Abstract
Statins (HMG-CoA reductase inhibitors) are a group of highly efficient pharmacological agents used for reducing blood cholesterol level and prevention/treatment of cardiovascular disease. Adverse reactions during statin treatment affect quite significant numbers of patients (reportedly from 5% to 20%), with more side effects occurring at higher doses. Reduced statin dosing can be achieved by improved bioavailability of statins, which is fairly low due to poor aqueous solubility, low permeability and high molecular weight of some members of the statin family. Moreover, since hepatic cholesterologenesis is a main target of statin action and extrahepatic inhibition of HMG-CoA reductase has no effect on plasma lipids, hepatic bioavailability, in our opinion, becomes a new important modality of statins maximizing their potential effect on the plasma lipid profile and diminishing their extrahepatic toxicity. Therefore efficient delivery systems of statins into hepatocytes need to be developed and introduced. Uses of nano-emulsifying statin delivery systems which may include vectors of intrahepatic transport, in particular lycopene, are discussed. As a proof of concept, some preliminary results revealing the effect of a lycopene-containing nanoformulation of simvastatin (designated as Lyco-Simvastatin) on LDL in mildly hypercholesterolemic patients are shown.
Keywords: statins, lycopene, bioavailability, cholesterol
Introduction
Cholesterol is an essential component of every living eukaryotic cell required for the formation of cell membranes and cellular organelles, also serving as a major metabolic precursor of all steroid hormones, bile acids and vitamin D [1]. Over 90% of cholesterol is located in the tissues and organs of an animal body, whereas 7–10% is associated with plasma and blood cells. Since cholesterol is a highly insoluble substance, it is transported in blood by particles, called lipoproteins, which are classified according to their density. Low-density lipoproteins (LDL) are known to be a major means of transport of cholesterol in human blood [2]. An overwhelming number of epidemiological studies suggest that elevated levels of LDL and total cholesterol in blood are linked to increased incidence of cardiovascular disease and higher mortality of cardiovascular patients [3, 4]. On the other hand, reduced LDL and total cholesterol values confer lower occurrence of atherosclerosis, stroke and myocardial infarction in patients with atherosclerosis and peripheral artery disease [5–7]. Therefore, reduction of blood cholesterol, especially its LDL fraction, represents the most popular and modern approach in the prevention and pharmacological management of cardiovascular disease [8, 9].
Statins
Approximately 80% of cholesterol in human blood originates from the liver [10]. Therefore a search for effective pharmacological inhibitors of hepatic lipogenesis initiated decades ago remains the most effective strategy in the prevention and treatment of hypercholesterolemia and atherosclerosis. Screening of different xenogenic compounds resulted 30 years ago in the discovery of selective inhibitors of 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMG-CoA reductase), a rate-limiting enzyme of the cholesterol biosynthetic pathway in the liver [11]. Notably, pharmacological inhibition of any other enzyme belonging to the cholesterol biosynthesis pathway in the liver is not as effective in terms of the cholesterol reduction in blood [12]. Subsequently, a new class of pharmacological compounds called statins emerged. This group recently includes lovastatin, fluvastatin, pitavastatin, pravastatin, rosuvastatin, simvastatin and atorvastatin. All of them have a closely related chemical structure and work exclusively by activation of the hepatic clearance of plasma lipoprotein via an LDL-receptor pathway and further elimination of cholesterol from the human body with bile [11–13]. Thus, the reduction of cholesterol in the blood of statin-treated patients is a strictly liver-mediated phenomenon [14]. It has to be emphasized that HMG-CoA reductase, the only known molecular target of statins mediating their effects on the lipid profile, is a liver-specific enzyme poorly expressed in other tissues [15]. Despite undisputable health benefits of the statin treatment proven in hundreds of research projects (reduction in heart attack/sudden cardiac death incidence by 60%, and strokes by 17%), there are some significant concerns related to their long-term use in clinical practice [16, 17]. It is believed that poor compliance and insufficient persistence in statin treatment does not confer measurable health benefits [18]. However, long-term intake of statins is associated with significant side effects. Adverse reactions during statin treatment affect quite significant numbers of patients (reportedly from 5% to 20%), with more side effects occurring at higher doses [19, 20]. Myopathies, memory impairment, neuropathies, increased risk of type 2 diabetes, elevated liver enzymes, general weakness and depression are reported in statin-treated patients [21]. Among them, muscle damage leading in extreme cases to fatal rhabdomyolysis is considered to be the most severe side effect of statin therapy [22, 23]. It has to be emphasized that toxic effects of statins develop in a strictly dose-dependent manner and often subside when the dose is reduced. Therefore, avoidance of unnecessarily intense treatment schedules and targeting the statin delivery to the liver might be very useful in the prevention of statin toxicity.
Bioavailability of statins
Reduced statin dosing can be achieved by improved bioavailability of statins, which is fairly low due to poor aqueous solubility, low permeability and high molecular weight of some members of the statin family. As an example, the bioavailability rate for lovastatin is astonishingly low, approximating 5% only, whereas the reported value for simvastatin is much higher, reaching up to 60% [24]. Although limited aqueous solubility of statins is considered to be an important cause of their low bioavailability, solubility enhancement, as well as maximizing intestinal absorption of statins used as a single approach, is not likely to be a successful strategy. A high concentration of statins in the systemic circulation may aggravate the statin side effects and toxicity in the long term.
It can be assumed that since hepatic cholesterologenesis is a main target of statin action, an efficient system of delivery of statins into hepatocyte needs to be developed. Therefore hepatic bioavailability becomes a new important modality of action of statins in the human body, maximizing their potential effect on the plasma lipid profile and diminishing their extrahepatic toxicity.
Intrahepatic availability as a keystone feature of statin action
Occurrence of statins in the systemic circulation upon absorption does not necessarily translate into an immediate lipid-lowering effect. At the onset of their action, statins have to become available for binding with HMG-CoA reductase inside hepatocytes. However, statins tend to be widely distributed among different internal organs and tissues (liver, spleen, adrenal glands, adipose tissue and muscles) after absorption [25]. Once again, the liver is a main target organ for HMG-CoA reductase inhibitors, and statin action in non-hepatic tissues has no known therapeutic benefits. Minimizing deposition of statins in extrahepatic tissues as well as enhancing statin delivery to the liver would be very beneficial for clinical practice. First-pass uptake of statins by hepatocytes is reported to be mediated by different mechanisms including passive diffusion, and active, carrier-mediated transport through the hepatocyte membrane with organic anion transport polypeptide-C is thought to be essential for hepatocellular delivery of hydrophilic statins [24, 26]. Strikingly, rosuvastatin, a known champion of hepatoselectivity among statins, whose intrahepatic delivery rate reaches 90% of the dose absorbed in the intestine, has the most prominent effect on plasma LDL as well as remarkably low toxicity [24].
Taking everything into consideration, it can be stated that an ideal statin delivery system has to meet at least two basic requirements. Firstly it has to provide efficient transport of statins through a gastrointestinal barrier, and secondly it has to be capable of effective intrahepatic delivery of the drug. Moreover, there is a huge and as yet poorly explored potential for use of endogenous receptor-mediated hepatic pathways to promote intrahepatic delivery of pharmaceuticals. Various receptors expressed on hepatocyte membranes have extreme selectivity and efficiency in the internalization of different ligands, in contrast to less efficient passive and active diffusion of xenobiotics through the hepatocyte membrane. Therefore construction of microparticles containing a xenobiotic compound bound to the ligands of receptor-mediated hepatic uptake may represent a new strategy in enhancing hepatoselectivity of statins.
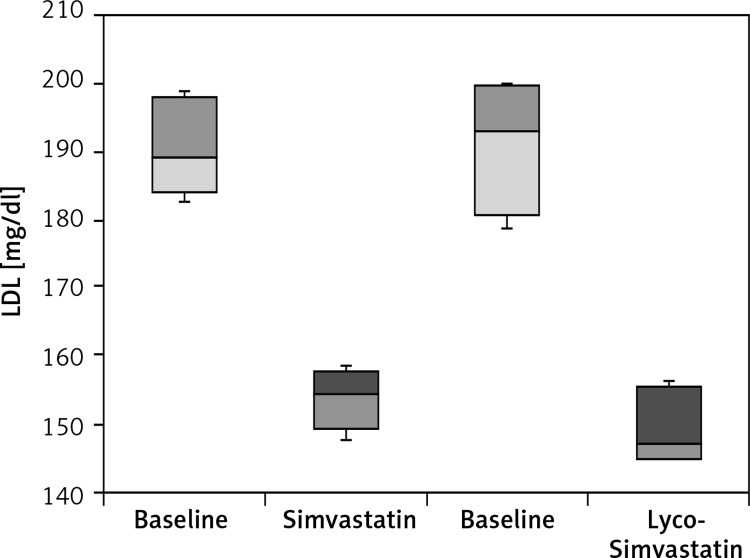
From this standpoint, a novel statin delivery system, designated as Lycostatin, was developed in our work [27]. Lycostatin is a new formulation of statins in which a HMG-CoA reductase inhibitor, in particular simvastatin (named Lyco-Simvastatin), is incorporated into a microemulsifying system using spray drying, ultrasound, and supercritical CO2 [28]. This system contains lycopene, a hydrophobic compound, which is used not only as a core-forming agent, but also as a vector with high tropism to hepatocytes, which are known to express abundantly a carotenoid receptor. In addition it contains amphiphilic phosphatidylcholine as a chaperone for lycopene, which also has hydrophilyzing as well as emulsifying properties and increases thereby intestinal absorption. In a water-free environment Lyco-Simvastatin is a composition of nano-sized lycosome particles. In experimental settings, the solubilized lycosome particles have an enhanced intestinal absorption rate and ability to bind hepatocyte membranes as compared to unmodified simvastatin (Petyaev et al., unpublished observation). The preliminary clinical results for Lyco-Simvastatin use are shown in Figure 1. As can be seen from our preliminary results (Figure 1), Lyco-simvastatin has superior activity in reducing LDL levels in patients with hypercholesterolemia at the same dosage level (20 mg daily) as compared to the unmodified simvastatin (p = 0.0049).
Figure 1.
LDL values following 4 week treatment with simvastatin versus of Lyco-Simvastatin. Ten patients of both genders aged from 47 to 65 years old with moderate increase in plasma LDL (from 150 to 200 mg/dl) were randomized and enrolled in the pilot clinical trial. Each patient received daily either 20 mg of unmodified simvastatin or 20 lycosome-formulated statin (Lyco-Simvastatin). Plasma samples were obtained after 30-day treatment and analyzed for lipids. The results are presented in box-and-whisker plots versus pre-treatment (baseline) values
Although further research related to pharmacology of Lyco-Simvastatin (as well as other lycosome-formulated statins) still needs to be done, these results allow us to assume that higher functional activity of Lyco-Simvastatin could be attributable to enhanced hepatic delivery of the drug arising from the specifics of the nanoparticle composition used. The interface area of lycosome-formulated statin microparticles contains lycopene, a carotenoid utilizing a unique transport system inside the human body. It is well acknowledged that upon absorption lycopene crystals and/or lycopene-containing nanoparticles (lycosomes) become incorporated into chylomicrons to be distributed in the human body by lymph and blood flows [29]. Inside the liver the lycosome-containing chylomicrons are likely to undergo a dual receptor-mediated uptake. Since lycosome-containing chylomicrons include in their core lycopene, a powerful ligand for carotenoid receptors, expressed by hepatocytes, they become more easily internalized by these cells via a carotenoid receptor mechanism, promoting thereby intrahepatic delivery of lycosome-formulated statins. Besides the carotenoid receptor, the enhanced hepatocellular delivery of Lycostatin can be confidently explained by an LDL-receptor mechanism, which represents, in our opinion, a second pathway of intrahepatic uptake. It is well known that chylomicrons and products of their enzymatic degradation (LDL and VLDL) are transported inside hepatocytes using the LDL receptor mediated by ApoB, an intrinsic component of low-density lipoprotein particles [30].
Conclusions
Discovery of statins and their further development started with scrupulous investigation and subsequent chemical modifications of compactin, a single naturally occurring small molecule produced by a fungus from the Penicillium family [31, 32]. In recent times the search for new statins has been virtually exhausted since computational chemistry does not predict any new statin derivate showing inhibitory activity towards HMG-CoA reductase [33]. Therefore, the developments in pharmacology of hypercholesterolemia will be limited in the foreseeable future to already known statins, while optimization of their delivery systems and bioavailability may offer new therapeutic benefits. However, the projected use of statins is likely to grow over the next decades as new indications for their use become substantiated [19, 34].
In these terms, development of statin formulations with increased hepatic bioavailability would be a significant step forward in the treatment of cardiovascular disease. Incorporation of simvastatin in the lycopene-containing microparticles, promoting their enhanced absorption and subsequent incorporation in chylomicrons with further hepatic intake via a dual carotenoid/LDL receptor mechanism ensures targeted hepatic delivery of the drug to the liver. It is possible that other vectors promoting efficient hepatic delivery can be used for new statin formulations with enhanced therapeutic efficiency. Redirecting a drug flow to the liver not only allows statin dose reduction but also minimizes exposure of the tissues vulnerable to statin action (muscles, nerve tissue, etc.), thereby reducing adverse effects. This would help to expand the use of this drug to the broader population to further reduce the prevalence of cardiovascular disease and other clinical complications of atherosclerosis.
Conflict of interest
The author declares no conflict of interest.
References
- 1.Coyan FC, Loussouarn G. Cholesterol regulation of ion channels: crosstalk in proteins, crosstalk in lipids. Channels (Austin) 2013;7:415–6. doi: 10.4161/chan.26683. [DOI] [PubMed] [Google Scholar]
- 2.Poirier S, Mayer G. The biology of PCSK9 from the endoplasmic reticulum to lysosomes: new and emerging therapeutics to control low-density lipoprotein cholesterol. Drug Des Devel Ther. 2013;7:1135–48. doi: 10.2147/DDDT.S36984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mark L, Paragh G, Karadi I, Reiber I, Pados G, Kiss Z. How can we further improve the LDL-cholesterol target level achievement rate based on the Hungarian MULTI GAP 2011 study results and considering the new European dyslipidemia guidelines? Arch Med Sci. 2012;8:608–13. doi: 10.5114/aoms.2012.30283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webb J, Gonna H, Ray KK. Lipid management: maximising reduction of cardiac risk. Clin Med. 2013;13:618–20. doi: 10.7861/clinmedicine.13-6-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katsiki N, Mikhailidis DP, Athyros VG, Hatzitolios AI, Karagiannis A, Banach M. Are we getting to lipid targets in real life? Arch Med Sci. 2010;6:639–41. doi: 10.5114/aoms.2010.17073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aronow WS. Peripheral arterial disease of the lower extremities. Arch Med Sci. 2012;8:375–88. doi: 10.5114/aoms.2012.28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorci-Thomas MG, Thomas MJ. Why targeting HDL should work as a therapeutic tool, but has not. J Cardiovasc Pharmacol. 2013;62:239–46. doi: 10.1097/FJC.0b013e31829d48a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hennekens CH, Drowos J. Statins: the high risks of discontinuation and large benefits of continuation. Arch Med Sci. 2011;7:931–2. doi: 10.5114/aoms.2011.26602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rizzo M, Battista Rini G. Ezetimibe, cardiovascular risk and atherogenic dyslipidaemia. Arch Med Sci. 2011;7:5–7. doi: 10.5114/aoms.2011.20597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poli G, Biasi F, Leonarduzzi G. Oxysterols in the pathogenesis of major chronic diseases. Redox Biol. 2013;1:125–30. doi: 10.1016/j.redox.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parhofer KG. Update: clinical lipidology. MMW Fortschr Med. 2013;155:49–52. doi: 10.1007/s15006-013-1050-6. quiz 53-4. [DOI] [PubMed] [Google Scholar]
- 12.Sharpe LJ, Brown AJ. Controlling cholesterol synthesis beyond 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) J Biol Chem. 2013;288:18707–15. doi: 10.1074/jbc.R113.479808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allian-Sauer MU, Falko JM. New treatments on the horizon for familial hypercholesterolemia. Expert Rev Cardiovasc Ther. 2012;10:1227–37. doi: 10.1586/erc.12.112. [DOI] [PubMed] [Google Scholar]
- 14.Tiwari R, Pathak K. Statins therapy: a review on conventional and novel formulation approaches. J Pharm Pharmacol. 2011;63:983–98. doi: 10.1111/j.2042-7158.2011.01273.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen GP, Yao L, Lu X, Li L, Hu SJ. Tissue-specific effects of atorvastatin on 3-hydroxy-3-methylglutarylcoenzyme A reductase expression and activity in spontaneously hypertensive rats. Acta Pharmacol Sin. 2008;29:1181–6. doi: 10.1111/j.1745-7254.2008.00855.x. [DOI] [PubMed] [Google Scholar]
- 16.Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ. 2003;326:1423–29. doi: 10.1136/bmj.326.7404.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banach M, Aronow WS, Serban C, et al. Lipids, blood pressure and kidney update 2014. Pharmacol Res. 2015;95-96C:111–25. doi: 10.1016/j.phrs.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Kiss Z, Nagy L, Reiber I, et al. Persistence with statin therapy in Hungary. Arch Med Sci. 2013;9:409–17. doi: 10.5114/aoms.2013.35327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olson EA, Hainsworth DP, Davis G, Hagan JC. Eye on statins: a comprehensive review. Mo Med. 2013;110:344–8. [PMC free article] [PubMed] [Google Scholar]
- 20.Banach M, Rizzo M, Toth PP, et al. Statin intolerance – an attempt at a unified definition. Position paper from an International Lipid Expert Panel. Arch Med Sci. 2015;11:1–23. doi: 10.5114/aoms.2015.49807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huddy K, Dhesi P, Thompson PD. Do the frequencies of adverse events increase, decrease, or stay the same with long-term use of statins? Curr Atheroscler Rep. 2013;15:301–8. doi: 10.1007/s11883-012-0301-9. [DOI] [PubMed] [Google Scholar]
- 22.Hohenegger M. Drug induced rhabdomyolysis. Curr Opin Pharmacol. 2012;12:335–40. doi: 10.1016/j.coph.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients: the PRIMO study. Cardiovasc Drugs Ther. 2005;19:403–14. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- 24.Schachter M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol. 2005;19:117–25. doi: 10.1111/j.1472-8206.2004.00299.x. [DOI] [PubMed] [Google Scholar]
- 25.Nezasa K, Higaki K, Matsumura T, et al. Liver-specific distribution of rosuvastatin in rats: comparison with pravastatin and simvastatin. Drug Metab Dispos. 2002;30:1158–63. doi: 10.1124/dmd.30.11.1158. [DOI] [PubMed] [Google Scholar]
- 26.Shirasaka Y, Suzuki K, Nakanishi T, Tamai I. Intestinal absorption of HMG-CoA reductase inhibitor pravastatin mediated by organic anion transporting polypeptide. Pharm Res. 2010;27:2141–9. doi: 10.1007/s11095-010-0216-5. [DOI] [PubMed] [Google Scholar]
- 27.Carotenoid Particles And Uses Thereof; GB Patent Application No. 1101669.8, PCT/GB2012/000075, 25.01.2012. [Google Scholar]
- 28.Patel D, Sawant KK. Self micro-emulsifying drug delivery system: formulation development and biopharmaceutical evaluation of lipophilic drugs. Curr Drug Deliv. 2009;6:419–24. doi: 10.2174/156720109789000519. [DOI] [PubMed] [Google Scholar]
- 29.Bravo E, Napolitano M. Mechanisms involved in chylomicron remnant lipid uptake by macrophages. Biochem Soc Trans. 2007;35:459–63. doi: 10.1042/BST0350459. [DOI] [PubMed] [Google Scholar]
- 30.Hoover-Plow J, Huang M. Lipoprotein(a) metabolism: potential sites for therapeutic targets. Metabolism. 2013;62:479–91. doi: 10.1016/j.metabol.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chakravarti R, Sahai V. Compactin – a review. Appl Microbiol Biotechnol. 2004;64:618–24. doi: 10.1007/s00253-003-1553-7. [DOI] [PubMed] [Google Scholar]
- 32.Roth BD. The discovery and development of atorvastatin, a potent novel hypolipidemic agent. Prog Med Chem. 2002;40:1–22. doi: 10.1016/s0079-6468(08)70080-8. [DOI] [PubMed] [Google Scholar]
- 33.Ling H, Burns TL, Hilleman DE. Novel strategies for managing dyslipidemia: treatment beyond statins. Postgrad Med. 2012;124:43–54. doi: 10.3810/pgm.2012.11.2612. [DOI] [PubMed] [Google Scholar]
- 34.Banach M, Mikhailidis DP, Kjeldsen SE, Rysz J. Time for new indications for statins? J Med Sci Monit. 2009;15:MS1–5. [PubMed] [Google Scholar]