-0493. [DOI] [PubMed] [Google Scholar]
- 8.Taniguchi T, D'Andrea AD. Molecular pathogenesis of Fanconi anemia: recent progress. Blood. 2006;107:4223–33. doi: 10.1182/blood-2005-10-4240. [DOI] [PubMed] [Google Scholar]
- 9.Mori R, Yoshida K, Tanahashi T, et al. Decreased FANCJ caused by 5FU contributes to the increased sensitivity to oxaliplatin in gastriccancer cells. Gastric Cancer. 2013;16:345–54. doi: 10.1007/s10120-012-0191-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deans AJ, West SC. DNA interstrand crosslink repair and cancer. Nat Rev Cancer. 2011;11:467–80. doi: 10.1038/nrc3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hegi ME, Sciuscio D, Murat A, et al. Epigenetic deregulation of DNA repair and its potential for therapy. Clin Cancer Res. 2009;15:5026–31. doi: 10.1158/1078-0432.CCR-08-1169. [DOI] [PubMed] [Google Scholar]
- 12.Palagyi A, Neveling K, Plinninger U, et al. Genetic inactivation of the Fanconi anemia gene FANCC identified in the hepatocellular carcinoma cell line HuH-7 confers sensitivity towards DNA-interstrand crosslinking agents. Mol Cancer. 2010;9:127. doi: 10.1186/1476-4598-9-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burkitt K, Ljungman M. Phenylbutyrate interferes with the Fanconi anemia and BRCA pathway and sensitizes head and neck cancer cells to cisplatin. Mol Cancer. 2008;7:24. doi: 10.1186/1476-4598-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kondo N, Takahashi A, Mori E, et al. FANCD1/BRCA2 plays predominant role in the repair of DNA damage induced by ACNU or TMZ. PLoS One. 2011;6:e19659. doi: 10.1371/journal.pone.0019659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hannemann J, Oosterkamp HM, Bosch CA, et al. Changes in gene expression assiociated with response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2005;23:3331–42. doi: 10.1200/JCO.2005.09.077. [DOI] [PubMed] [Google Scholar]
- 16.Plummer R. Perspective on the pipeline of drugs being developed with modulation of DNA damage as a target. Clin Cancer Res. 2010;16:4527–31. doi: 10.1158/1078-0432.CCR-10-0984. [DOI] [PubMed] [Google Scholar]
- 17.Annunziata CM, O'Shaughnessy J. Poly (ADP-ribose) polymerase as a novel therapeutic target in cancer. Clin Cancer Res. 2010;16:4517–26. doi: 10.1158/1078-0432.CCR-10-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidson D, Wang Y, Aloyz R, et al. The PARP inhibitor ABT-888 synergizes irinotecan treatment of colon cancer cell lines. Invest New Drugs. 2013;31:461–8. doi: 10.1007/s10637-012-9886-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng H, Zhang Z, Borczuk A, et al. PARP inhibition selectively increases sensitivity to cisplatin in ERCC1-low non-small cell lung cancer cells. Carcinogenesis. 2013;34:739–49. doi: 10.1093/carcin/bgs393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cavallo F, Graziani G, Antinozzi C, et al. Reduced proficiency in homologous recombination underlies the high sensitivity of embryonal carcinoma testicular germ cell tumors to Cisplatin and poly (adp-ribose) polymerase inhibition. PLoS One. 2012;7:51563. doi: 10.1371/journal.pone.0051563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donawho CK, Luo Y, Luo Y, et al. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res. 2007;13:2728–37. doi: 10.1158/1078-0432.CCR-06-3039. [DOI] [PubMed] [Google Scholar]
- 22.Mah LJ, Orlowski C, Ververis K, et al. Utility of γH2AX as a molecular marker of DNA double-strand breaks in nuclear medicine: applications to radionuclide therapy employing auger electron-emitting isotopes. Curr Radiopharm. 2011;4:59–67. doi: 10.2174/1874471011104010059. [DOI] [PubMed] [Google Scholar]