Abstract
The trigeminocardiac reflex (TCR) has previously been described in the literature as a reflexive response of bradycardia, hypotension, and gastric hypermotility seen upon mechanical stimulation in the distribution of the trigeminal nerve. The diving reflex (DR) in humans is characterized by breath-holding, slowing of the heart rate, reduction of limb blood flow and a gradual rise in the mean arterial blood pressure. Although the two reflexes share many similarities, their relationship and especially their functional purpose in humans have yet to be fully elucidated. In the present review, we have tried to integrate and elaborate these two phenomena into a unified physiological concept. Assuming that the TCR and the DR are closely linked functionally and phylogenetically, we have also highlighted the significance of these reflexes in humans.
Keywords: reflexes, oxygen-conserving effects, breath-hold, brain, trigeminocardiac reflex, diving reflex
Introduction
The trigeminal cardiac reflex (TCR) and diving reflex (DR) are neurogenic reflexes that share many similarities in their clinical presentations and mechanisms of action; however, their relationship and especially their functional purpose in humans have yet to be fully elucidated [1–10]. In the present review, we have tried to integrate and elaborate these two phenomena into a unified physiological concept. We have also highlighted the significance and current role of these reflexes in humans.
Definitions
The trigeminocardiac reflex
The TCR is a well-established neurogenic reflex which manifests as bradycardia, hypotension, and gastric hypermotility seen upon mechanical stimulation in the distribution of the trigeminal nerve [1–3]. Initial reports were based on animal experiments; however, TCR in neurosurgical patients was first elaborated by Schaller et al. in 1999 [3–6]. In their key work, Schaller et al. meticulously defined TCR, and their observations are still used and commonly appreciated by researchers worldwide [1–3, 5–7]. The incidence of the TCR in neurosurgical procedures involving or near the trigeminal nerve vicinity was reported to be about 10–18% [7, 8]. These studies have usually taken a 20% decrease in hemodynamic changes as the cut-off limit to define TCR; therefore the true incidence may be even higher. Moreover, the intensity of TCR is also a factor of the intensity of stimuli like the DR, which increases proportionally to decreasing water temperature [1–8]. At the molecular level, TCR is believed to be a physiological oxygen conserving reflex which when incited initiates a powerful sympathetic system response (within a few seconds) and thus increases the cerebral blood flow (CBF). This regional elevation of CBF without accompanying changes in the cerebral metabolic rate of oxygen or glucose rapidly provides oxygen to the brain in a more efficient manner. External stimuli and individual factors have a variable influence on inciting the TCR in humans [8].
The diving reflex
The diving reflex in humans is characterized by breath-holding, slowing of the heart rate, a decrease in cardiac output with sympathetic mediated peripheral vasoconstriction, an increase in mean arterial blood pressure (MABP) and splenic contraction [9–16]. Blood is re-directed to more vital organs (heart, brain and lung) while at the peripheral level poor irrigation of tissues manifests as lactate accumulation. A simultaneous splenic contraction is also observed which indeed increases the static apnea duration and helps in further resurgence of red blood cells in the blood circulation [17, 18]. These phenomena also exist during repeated episodes of dynamic apnea [13]. Diving reflex is firstly triggered by breath-holding and is augmented further by immersion of the face into cold water [19, 20]. This response is also very prompt; therefore, it can be considered as a reflex. The inhibition of the respiratory centers and the absence of afferent input from pulmonary stretch receptors increase vasomotor and cardio-inhibitory activity [21–23]. All these modifications allow economizing of the oxygen stores for the breath-hold diver (BHD) [18, 21, 24–27]. Similar to the TCR, the DR also acts as a protective oxygen-conserving reflex (OCR) and aims to keep the body alive during cold water immersion. Thus it protects the vital organs (the heart and the brain) from extreme hypoxia. The DR in humans is modifiable by various factors including water temperature, exercise, partial pressure of arterial oxygen (PaO2), carbon dioxide tension (PaCO2) and psychological factors [22].
Though the two reflexes share a lot of similarities, their relationship and especially their functional purpose in humans have not yet been fully elucidated. In the present article, we have tried to elaborate the similarities as well as dissimilarities between these two unique reflexes and therefore possibly integrate these into one common mechanism. We assume that partially, if not substantially, the TCR and the DR are closely linked functionally as well as phylogenetically and represent old reflexes that are physiological in humans in the first few months of life.
General similarities
There are obvious strong links between the TCR and the DR that are generally accepted. Both reflexes are based on the integrity of the trigeminal-brain stem reflex arc [2, 3]. In both, bradycardia is a common manifestation and is induced via reflex centers located in the medulla oblongata [1, 28]. Efferent parasympathetic pathways mediate bradycardia and similarly efferent sympathetic pathways mediate peripheral vasoconstriction.
General differences
The TCR occurs due to either peripheral or central stimulation [3]. Stimulation of the trigeminal nerve anywhere along the face including the nose, orbit, eyeball and scalp (the area supplied by the trigeminal nerve) up to the gasserian ganglion (entry into the intracranial compartment) is considered as a peripheral TCR. The DR can be considered as a peripheral TCR. The gasserian ganglion to the brainstem constitutes the rest of the trigeminal nerve course, and stimulation along this part is considered as the central TCR. Peripheral TCR stimulation may present with bradycardia with or without hypotension, whereas central TCR stimulation is usually followed by severe bradycardia/asystole and hypotension [3, 8]. What differs is that in the TCR there is a secondary decrease in MABP, whereas in the DR the MABP gradually increases. The difference might simply reflect the fact that the stimulus in the DR is not ceased as immediately as in the TCR. At present it is still not clear whether this increase in MABP is specific for all peripheral TCRs or only for a subgroup.
Bradycardia
It is a well-established fact that during breath-hold diving, the heart rate (HR) becomes slower. The reduction in HR is brought about by the involvement of both central inspiratory and phasic pulmonary afferent mechanisms [29]. Similarly, it has been reported that tracheal intubation imposed during surgical procedures may give some protection against activation of the TCR [30]. Babies under 6 months of age are excellent swimmers due to their DR: a baby's air passage blocks in contact with water, which explains the common observation of babies “swimming” under water with open mouths.
Both reflexes, the TCR and the DR, have a well-known reciprocal influence on cardiac vagal and sympathetic activity in adults resulting in bradycardia [31, 32]. Despite the above-mentioned brainstem changes, the striking age-related decline in occurrence of the TCR/DR in adults could be the result of increased arterial stiffness [32].
In fact, in some instances the HR may rebound to produce a delayed tachycardia being indicative of a temporal difference in the activation of the autonomic outflows with the increase in cardiac sympathetic activity outlasting the vagal effect. Indeed, for the DR, administration of methyl scopolamine may unmask tachycardia that may then be abolished by subsequent β-adrenoceptor blockade with propranolol. In addition, vagally mediated bradycardia evoked by stimulation of nasopharyngeal receptors was associated with simultaneous shortening of the electrocardiogram QT interval, a measure of ventricular repolarization. Paton et al. suggested that simultaneous co-activation may lead to a more efficient cardiac function, giving greater cardiac output than activation of the sympathetic limb alone, which is important when pumping blood into a constricted vascular tree as in the case of the DR and TCR [33].
Arterial blood pressure
Despite the increasing clinical reports about the TCR, the physiological function of this brainstem reflex is not yet fully explored [3, 33]. The one important difference is that the typical response of the DR – or peripheral TCR – is characterized by arterial hypertension, whereas the “classical” and central TCR leads to arterial hypotension. However, most of the measurements during breath-holding of blood pressure have shown no or a modest increase in blood pressure, indicating that the DR is less effective in humans during breath-hold diving than in mammals.
Cerebral blood flow
Another physiological similarity of both reflexes underlines the strong link between both: The DR results in an increase in cerebral blood flow, although there is some constriction of the cerebral resistance vessels [34]. A study revealed that in untrained BHDs and for apnea of 30 s, the CBF was increased by 60%; in elite BHDs and for the same apnea time, the CBF could be increased by 200%. The increase in CBF is thus trainable [35].
Trigeminocardiac reflex, diving reflex and the autonomous nervous system
From a phylogenetic standpoint, the autonomic nervous system may be considered as a structure that progressively formed in the course of evolution in order to increase cardiorespiratory survival. This hypothesis is further underlined by the fact that the two main divisions of the autonomic nervous system – the sympathetic and parasympathetic system – support different types of exchange with the external environment. The main function of the sympathetico-adrenal system is to organize the function of the visceral organs for an action to be performed by the organism in response to the (unexpected) requirements of the environment (“fight or flight”). On the other hand, the role of the parasympathetic system is to prepare the visceral organs for an action to be performed by the organism on itself: self-protection (homeostasis), regeneration and recovery and reproduction. This system strongly underlies phylo- and ontogenetically determined patterns.
The fact that cardiac vagal activity is similar after stimulation of the TCR and DR supports the hypothesis that the DR and TCR are closely linked. Whereas the goal of the DR may be clear (saving the organism from drowning with an oxygen-conserving effect), the purpose of the TCR remains less obvious.
Trigeminocardiac reflex and diving reflex as the basis of other pathologies?
The DR is the reflex mechanism most frequently considered in the etiopathogenesis of sudden infant death syndrome (SIDS) or crib death [14, 36–38]. This is defined as the sudden, unexpected death of an infant younger than 1 year of age which remains unexplained after a thorough investigation, including a complete autopsy, examination of the death scene, and a review of the clinical history [39].
Recent studies on the pathophysiology of SIDS have focused on the autonomic nervous system and have disclosed anomalies – mostly congenital – located in the brainstem [40–46]. In over 50% of SIDS cases, histological examination of the brainstem on serial sections showed underdevelopment of the brainstem, e.g. mono-, bilateral or partial hypoplasia, delayed neuronal maturation or decreased neuronal density of the arcuate nucleus, which is an important center controlling breathing activity. Underdevelopment of the pre-Bötzinger, of the parabrachial Kölliker-Fuse complex and of the hypoglossal nucleus was also detected [38, 41, 42, 44, 46]. Overall, the abnormalities of the autonomic nervous system described in SIDS may explain the occurrence of SIDS by vagal inhibition elicited by the DR [40, 43]. In the case of SIDS, a possible role of parenteral cigarette smoking in the pathogenesis of arcuate nucleus hypoplasia is discussed, suggesting a similar defect in patients who are susceptible to the TCR during neurosurgical operations [43].
Trigeminocardiac reflex, diving reflex and phylogenesis
As a matter of fact, the oxygen-conserving DR and its subsets seem to persist in humans [7, 8, 31]. In man, DR may be considered as an archaic relict which has functional importance in phylogenetically lower-ranked animals such as diver birds or amphibians. The DR is indeed particularly developed in birds to provide inhibition of cardiac and breathing activity during underwater feeding, necessary for individual and species survival [38]. In mammals, the DR is elicited by contact of the face with cold water and involves breath-holding, decreased ventilation, bradycardia, intense peripheral vasoconstriction, and increased MABP, with the purpose of preventing drowning and providing an oxygen reservoir in the lungs, maintaining the heart and the brain adequately oxygenated at the expense of less hypoxia-sensitive organs [47].
In humans, washing the face or plunging into cold water results in profound bradycardia and redistribution of the blood flow to the lungs, brain and heart [36]. Though considered to be the most powerful autonomic reflex, the purpose of this reflex, especially the breath-hold (BH) response in humans, is equivocal [47]. In newborns and infants with a developmental defect of the brainstem and its reflexogenic centers, there were found more deaths due to apnea or cessation of breathing [14, 36, 38]. Thus the role of the brainstem as part of the reflex cannot be ignored.
There is fine tuning between the reflex and the bodily response; however, exaggeration of this protective response could be detrimental and has been implicated in causing SIDS. This reflex can also become manifest in adult with acquired bulbospinal disease [38]. In elite BHDs, such problems after a long period of BH training are unknown.
In humans, the DR may be psychologically mediated and paradoxically may be lethal. Wolf in 1978 described sudden cardiac death from the DR in subjects who developed sinus arrest while thinking of and/or preparing to dive [38, 48]. Thereafter, no similar case has been reported, so this is a rare occurrence. But the DR may be trainable. It was found that BHDs presented biphasic HR kinetics and two heart rate decreases [49]. The second HR decrease, which was concomitant to the pronounced arterial oxygen saturation (SaO2) decrease, was also simultaneous with a marked increase in the root mean square successive difference of the R-R intervals (RMSSD), a vagal index. On the other hand, untrained BHDs showed only one HR decrease, which appeared before the concomitant SaO2 and RMSSD changes. This study indicates that baroreceptor reflex stimulation and hypoxia may be the key mechanisms involved in producing such a biphasic HR response of BHDs and thus help in prolongation of BH duration [49].
In fact, the DR had been shown to be effective in conserving oxygen in humans during BH at rest [21, 50]. Trained BHDs with severe bradycardia were able to slow the arterial desaturation by a factor of two or three [50]. This bradycardia is also accentuated with larger BHD pulmonary volumes before apnea, stimulating the activity in the slowly adapting pulmonary stretch receptors more powerfully and resulting in a lower HR decrease in the first phase. The decreasing HR in phase one also probably accounted for the slight diminution in cardiac output and, consequently, a slight increase in total peripheral resistance at the end of this first phase [51]. It would also account for an increase in stroke volume, which in turn would be a stimulus for the high-pressure aortic and carotid baroreceptor [52]. During the second phase, as BH duration increased and the alveolar volume decreased, the likely pulmonary volume decreases may have further reduced the activity in the pulmonary stretch receptors and, consequently, further reduced the HR [53]. It can be speculated that hypoxia would result in greater arterial chemoreceptor stimulation and thus would further accentuate bradycardia (Figure 1) [15].
Figure 1.
Static apnea of an elite breath-hold diver. The position is not conventional because usually they are lying at the surface
Rossi described a case of lethal cardiac arrest in a young army recruit who succumbed to the common barracks prank of pouring a messtin of cold water on the face of a sleeping comrade [38]. Abrupt vagal hyperexcitation from an aberrant DR due to trigeminal-ophthalmic triggering was the most plausible explanation, but no aimed control of the central nervous system was carried out, suggesting that there may be another reflexogenic arc than in the TCR.
The higher purpose of the TCR in mammals – especially humans – is at present not fully understood. We think that a very plausible explanation may be the following: The TCR may be important for breast-feeding during the first months of life. At that time the newborn drinks for a relatively long period of time with its face literally against the mother. Consequently, the upper airways are partially obstructed by the mother's body, resulting in hypoventilation. The TCR which is elicited by mechanical stimulation results in bradycardia, hypotension and increase in the cerebral blood flow in order to avoid damage to the developing brain. The role of the gastric hypermobility, another typical reaction elicited by stimulation of the TCR, also becomes evident from this view. This also may explain the psychological or emotional dimension of these reflexes.
Schaller et al. in their studies have also hypothesized on grounds of precise clinical and physiological observations that the term TCR subsumes the “classical” central TCR and the peripheral DR or oculocardiac reflex [54]. Grogaard and Sundell studied the “trigeminal diving reflex” in newborn lambs, reporting that this reflex is significantly reduced after treatment with β-adrenergic agonists [55]. As discussed above, we think that even from a phylogenetic standpoint there is a lot of evidence that both reflexes are closely linked and may interact with each other. They are phylogenetically old reflexes especially useful for the underwater feeding of diver-birds and amphibians. In humans they may be important during the breast feeding period where the babies’ upper airways are partially obstructed by the close body contact with the mother. Their role in the pathogenesis of SIDS and for potential complications during neurosurgical procedures is also highly important. In fact, a better understanding of these reflexes will result in better patient care.
Trigeminocardiac reflex and diving reflex clinical cases
Trigeminocardiac reflex case
A 60-year-old male patient with a diagnosis of right-sided vestibular schwannoma underwent tumor resection via a retrosigmoid (suboccipital approach) approach. His medical history was significant for long standing hypertension (on irbesartan – an angiotensin receptor blocker) and a history of smoking (14 PPD). His baseline MABP was 73 mm Hg and heart rate was 65 beats per minute. Two hours after skin incision, his MABP dropped to 43 mm Hg (40.8% drop from baseline) and concomitantly, his heart rate dropped to 40 beats per minute (38% drop from baseline). Then, the surgical procedure was discontinued; he was given atropine 0.6 mg intravenously. After 5 min, his MABP and heart rate stabilized to physiological values and the surgical procedure was carried out successfully to the end without any further episodes of TCR. His oxygen saturation was 100% and no hypercarbia occurred. The postoperative course was uneventful.
Diving reflex case
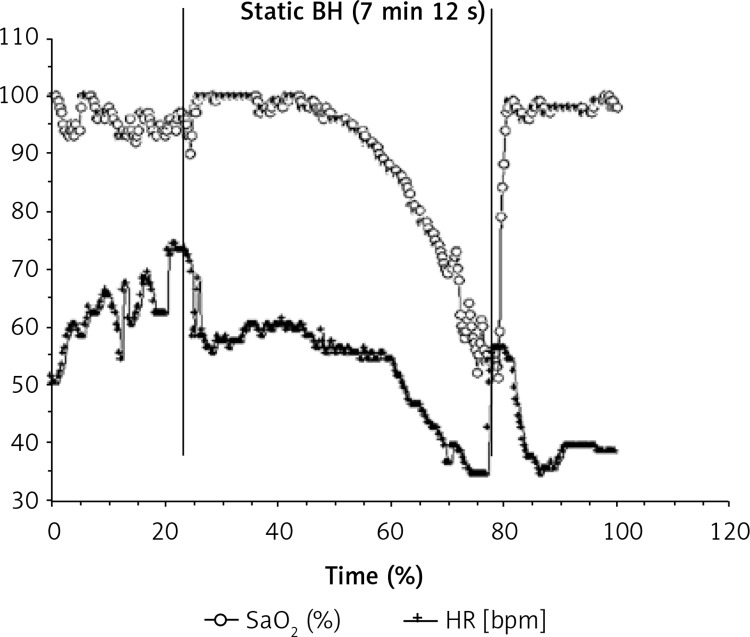
An expert BHD (man), 33 years old, with 11 years of BH practice and a forced vital capacity (FVC) of 6.7 l (124% of predicted values), performed a static BH of 7 min 12 s lying on the surface in a swimming pool. Heart rate behavior and SaO2 were continuously recorded during one maximal BH. Short-term changes in SaO2, HR, the root mean square successive difference of the R-R intervals (RMSSD), and the time-domain heart rate variability (HRV) index were calculated over the complete BH duration. This BHD presented biphasic HR kinetics (Figure 2), with two HR decreases (32% and 64% of initial HR). The second HR decrease, which was concomitant to the pronounced SaO2 decrease, was also simultaneous with a marked increase in RMSSD. A classically untrained BHD showed only one HR decrease (about 20–30% of initial HR), which appeared before the concomitant SaO2 and RMSSD changes [49].
Figure 2.
Static BH of 7 min and 12 s in one expert BHD. Heart rate [2] and SaO2 were recorded continuously before, during and after the BH duration (presented between the two vertical lines)
In fact, for this BHD the cardiovascular diving response was effective in conserving oxygen during BH at rest. This elite BHD with a strong bradycardia was able to slow the arterial desaturation according to previous studies [50]. The BHD also had higher FVC than predicted values, which can at the beginning of the BH powerfully stimulate the activity in the slowly adapting pulmonary stretch receptors and result in a strong HR decrease in the first phase. The decreasing HR in phase one also probably accounted for the slight diminution in cardiac output with an increase in stroke volume, which in turn would be a stimulus for the high-pressure aortic and carotid baroreceptors [52]. During the second phase, as BH duration increased, the activity in the pulmonary stretch receptors would decrease and, consequently, reduce HR even more [53]. Hypoxia would also result in greater arterial chemoreceptor stimulation and would accentuate bradycardia [15]. Thus hypoxia could enhance vasoconstriction and thus the venous return, stimulating baroreceptors and again reducing HR [15]. This case indicates that baroreflex stimulation and hypoxia may be involved in the biphasic HR response and thus in the long BH duration and that the DR may be trained.
Current role of trigeminocardiac reflex vs. diving reflex
A recent review highlighted the clinical implications of these reflexes. The DR has been used to treat supraventricular tachycardia (SVT) [56]. This reflex is incited by various maneuvers including immersion of the face in preset cold water and breath holding exercises. Even nasopharyngeal suction aborted the episodes of SVT in pediatric patients too [57]. However, a recent animal (pontomedullary transaction) experiment suggested that the medulla and spinal cord may be the primary target site to complete the reflex arc [58]. On the other hand, the TCR has been investigated as a protective reflex during sleep bruxism [59]. Due to periods of micro-arousals, tachycardia starts; however, masticatory movements rapidly incite the TCR and slow down the heart rate. Although it was previously thought that local anesthetic could block incitation of the TCR, many studies have shown that local anesthetic is not effective to blunt the TCR [60, 61]. Similarly, local anesthetic could not blunt the DR either. These features indeed highlight the possibility of differential sensitivity of nerve fibers for inciting these reflexes, and the stretch could still be reflexogenic even with the blocked nerve. The reflexogenic mechanisms of both the reflexes, if not substantially, may partially share the pathways. The different receptors and related stimuli may possibly play a complex role to elucidate the two different mechanisms; however, these are still not fully elucidated and warrant further research [62].
Conclusions
There exists a close association between these two unique neurogenic reflexes. Both are physiological, protective reflexes and pose some clinical significance; however, both can be detrimental if not properly regulated either due to absence of physiological control or anatomical defect. The DR can be considered as peripheral a sub-form of the TCR. However, so far, there are no convincing experimental or histopathological data to prove these connections, and thus further studies are warranted. In cases of sudden death attributed to diving or trigeminal stimulation, meticulous examination of the brainstem on serial sections may have a crucial role in indentifying morphological substrates responsible for these reflexes [38, 43, 44, 63–68]. In the future, it will be very interesting to examine the TCR in elite breath-hold divers who develop a strong DR.
Conflict of interest
Dr Bernhard Schaller, one of the co-authors of this review, is the co-editor of the journal (“Archives of Medical Science”).
The authors declare no conflict of interest.
References
- 1.Schaller B, Probst R, Strebel S, Gratzl O. Trigeminocardiac reflex during surgery in the cerebellopontine angle. J Neurosurg. 1999;90:215–20. doi: 10.3171/jns.1999.90.2.0215. [DOI] [PubMed] [Google Scholar]
- 2.Schaller B. Trigeminocardiac reflex. A clinical phenomenon or a new physiological entity? J Neurol. 2004;251:658–65. doi: 10.1007/s00415-004-0458-4. [DOI] [PubMed] [Google Scholar]
- 3.Schaller B, Cornelius JF, Prabhakar H, et al. The trigemino-cardiac reflex: an update of the current knowledge. J Neurosurg Anesthesiol. 2009;21:187–95. doi: 10.1097/ANA.0b013e3181a2bf22. [DOI] [PubMed] [Google Scholar]
- 4.Kumada M, Dampney RA, Reis DJ. The trigeminal depressor response: a novel vasodepressor response originating from the trigeminal system. Brain Res. 1977;119:305–26. doi: 10.1016/0006-8993(77)90313-4. [DOI] [PubMed] [Google Scholar]
- 5.Schaller B. Trigemino-cardiac reflex during microvascular trigeminal decompression in cases of trigeminal neuralgia. J Neurosurg Anesthesiol. 2005;17:45–8. [PubMed] [Google Scholar]
- 6.Schaller B. Trigemino-cardiac reflex during transsphenoidal surgery for pituitary adenomas. Clin Neurol Neurosurg. 2005;107:468–74. doi: 10.1016/j.clineuro.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Schaller B, Cornelius JF, Sandu N, Ottaviani G, Perez-Pinzon MA. Oxygen-conserving reflexes of the brain: the current molecular knowledge. J Cell Mol Med. 2009;13:644–7. doi: 10.1111/j.1582-4934.2009.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaller B, Sandu N, Cornelius J. Trigemino-Cardiac-Reflex-Examination-Group (T.C.R.E.G.). Oxygen-conserving implications of the trigemino-cardiac reflex in the brain: the molecular basis of neuroprotection? Mol Med. 2009;15:125–6. doi: 10.2119/molmed.2009.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heusser K, Dzamonja G, Tank J, et al. Cardiovascular regulation during apnea in elite divers. Hypertension. 2009;53:719–24. doi: 10.1161/HYPERTENSIONAHA.108.127530. [DOI] [PubMed] [Google Scholar]
- 10.Muth CM, Ehrmann U, Radermacher P. Physiological and clinical aspects of apnea diving. Clin Chest Med. 2005;26:381–94. doi: 10.1016/j.ccm.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Ferretti G. Extreme human breath-hold diving. Eur J Appl Physiol. 2001;84:254–71. doi: 10.1007/s004210000377. [DOI] [PubMed] [Google Scholar]
- 12.Ferrigno M, Ferretti G, Ellis A, et al. Cardiovascular changes during deep breath-hold dives in a pressure chamber. J Appl Physiol. 1997;83:1282–90. doi: 10.1152/jappl.1997.83.4.1282. [DOI] [PubMed] [Google Scholar]
- 13.Lemaitre F, Polin D, Joulia F, et al. Physiological responses to repeated apneas in underwater hockey players and controls. Undersea Hyperb Med. 2007;34:407–14. [PubMed] [Google Scholar]
- 14.Lobban CD. The oxygen-conserving dive reflex re-examined as the principal contributory factor in sudden infant death. Med Hypotheses. 1995;44:273–7. doi: 10.1016/0306-9877(95)90179-5. [DOI] [PubMed] [Google Scholar]
- 15.Lin YC, Shida KK, Hong SK. Effects of hypercapnia, hypoxia, and rebreathing on heart rate response during apnea. J Appl Physiol. 1983;54:166–71. doi: 10.1152/jappl.1983.54.1.166. [DOI] [PubMed] [Google Scholar]
- 16.Liner MH. Tissue gas stores of the body and head-out immersion in humans. J Appl Physiol. 1993;75:1285–93. doi: 10.1152/jappl.1993.75.3.1285. [DOI] [PubMed] [Google Scholar]
- 17.Bakovic D, Valic Z, Eterovic D, et al. Spleen volume and blood flow response to repeated breath-hold apneas. J Appl Physiol. 2003;95:1460–6. doi: 10.1152/japplphysiol.00221.2003. [DOI] [PubMed] [Google Scholar]
- 18.Palada I, Eterovic D, Obad A, et al. Spleen and cardiovascular function during short apneas in divers. J Appl Physiol. 2007;103:1958–63. doi: 10.1152/japplphysiol.00182.2007. [DOI] [PubMed] [Google Scholar]
- 19.Andersson J, Schagatay E, Gislen A, Holm B. Cardiovascular responses to cold-water immersions of the forearm and face, and their relationship to apnoea. Eur J Appl Physiol. 2000;83:566–72. doi: 10.1007/s004210000317. [DOI] [PubMed] [Google Scholar]
- 20.Schuitema K, Holm B. The role of different facial areas in elicting human diving bradycardia. Acta Physiol Scan. 1988;132:119–20. doi: 10.1111/j.1748-1716.1988.tb08306.x. [DOI] [PubMed] [Google Scholar]
- 21.Delahoche J, Delapille P, Lemaitre F, Verin E, Tourny-Chollet C. Arterial oxygen saturation and heart rate variation during breath-holding: comparison between breath-hold divers and controls. Int J Sports Med. 2005;26:177–81. doi: 10.1055/s-2004-820976. [DOI] [PubMed] [Google Scholar]
- 22.Gooden BA. Mechanism of the human diving response. Integr Physiol Behav Sci. 1994;29:6–16. doi: 10.1007/BF02691277. [DOI] [PubMed] [Google Scholar]
- 23.Manley L. Apnoeic heart rate responses in humans. A review. Sports Med. 1990;9:286–310. doi: 10.2165/00007256-199009050-00004. [DOI] [PubMed] [Google Scholar]
- 24.Andersson JP, Liner MH, Runow E, Schagatay EK. Diving response and arterial oxygen saturation during apnea and exercise in breath-hold divers. J Appl Physiol. 2002;93:882–6. doi: 10.1152/japplphysiol.00863.2001. [DOI] [PubMed] [Google Scholar]
- 25.Foster GE, Sheel AW. The human diving response, its function, and its control. Scand J Med Sci Sports. 2005;15:3–12. doi: 10.1111/j.1600-0838.2005.00440.x. [DOI] [PubMed] [Google Scholar]
- 26.Schagatay E, Andersson J. Diving response and apneic time in humans. Undersea Hyperb Med. 1998;25:13–9. [PubMed] [Google Scholar]
- 27.Schagatay E, van Kampen M, Emanuelsson S, Holm B. Effects of physical and apnea training on apneic time and the diving response in humans. Eur J Appl Physiol. 2000;82:161–9. doi: 10.1007/s004210050668. [DOI] [PubMed] [Google Scholar]
- 28.Khurana RK, Watabiki S, Hebel JR, Toro R, Nelson E. Cold face test in the assessment of trigeminal-brainstem-vagal function in humans. Ann Neurol. 1980;7:144–9. doi: 10.1002/ana.410070209. [DOI] [PubMed] [Google Scholar]
- 29.Bamford OS, Jones DR. Respiratory and cardiovascular interactions in ducks: the effect of lung denervation on the initation of and recovery from some cardiovascular responses to submergence. J Physiol. 1976;259:575–96. doi: 10.1113/jphysiol.1976.sp011484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gandevia SC, McCloskey DI, Potter EK. Reflex bradycardia occurring in response to diving, nasopharyngeal stimulation and ocular pressure, and its modification by respiration and swallowing. J Physiol. 1978;276:383–94. doi: 10.1113/jphysiol.1978.sp012241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaller B, Buchfelder M. Trigemino-cardiac reflex: A recently discovered “oxygen-conserving” response? The potential therapeutic role of a physiological reflex. Arch Med Sci. 2006;2:3–5. [Google Scholar]
- 32.Schaller B, Filis A, Buchfelder M. Cardiac autonomic control in neurosurgery. Arch Med Sci. 2007;3:287–92. [Google Scholar]
- 33.Paton JF, Boscan P, Pickering AE, Nalivaiko E. The yin and yang of cardiac autonomic control: vago-sympathetic interactions revisited. Brain Res Brain Res Rev. 2005;49:555–65. doi: 10.1016/j.brainresrev.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Brown CM, Sanya EO, Hilz MJ. Effect of cold face stimulation on cerebral blood flow in humans. Brain Res Bull. 2003;61:81–6. doi: 10.1016/s0361-9230(03)00065-0. [DOI] [PubMed] [Google Scholar]
- 35.Joulia F, Lemaitre F, Fontanari P, Mille ML, Barthelemy P. Circulatory effects of apnoea in elite breath-hold divers. Acta Physiol (Oxf) 2009;197:75–82. doi: 10.1111/j.1748-1716.2009.01982.x. [DOI] [PubMed] [Google Scholar]
- 36.Lobban CD. The human dive reflex as a primary cause of SIDS. A review of the literature. Med J Aust. 1991;155:561–3. [PubMed] [Google Scholar]
- 37.Berlin Heidelberg. Crib Death; Sudden Unexplained Death of Infants: the Pathologist's Viewpoint; Germany: Springer-Verlag; 2007. [Google Scholar]
- 38.Rossi L, Matturri L. Clinicopathological approach to cardiac arrhythmias. In: Torino, editor. New-York: Futura; 1990. pp. 234–50. [Google Scholar]
- 39.Willinger M, James LS, Catz C. Defining the sudden infant death syndrome (SIDS): deliberations of an expert panel convened by the National Institute of Child Health and Human Development. Pediatr Pathol. 1991;11:677–84. doi: 10.3109/15513819109065465. [DOI] [PubMed] [Google Scholar]
- 40.Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol Neonate. 1994;65:194–7. doi: 10.1159/000244052. [DOI] [PubMed] [Google Scholar]
- 41.Lavezzi AM, Ottaviani G, Matturri L. Adverse effects of prenatal tobacco smoke exposure on biological parameters of the developing brainstem. Neurobiol Dis. 2005;20:601–7. doi: 10.1016/j.nbd.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 42.Matturri L, Ottaviani G, Alfonsi G, Crippa M, Rossi L, Lavezzi AM. Study of the brainstem, particularly the arcuate nucleus, in sudden infant death syndrome (SIDS) and sudden intrauterine unexplained death (SIUD) Am J Forensic Med Pathol. 2004;25:44–8. doi: 10.1097/01.paf.0000113813.83779.21. [DOI] [PubMed] [Google Scholar]
- 43.Matturri L, Ottaviani G, Lavezzi AM. Sudden infant death triggered by dive reflex. J Clin Pathol. 2005;58:77–80. doi: 10.1136/jcp.2004.020867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matturri L, Ottaviani G, Lavezzi AM. Techniques and criteria in pathologic and forensic-medical diagnostics in sudden unexpected infant and perinatal death. Am J Clin Pathol. 2005;124:259–68. doi: 10.1309/J6AR-EY41-HKBE-YVHX. [DOI] [PubMed] [Google Scholar]
- 45.Ottaviani G, Matturri L, Bruni B, Lavezzi AM. Sudden infant death syndrome “gray zone” disclosed only by a study of the brain stem on serial sections. J Perinat Med. 2005;33:165–9. doi: 10.1515/JPM.2005.031. [DOI] [PubMed] [Google Scholar]
- 46.Ottaviani G, Matturri L, Mingrone R, Lavezzi AM. Hypoplasia and neuronal immaturity of the hypoglossal nucleus in sudden infant death. J Clin Pathol. 2006;59:497–500. doi: 10.1136/jcp.2005.032037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giesbrecht GG. Cold stress, near drowning and accidental hypothermia: a review. Aviat Space Environ Med. 2000;71:733–52. [PubMed] [Google Scholar]
- 48.Wolf S. Psychophysiological influences of the dive reflex in man; New York: Raven Press; 1978. [Google Scholar]
- 49.Lemaitre F, Buchheit M, Joulia F, Fontanari P, Tourny-Chollet C. Static apnea effect on heart rate and its variability in elite breath-hold divers. Aviat Space Environ Med. 2008;79:99–104. doi: 10.3357/asem.2142.2008. [DOI] [PubMed] [Google Scholar]
- 50.Stewart IB, Bulmer AC, Sharman JE, Ridgway L. Arterial oxygen desaturation kinetics during apnea. Med Sci Sports Exerc. 2005;37:1871–6. doi: 10.1249/01.mss.0000176305.51360.7e. [DOI] [PubMed] [Google Scholar]
- 51.Trzebski A, Smietanowski M. Cardiovascular periodicities in healthy humans in the absence of breathing and under reduced chemical drive of respiration. J Auton Nerv Syst. 1996;57:144–8. doi: 10.1016/0165-1838(95)00076-3. [DOI] [PubMed] [Google Scholar]
- 52.Duprez D, De Buyzere M, Trouerbach J, Ranschaert W, Clement DL. Continuous monitoring of haemodynamic parameters in humans during the early phase of simulated diving with and without breathholding. Eur J Appl Physiol. 2000;81:411–7. doi: 10.1007/s004210050062. [DOI] [PubMed] [Google Scholar]
- 53.Mithoefer JC. Lung volume restriction as a ventilatory stimulus during breath holding. J Appl Physiol. 1959;14:701–5. doi: 10.1152/jappl.1959.14.5.701. [DOI] [PubMed] [Google Scholar]
- 54.Schaller BJ, Buchfelder M, Knauth M. Trigemino-cardiac reflex during skull base surgery: a new entity of ischaemic preconditioning? The potential role of imaging. Eur J Nucl Med Mol Imaging. 2006;33:384–5. doi: 10.1007/s00259-005-1964-z. [DOI] [PubMed] [Google Scholar]
- 55.Grogaard J, Sundell H. Effect of beta-adrenergic agonists on apnea reflexes in newborn lambs. Pediatr Res. 1983;17:213–9. doi: 10.1203/00006450-198303000-00010. [DOI] [PubMed] [Google Scholar]
- 56.Smith G, Morgans A, Taylor DM, Cameron P. Use of the human dive reflex for the management of supraventricular tachycardia: a review of the literature. Emerg Med J. 2012;29:611–6. doi: 10.1136/emermed-2011-200877. [DOI] [PubMed] [Google Scholar]
- 57.Bjelakovic B, Vukovic B, Vojinovic J, et al. Deep nasopharyngeal aspiration as a treatment option for conversion of supraventricular paroxysmal tachycardia in infants: first experiences. Pediatr Crit Care Med. 2011;12:402–3. doi: 10.1097/PCC.0b013e3181fe3417. [DOI] [PubMed] [Google Scholar]
- 58.Panneton WM, Gan Q, Sun DW. Persistence of the nasotrigeminal reflex after pontomedullary transection. Respir Physiol Neurobiol. 2012;180:230–6. doi: 10.1016/j.resp.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schames SE, Schames J, Schames M, Chagall-Gungur SS. Sleep bruxism, an autonomic self-regulating response by triggering the trigeminal cardiac reflex. J Calif Dent Assoc. 2012;40:674–6. [PubMed] [Google Scholar]
- 60.Chowdhury T, West M. Intraoperative asystole in a patient undergoing craniotomy under monitored anesthesia care: is it TCR? J Neurosurg Anesthesiol. 2013;25:92–3. doi: 10.1097/ANA.0b013e318277d38a. [DOI] [PubMed] [Google Scholar]
- 61.Chowdhury T, Cappellani RB. Isolated bradycardia due to skull pin fixation: raised ICP or TCR? J Neurosurg Anesthesiol. 2013;25:207. doi: 10.1097/ANA.0b013e3182897fce. [DOI] [PubMed] [Google Scholar]
- 62.Yorgancilar E, Gun R, Yildirim M, Bakir S, Akkus Z, Topcu I. Determination of trigeminocardiac reflex during rhinoplasty. Int J Oral Maxillofac Surg. 2012;41:389–93. doi: 10.1016/j.ijom.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 63.Meuwly C, Golanov E, Chowdhury T, Erne P, Schaller B. Trigeminal cardiac reflex: new thinking model about the definition based on a literature review. Medicine (Baltimore) 2015;94:e484. doi: 10.1097/MD.0000000000000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chowdhury T, Mendelowitz D, Golanov E, et al. Trigeminocardiac reflex: the current clinical and physiological knowledge. J Neurosurg Anesthesiol. 2015;27:136–4. doi: 10.1097/ANA.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 65.Chowdhury T, Sandu N, Meuwly C, Cappellani RB, Schaller B. Trigeminocardiac reflex: differential behavior and risk factors in the course of the trigeminal nerve. Future Neurol. 2014;9:41–7. [Google Scholar]
- 66.Sandu N, Chowdhury T, Sadr-Eshkevari P, et al. Trigeminocardiac reflex during cerebellopontine angle surgery: anatomical location as a new risk factor. Future Neurol. 2015;10:7–13. [Google Scholar]
- 67.Koźluk E, Cybulski G, Piątkowska A, et al. Early hemodynamic response to the tilt test in patients with syncope. Arch Med Sci. 2014;10:1078–85. doi: 10.5114/aoms.2014.47820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chudzik M, Cygankiewicz I, Klimczak A, Lewek J, Bartczak K, Wranicz JK. Short-term ECG recordings for heart rate assessment in patients with chronic atrial fibrillation. Arch Med Sci. 2014;10:676–83. doi: 10.5114/aoms.2014.44859. [DOI] [PMC free article] [PubMed] [Google Scholar]