Abstract
Introduction
Acute lung injury (ALI) is an acute inflammatory disease characterized by excess production of inflammatory factors in lung tissue. Quercetin, a herbal flavonoid, exhibits anti-inflammatory and anti-oxidative properties. This study was performed to assess the effects of quercetin on lipopolysaccharide (LPS)-induced ALI.
Material and methods
Sprague-Dawley rats were randomly divided into 3 groups: the control group (saline alone), the LPS group challenged with LPS (Escherichia coli 026:B6; 100 µg/kg), and the quercetin group pretreated with quercetin (50 mg/kg, by gavage) 1 h before LPS challenge. Bronchoalveolar lavage fluid (BALF) samples and lung tissues were collected 6 h after LPS administration. Histopathological and biochemical parameters were measured.
Results
The LPS treatment led to increased alveolar wall thickening and cellular infiltration in the lung, which was markedly prevented by quercetin pretreatment. Moreover, quercetin significantly (p < 0.05) attenuated the increase in the BALF protein level and neutrophil count and lung wet/dry weight ratio and myeloperoxidase activity in LPS-challenged rats. The LPS exposure evoked a 4- to 5-fold rise in BALF levels of tumor necrosis factor-α and interleukin-6, which was significantly (p < 0.05) counteracted by quercetin pretreatment. Additionally, quercetin significantly (p < 0.05) suppressed the malondialdehyde level and increased the activities of superoxide dismutase, catalase, and glutathione peroxidase in the lung of LPS-treated rats.
Conclusions
Quercetin pretreatment effectively ameliorates LPS-induced ALI, largely through suppression of inflammation and oxidative stress, and may thus have therapeutic potential in the prevention of this disease.
Keywords: quercetin, acute lung injury, inflammation, oxidative stress
Introduction
Acute lung injury (ALI) is an acute inflammatory disease, characterized by excess production of inflammatory factors in lung tissue, and followed by non-cardiogenic dyspnea, severe hypoxemia, and pulmonary edema, thus leading to both high morbidity and mortality [1, 2]. A major cause of the development of ALI is sepsis, wherein Gram-negative bacteria are a prominent cause [3]. The intraperitoneal injection of lipopolysaccharide (LPS), a component of the outer cell wall of most Gram-negative bacteria, mimics human Gram-negative ALI and is one of the most commonly accepted models for ALI [4]. Lipopolysaccharide, binding to its receptor, toll-like receptor 4, provokes the activation of a key pro-inflammatory transcription factor, nuclear factor κB, which induces the expression of various pro-inflammatory cytokines and chemokines, such as tumor necrosis factor-α (TNF-α), interleukin-1β, and macrophage inflammatory protein-2 [5]. As a consequence of the strong inflammatory response, alveolar structures change, endothelial and alveolar permeability increase and alveolar fluid clearance decreases, thus critically impairing lung function [3, 6]. Despite significant advances in understanding of the pathophysiology of ALI, currently available treatment options have failed to significantly reduce ALI-related mortality. Therefore, new strategies are still needed to achieve effective treatment of ALI.
Flavonoids are a group of natural substances with a wide range of biological effects, including anti-inflammatory and anti-oxidative effects [7]. For instance, licochalcone A, a flavonoid in licorice root (Glycyrrhiza glabra), attenuates alveolar wall thickening, alveolar hemorrhage, and interstitial edema [8]. Quercetin is a flavonoid present in many vegetables, fruits, and beverages and possesses a broad range of pharmacological properties, including anti-inflammatory [9], anti-proliferative [10], and anti-oxidative effects [11]. Recent evidence demonstrates that quercetin can elicit protective effects in different lung injury models [12, 13]. Quercetin administration suppresses paraquat-induced pulmonary injury through its antioxidant activity [12]. Quercetin attenuates cigarette smoke-induced airway inflammation and mucus production in rats [13]. In addition, at the molecular level, quercetin inhibits pulmonary oxidant stress in lung epithelial cells through inducing heme oxygenase-1 (HO-1) and reduces paraquat-mediated oxidative damage via modulating the expression of antioxidant genes [14, 15].
Therefore, we postulated that quercetin could protect against LPS-induced lung injury. In the present study, we tested this hypothesis by using a rat model of LPS-induced ALI.
Material and methods
Animals and experimental groups
All animal experiments were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals, and were approved by the Animal Care and Use Committee of Wenzhou Central Hospital (Wenzhou, China). A total of 36 adult male Sprague-Dawley rats weighing 280–300 γ were obtained from the Animal Center of Wenzhou Medical College (Wenzhou, China). They were housed in a temperature-and humidity-controlled environment with a 12-h light-dark cycle and allowed free access to water and standard laboratory chow. The animals were randomly divided into 3 groups: the control group, the LPS group challenged with LPS alone, and the quercetin group pretreated with quercetin 1 h before LPS challenge.
LPS-induced ALI model
A rat model of ALI was generated as described previously [16]. Briefly, rats were fasted overnight and anesthetized with pentobarbital sodium (50 mg/kg body weight, i.p.). A tracheostomy was performed and LPS (from Escherichia coli 026:B6; 100 µg/kg body weight; Sigma, St. Louis, MO, USA) was injected intratracheally through a 24-gauge catheter. Sham-treated animals (control group) received an equal volume of saline alone. In the quercetin group, animals were given a single dose of quercetin (50 mg/kg, by gavage) 1 h before LPS challenge. The quercetin concentration was used according to a previous report [17].
At 6 h after LPS instillation, rats were killed with an overdose of pentobarbital sodium. The left lung was collected from 5 animals of each group for histopathological examination, and the right lung for analysis of lung wet/dry weight ratio. The left lung from the other 7 animals of each group was subjected to bronchoalveolar lavage (BAL), and the right lung was excised for measurement of biochemical parameters.
BAL and differential cell counts
Bronchoalveolar lavage fluid was performed as described previously [18]. The left lung was lavaged 3 times with 2.0 ml of ice-cold phosphate buffered saline (PBS). The BAL fluid (BALF) was immediately centrifuged at 500 × γ for 10 min at 4°C, and the cell-free supernatants were stored at –80°C for cytokine and protein analysis. The cell pellets were resuspended in PBS, and the total cell number was determined using a hemocytometer. Cytospin of BAL was prepared and stained with the Wright-Giemsa method. Differential cell counts were evaluated by counting at least 500 cells for the determination of the relative percentage of a specific cell type. Number of neutrophils was calculated as the percentage of neutrophils multiplied by the total number of cells in the BAL.
Protein determination in BALF
Total protein content in BALF was determined by the Bradford method according to the manufacturer's instructions (Tiangen, Beijing, China) using bovine serum albumin as a standard.
Measurement of cytokines in BALF
The concentrations of TNF-α and interleukin-6 (IL-6) in the BALF were measured by standard enzyme-linked immunosorbent assay (ELISA) using commercially available kits according to the manufacturer's instructions (R&D system, Minneapolis, MN, USA).
Lung wet/dry weight ratio
Lung edema was assessed by measuring the tissue wet/dry weight ratio. The right lung was excised and the wet weight was recorded. The dry weight was determined after the lung was placed in an incubator at 80°C for 24 h, and the wet/dry weight ratios were then calculated.
Myeloperoxidase assay
Myeloperoxidase (MPO) activity, a common indicator of neutrophil sequestration, was measured as described previously [19]. In brief, snap-frozen lung tissues were homogenized in PBS solution (pH 6.0) containing 0.5% hexadecyltrimethylammonium bromide, sonicated twice for 30 s on ice, and centrifuged at 12,000 × γ for 15 min at 4°C. The supernatant was then collected and mixed 1: 30 (v/v) with assay buffer containing the substrate o-dianisidine hydrochloride (0.2 mg/ml) and 0.2 mM hydrogen peroxide. Standard MPO (Sigma) was used in parallel to determine MPO activity in the sample. Absorbance change was measured at 460 nm for 5 min, and MPO activity was expressed as unit per gram of wet lung tissue per min.
Oxidative stress measurements
Oxidative stress parameters were measured as described previously [20]. A 10% (w/vol) lung homogenate was prepared and centrifuged, and the supernatant was used for assays with commercially available kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Malondialdehyde (MDA) levels were measured with the thiobarbituric acid reaction. Superoxide dismutase (SOD) activity was measured at 550 nm by the hydroxylamine method. Catalase (CAT) activity was determined by the breakdown of hydrogen peroxide at 37°C. Glutathione peroxidase (GPx) activity was determined by measuring the oxidation of NADPH at 37°C.
Lung histopathology
Lung tissue was fixed in 10% paraformaldehyde for 24 h, dehydrated by ethanol, embedded in paraffin, and sectioned into 4-µm slices. The deparaffinized sections were stained with hematoxylin and eosin (H + E). Evaluation of lung injury was performed under a light microscope.
Statistical analysis
Data are expressed as means ± standard deviation (SD). Statistical significance was determined by one-way analysis of variance followed by Tukey's multiple comparison test. A p value < 0.05 was considered statistically significant.
Results
Effects of quercetin on LPS-induced histological changes in the lung
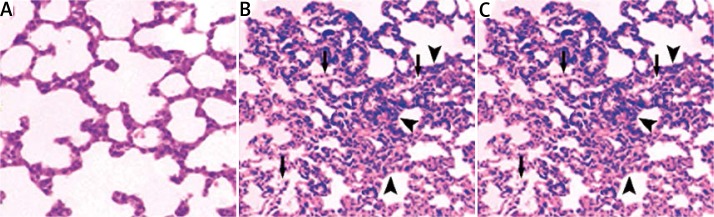
As shown in Figure 1A, sham-operated rats (control group) had normal lung morphology, with a thin alveolar wall and no evident infiltration. In contrast, LPS-treated animals without quercetin pretreatment exhibited extensive lung damage, manifested by profound infiltration of inflammatory cells into lung interstitium and alveolar spaces and alveolar wall thickening (Figure 1B). Notably, such lung histopathological changes were markedly attenuated by quercetin pretreatment (Figure 1C).
Figure 1.
Quercetin pretreatment ameliorates LPS-induced pathological changes in the lung. The lungs from the control group (A), LPS-treated group (B), and quercetin group (C) with quercetin pretreatment 1 h before LPS treatment were subjected to H + E staining. Representative images of H + E-stained lung sections from each group are shown (magnification: 200×). B – LPS exposure led to infiltration of inflammatory cells into lung interstitium and alveolar spaces (arrow) and alveolar wall thickening (arrowhead). C – Such pathological changes were attenuated by quercetin pretreatment
Effects of quercetin on LPS-induced pulmonary hyperpermeability and edema
Lipopolysaccharide exposure resulted in a > 4-fold rise in BALF protein levels and a significant (p < 0.05; Figure 2A) increase in lung wet/dry weight ratios, when compared to sham-operated rats (Figure 2B). The LPS-induced protein leakage and pulmonary edema were significantly (p < 0.05) reduced by quercetin pretreatment (Figure 2).
Figure 2.
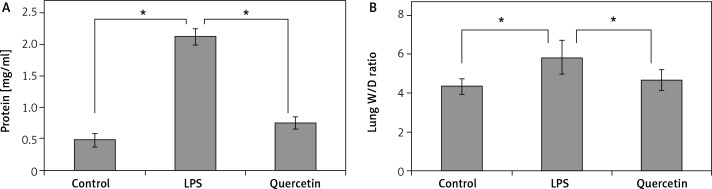
Quercetin inhibits LPS-induced pulmonary hyperpermeability and edema. After LPS challenge, the BALF and the right lung from each group were collected and assessed for the protein concentration (A) and wet/dry weight ratio (B), respectively
Data are presented as mean ± SD; *p < 0.05.
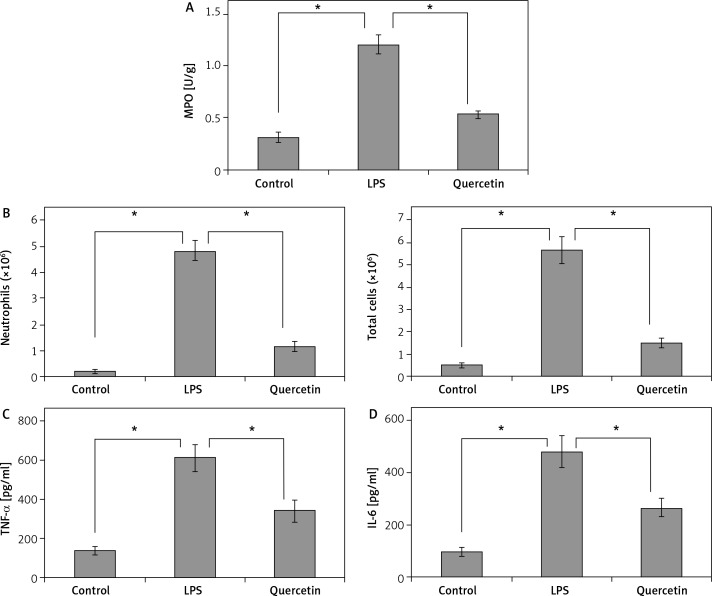
Effects of quercetin on LPS-induced pulmonary inflammation
Lung MPO activity and BAL neutrophil numbers were significantly (p < 0.05) higher in animals after LPS exposure than in sham-operated animals (Figure 3A, B). Quercetin pretreatment significantly (p < 0.05) reduced the MPO activity and BAL neutrophil count by 58% and 79%, respectively (Figure 3A, B).
Figure 3.
Quercetin pretreatment suppresses LPS-induced neutrophil infiltration and pro-inflammatory cytokine release. The MPO activity in the lung (A), the number of neutrophils and total cells (B), and the concentrations of TNF-α (C) and IL-6 (D) in BALF were measured in each group after LPS challenge
Data are presented as mean ± SD; *p < 0.05.
As shown in Figures 3C and D, LPS exposure evoked a 4- to 5-fold rise in the BALF levels of TNF-α and IL-6 in comparison with sham-operated animals. The LPS-induced release of the two cytokines was significantly (p < 0.05) counteracted by quercetin pretreatment.
Effects of quercetin on LPS-induced lung oxidative stress
As shown in Table I, the level of MDA, a lipid peroxidation end product, was significantly (p < 0.05) increased in LPS-treated animals relative to sham-operated animals. Quercetin pretreatment diminished the MDA level by 43%, when compared to the LPS group. The activities of the 3 antioxidant enzymes (SOD, CAT, and GPx) were significantly (p < 0.05) lower in LPS-treated animals than in sham-operated animals (Table I). Notably, such a decline in the activities of these enzymatic antioxidants was significantly (p < 0.05) prevented by quercetin pretreatment.
Table I.
Measurement of oxidative stress biomarkers in the lung
| Variable | Control group | LPS group | Quercetin group |
|---|---|---|---|
| MDA [nmol/mg protein] | 8.21 ±1.54 | 21.08 ±1.65* | 12.48 ±1.95*,# |
| SOD [U/mg protein] | 260.36 ±17.89 | 159.75 ±15.05* | 193.26 ±11.32*,# |
| GPx [U/mg protein] | 190.14 ±42.61 | 136.39 ±61.47* | 171.28 ±30.86*,# |
| CAT [U/mg protein] | 21.26 ±2.54 | 14.70 ±3.05* | 18.37 ±2.81*,# |
MDA – malondialdehyde, SOD – superoxide dismutase, GPx – glutathione peroxidase, CAT – catalase. Value are expressed as mean ± SD
p < 0.05 versus the control group
p < 0.05 versus the LPS group.
Discussion
In the present study, we found that pretreatment with quercetin attenuated LPS-induced alveolar wall thickening and inflammatory cell infiltration. Quercetin pretreatment diminished the lung wet/dry weight ratio and BALF protein level, indicating a reduction in pulmonary hyperpermeability and edema. LPS-induced TNF-α and IL-6 secretion and neutrophil cell influx in BALF were inhibited by quercetin, accompanied by reduced MPO activity in the lung. Additionally, quercetin pretreatment resulted in a decline in the MDA level and elevation in the activities of SOD, CAT, and GPx. Taken together, our results show that quercetin prevents LPS-induced ALI, at least partially, via suppression of inflammation and oxidative stress.
Release of various cytokines/chemokines plays a critical role in the pathogenesis of ALI, leading to an increase in the pulmonary permeability of the alveolar-capillary barrier and a consequent impairment in arterial oxygenation [3, 21]. Thus, inhibition of lung inflammation has been a primary target in pharmacologic treatment of ALI patients. Many natural flavonoids such as licochalcone A [8], oroxylin A [22], and astragalin [23] have shown benefits in protecting the lung from LPS-induced ALI via suppression of pro-inflammatory cytokine secretion. Consistently, we found that the natural flavonoid quercetin also ameliorated LPS-induced ALI, which was associated with negative modulation of pro-inflammatory factors including TNF-α and IL-6. Neutrophil recruitment into the lung is a hallmark of ALI. It has been widely accepted that neutrophils are an important source of pro-inflammatory cytokines and contribute to inflammation-induced lung injury [4, 24]. Our data revealed that quercetin pretreatment significantly decreased the number of neutrophils in BALF and the MPO activity in the lung. Tissue MPO activity is a well-established indicator for neutrophil infiltration [25]. Taken together, these results indicate that quercetin exerts anti-inflammatory effects against LPS-induced ALI.
A large body of evidence supports the role of oxidants and oxidative injury in the pathogenesis of ALI [26, 27]. Activated neutrophils produce vast quantities of reactive oxygen species and nitrogen species, which cause cell injury through direct damage to DNA, lipid peroxidation with formation of vasoactive molecules, and oxidation of proteins [28]. Our results also demonstrated that LPS-induced neutrophil recruitment and activation in the lung was accompanied by occurrence of pulmonary oxidative stress, as evidenced by increased MDA levels and reduced antioxidant enzymes. Many efforts have been made to develop antioxidant therapy for the treatment of ALI. Quercetin has shown anti-oxidative activity in various oxidative diseases [29, 30]. Park et al. [12] reported that quercetin can effectively inhibit paraquat-induced lung injury in rats through reduction of oxidative stress. Hayashi et al. [14] demonstrated that quercetin treatment protects against pulmonary oxidant stress via induction of HO-1 in lung epithelial cells. In a rat model of cigarette smoke-induced lung injury, quercetin pretreatment was found to suppress goblet cell hyperplasia, inflammation, and oxidative stress in the lung [13]. Consistently, our present data demonstrated that pretreatment with quercetin significantly inhibited LPS-induced increase of pulmonary hyperpermeability and lung edema, inflammatory cytokine secretion and neutrophil influx in BALF. Moreover, quercetin administration restored the activities of SOD, CAT, and GPx and reduced the MDA level in the lung. These findings collectively demonstrate that quercetin pretreatment has a preventive effect against LPS-induced ALI, which is largely mediated through elevation of antioxidant enzymes and reduction of oxidative stress.
In conclusion, our results indicate that quercetin confers protection against LPS-induced ALI, which involves the reduction of oxidative stress and lung inflammation. These findings warrant further exploration of the clinical applicability of quercetin in the prevention and treatment of ALI.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Martínez O, Nin N, Esteban A. Prone position for the treatment of acute respiratory distress syndrome: a review of current literature. Arch Bronconeumol. 2009;45:291–6. doi: 10.1016/j.arbres.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Wozniak K, Sleszycka J, Safianowska A, Wiechno W, Domagala-Kulawik J. Systemic inflammation in peripheral arterial disease with or without coexistent chronic obstructive pulmonary disease: analysis of selected markers. Arch Med Sci. 2012;8:477–83. doi: 10.5114/aoms.2012.29403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L379–99. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–49. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 5.Beutler B, Rietschel ET. Innate immune sensing and its roots: the story of endotoxin. Nat Rev Immunol. 2003;3:169–76. doi: 10.1038/nri1004. [DOI] [PubMed] [Google Scholar]
- 6.Piotrowski WJ, Majewski S, Marczak J, Kurmanowska Z, Górski P, Antczak A. Exhaled breath 8-isoprostane as a marker of asthma severity. Arch Med Sci. 2012;8:515–20. doi: 10.5114/aoms.2012.28639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74:418–25. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- 8.Chu X, Ci X, Wei M, et al. Licochalcone a inhibits lipopolysaccharide-induced inflammatory response in vitro and in vivo. J Agric Food Chem. 2012;60:3947–54. doi: 10.1021/jf2051587. [DOI] [PubMed] [Google Scholar]
- 9.Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008;585:325–37. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Borska S, Gebarowska E, Wysocka T, Drag-Zalesińska M, Zabel M. The effects of quercetin vs cisplatin on proliferation and the apoptotic process in A549 and SW1271 cell lines in vitro conditions. Folia Morphol (Warsz) 2004;63:103–5. [PubMed] [Google Scholar]
- 11.Saito A, Sugisawa A, Umegaki K, Sunagawa H. Protective effects of quercetin and its metabolites on H2O2-induced chromosomal damage to WIL2-NS cells. Biosci Biotechnol Biochem. 2004;68:271–6. doi: 10.1271/bbb.68.271. [DOI] [PubMed] [Google Scholar]
- 12.Park HK, Kim SJ, Kwon do Y, Park JH, Kim YC. Protective effect of quercetin against paraquat-induced lung injury in rats. Life Sci. 2010;87:181–6. doi: 10.1016/j.lfs.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Yang T, Luo F, Shen Y, et al. Quercetin attenuates airway inflammation and mucus production induced by cigarette smoke in rats. Int Immunopharmacol. 2012;13:73–81. doi: 10.1016/j.intimp.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi Y, Matsushima M, Nakamura T, et al. Quercetin protects against pulmonary oxidant stress via heme oxygenase-1 induction in lung epithelial cells. Biochem Biophys Res Commun. 2012;417:169–74. doi: 10.1016/j.bbrc.2011.11.078. [DOI] [PubMed] [Google Scholar]
- 15.Zerin T, Kim YS, Hong SY, Song HY. Quercetin reduces oxidative damage induced by paraquat via modulating expression of antioxidant genes in A549 cells. J Appl Toxicol. 2013;33:1460–7. doi: 10.1002/jat.2812. [DOI] [PubMed] [Google Scholar]
- 16.McCarter SD, Mei SH, Lai PF, et al. Cell-based angiopoietin-1 gene therapy for acute lung injury. Am J Respir Crit Care Med. 2007;175:1014–26. doi: 10.1164/rccm.200609-1370OC. [DOI] [PubMed] [Google Scholar]
- 17.Francescato HD, Coimbra TM, Costa RS, Bianchi Mde L. Protective effect of quercetin on the evolution of cisplatin-induced acute tubular necrosis. Kidney Blood Press Res. 2004;27:148–58. doi: 10.1159/000078309. [DOI] [PubMed] [Google Scholar]
- 18.Ci X, Chu X, Wei M, Yang X, Cai Q, Deng X. Different effects of farrerol on an OVA-induced allergic asthma and LPS-induced acute lung injury. PLoS One. 2012;7:e34634. doi: 10.1371/journal.pone.0034634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Kovacs EJ, Schwacha MG, Chaudry IH, Choudhry MA. Acute alcohol intoxication increases interleukin-18-mediated neutrophil infiltration and lung inflammation following burn injury in rats. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1193–201. doi: 10.1152/ajplung.00408.2006. [DOI] [PubMed] [Google Scholar]
- 20.Trocha M, Merwid-Ląd A, Chlebda E, et al. Influence of ezetimibe on selected parameters of oxidative stress in rat liver subject to ischemia/reperfusion. Arch Med Sci. 2014;10:817–24. doi: 10.5114/aoms.2013.38087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodman RB, Pugin J, Lee JS, Matthay MA. Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev. 2003;14:523–35. doi: 10.1016/s1359-6101(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 22.Tseng TL, Chen MF, Tsai MJ, Hsu YH, Chen CP, Lee TJ. Oroxylin-A rescues LPS-induced acute lung injury via regulation of NF-κB signaling pathway in rodents. PLoS One. 2012;7:e47403. doi: 10.1371/journal.pone.0047403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soromou LW, Chen N, Jiang L, et al. Astragalin attenuates lipopolysaccharide-induced inflammatory responses by down-regulating NF-kappaB signaling pathway. Biochem Biophys Res Commun. 2012;419:256–61. doi: 10.1016/j.bbrc.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31(4 Suppl):S195–9. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- 25.Sun L, Guo RF, Newstead MW, Standiford TJ, Macariola DR, Shanley TP. Effect of IL-10 on neutrophil recruitment and survival after Pseudomonas aeruginosa challenge. Am J Respir Cell Mol Biol. 2009;41:76–84. doi: 10.1165/rcmb.2008-0202OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang CH, Yang ML, Tsai CH, Li YC, Lin YJ, Kuan YH. Ginkgo biloba leaves extract (EGb 761) attenuates lipopolysaccharide-induced acute lung injury via inhibition of oxidative stress and NF-kappaB-dependent matrix metalloproteinase-9 pathway. Phytomedicine. 2013;20:303–9. doi: 10.1016/j.phymed.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Kratzer E, Tian Y, Sarich N, et al. Oxidative stress contributes to lung injury and barrier dysfunction via microtubule destabilization. Am J Respir Cell Mol Biol. 2012;47:688–97. doi: 10.1165/rcmb.2012-0161OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Czapski GA, Avram D, Sakharov DV, Wirtz KW, Strosznajder JB, Pap EH. Activated neutrophils oxidize extracellular proteins of endothelial cells in culture: effect of nitric oxide donors. Biochem J. 2002;365:897–902. doi: 10.1042/BJ20011206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mariee AD, Abd-Allah GM, El-Beshbishy HA. Protective effect of dietary flavonoid quercetin against lipemic-oxidative hepatic injury in hypercholesterolemic rats. Pharm Biol. 2012;50:1019–25. doi: 10.3109/13880209.2012.655424. [DOI] [PubMed] [Google Scholar]
- 30.Pandey AK, Patnaik R, Muresanu DF, Sharma A, Sharma HS. Quercetin in hypoxia-induced oxidative stress: novel target for neuroprotection. Int Rev Neurobiol. 2012;102:107–46. doi: 10.1016/B978-0-12-386986-9.00005-3. [DOI] [PubMed] [Google Scholar]