Abstract
Introduction
Different clinical conditions can compromise the urinary bladder function and structure. Routine regenerative practices in urology for bladder augmentation have been associated with diverse side effects. The internal lining of the bladder, the urothelium, plays an integral role in normal bladder function. Tissue engineering has provided novel therapeutic strategies through scaffolding and cell transplantation. Nano-scale surface features of scaffolds are valuable parameters for enhancement of cell behavior and function.
Material and methods
We fabricated a new hybrid scaffold of poly ɛ-caprolactone (PCL) and poly-L-lactide acid (PLLA) using an electrospinning system to exploit each polymer's advantages at nano-scale in the same scaffold. Dog urothelial cells were isolated, characterized by immunocytochemistry, and expanded for loading on the scaffold. Cell viability and proliferation on the scaffold surface were assessed by 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Furthermore, cytoarchitecture, distribution and detailed morphology of cells, and expression of cell specific markers were examined using hematoxylin and eosin (H + E) staining, scanning electron microscopy (SEM), and immunohistochemistry, respectively.
Results
According to MTT results, the scaffold did not exert any cytotoxic effect, and also supported cell proliferation and viability for 14 days of culture, which led to a significant increase in the number of cells. Scanning electron microscopy images revealed evenly distributed and normal appearing colonies of urothelial cells. A well-defined layer of cells was observed using H + E staining, which preserved their markers (pan-cytokeratin and uroplakin III) while growing on the scaffold.
Conclusions
Our findings confirmed favorable properties of PCL/PLLA regarding biocompatibility and applicability for upcoming new methods of bladder augmentation and engineering.
Keywords: urothelial cells, poly ɛ-caprolactone/poly-L-lactide acid, hybrid scaffold, tissue engineering
Introduction
Normal function of the urinary bladder can be damaged due to various conditions, including bladder cancer, trauma, neuropathic or vascular damage, congenital abnormalities of the genitourinary system, and chronic inflammatory uropathies [1]. Enterocystoplasty, as the most common practice of urology for bladder reconstruction, is associated with serious complications because of long-term contact of urine with absorptive epithelium of the intestine [2, 3].
Normal human urothelium is a multilayered transitional epithelium with unique architecture and expression pattern of markers. Pan-cytokeratin and uroplakins (forming plaques of asymmetric unit membrane in the superficial cell layer of native urothelium) are specifically and prominently expressed in native urothelium [4]. Normal urothelium plays the critical role of imposing a barrier between urine and blood; hence, an integral urothelium layer is indispensable to engineer the urinary bladder. Recent advances in tissue engineering have provided promising vistas in reconstructive urology. Cell sources with the capability of impaired function regeneration and appropriate matrices are essential parts that have to be considered to engineer a tissue.
Finding supportive matrices for bladder reconstruction is an ongoing challenge. To this end, different matrices have been studied in vitro and in animal studies [5–10]. Naturally derived materials (e.g., collagen) [11], acellular tissue matrices (e.g., the bladder submucosa and the small intestine submucosa) [12, 13], and composite cystoplasty [14] have their own limitations and possible side effects. Nevertheless, synthetic polymeric materials possess the advantage of control over their features, such as strength, degradation rate, biodegradability, and microstructure.
Electrospun nanofibers have emerged as novel matrices to engineer the urinary tract due to their superior characteristics in comparison to scaffolds, which are prepared with other methods in a high surface/volume ratio, and mimic extracellular matrix structure [15, 16]. Previous studies have reported that nanometer surface features of scaffolds can promote cell functions and enhance cell adhesion and proliferation [17].
Poly ɛ-caprolactone (PCL)'s favorable flexibility as well as its biocompatibility and biodegradability has brought about its extensive application in different contexts of tissue engineering [18, 19]. Poly-L-lactide (PLLA), as a Food and Drug Administration approved synthetic polymer, in spite of poor biocompatibility and mechanical features, could be a suitable option in scaffolding owing to its superior degradability [20–22]. Herein, we fabricated a new hybrid scaffold of PCL and PLLA using the electrospinning method to utilize their beneficial aspects in the same scaffold for bladder tissue engineering.
Therefore, we aim to study PCL/PLLA scaffold biocompatibility and urothelial cell behavior, proliferation, and phenotype on PCL/PLLA to evaluate its applicability in future studies.
Material and methods
Fabrication and preparation of PCL/PLLA nanofibrous scaffold
PCL/PLLA (60: 40) nanofibrous hybrid scaffolds were fabricated by the double-jet electrospinning method. PCL (Mn 80000) was dissolved (8% w/v) in chloroform/N, N-dimethylformamide (Sigma-Aldrich; St Louis, MO, USA) solution. PLLA (MW 258700) was dissolved (4% w/v) in chloroform/N,N-dimethylformamide solution. The polymer solutions were poured into two syringe pumps (SP-500; JMS, Tokyo, Japan) connected to a 20-gauge syringe nozzle through polyethylene extension tubing. The needle tip moved in a limited space along the direction of the deposition area while the collector (stainless steel cylinder) was rotating at 2300 rpm, leading to a uniform mat. After termination of the fabrication process to increase hydrophilicity, oxygen plasma treatment was performed. The bare materials were exposed to oxygen plasma at 13.6 MHz for 5 min using a Diener electronic plasma device. For stabilization, the nanofibrous mats were treated with methanol, and rinsed with sodium hydroxide solution and deionized water. The nanostructured scaffolds were then freeze-dried and kept until use. Only plasma-treated scaffolds were utilized in the current study and designated as PCL/PLLA.
Cell isolation and characterization
All the protocols and procedures for animal studies were approved by the ethics committee at the Urology and Nephrology Research Center affiliated to Shahid Beheshti University of Medical Sciences. All the cell culture products, except where otherwise specified, were purchased from PAA, Pasching, Austria. Full thickness biopsies of dog bladder (n = 5) were transferred to a 50 ml conical tube containing Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 2% penicillin/streptomycin and 2% Fungizone. After thoroughly washing in Hank's Buffered Salt Solution (HBSS), the mucosal layer was dissected from the smooth muscle layer aseptically. The mucosal layer was minced and digested in enzyme solution (2 mg/ml collagenase IV and 1 mg/ml dispase (Invitrogen Corp., Grand Island, NY)) at 37°C for 1 h. The cell suspension was passed thorough a 70 µm cell strainer to filter out tissue debris. Following centrifugation at 1500 rpm at 4°C for 5 min, the cell pellet was resuspended in keratinocyte serum-free medium (KSFM) supplemented with 30 ng/ml cholera toxin, 0.5 µg/ml hydrocortisone, 5 µg/ml insulin (Sigma-Aldrich; St. Louis, MO, USA), 5 ng/ml epidermal growth factor, 50 µg/ml bovine pituitary extract (Invitrogen Corp., Grand Island, NY), and 1% penicillin/streptomycin, and was plated in a T-75 cell culture flask (SPL Life Sciences, Pochun, South Korea). After establishment of colonies, cells were passaged with 0.05% trypsin-ethylenediaminetetraacetic acid (EDTA) and cultured in a medium with 50% KSFM containing all supplements and 50% 3T3 conditioned medium.
To study expression of urothelial cell markers in our cultured cells, immunodetection of uroplakin III and pan-cytokeratin was used. Briefly, after fixation in 4% paraformaldehyde for 10 min, cell permeabilization was carried out with 0.2% Triton X-100 (Sigma-Aldrich; St. Louis, MO, USA) in phosphate buffered saline (PBS). Following 1 h blocking of nonspecific binding sites with 1% bovine serum albumin (Sigma-Aldrich; St. Louis, MO, USA), rabbit polyclonal uroplakin III or pan-cytokeratin antibody (Santa Cruz Biotechnology, Inc, Santa Cruz, CA) was added overnight at 4°C. To detect primary antibody binding sites, biotinylated universal pan-specific secondary antibody (Vector Laboratories, Inc., Burlingame, CA) and 5 µl/ml fluorescein-conjugated avidin dye (Vector Laboratories, Inc., Burlingame, CA) were applied. Nuclear counterstaining was performed using propidium iodide (Sigma-Aldrich; St. Louis, MO, USA). The fluorescent signal was analyzed with a fluorescent microscope (CKX 41, Olympus, Japan). Fluorescent images were merged using ImageJ software.
Cell seeding
Scaffolds were punched into discs that exactly fitted 24-well plates. Following 1 h of sterilization in 70% ethanol and several rinses with PBS, urothelial cells (passage 2 to 5) with a density of 10 × 104 cells/cm2were loaded on scaffolds in 250 µl of supplemented medium. Seeded scaffolds were maintained at 37°C and 5% CO2. The medium was refreshed every other day.
Cell viability and propagation
To appraise live cells on scaffolds at different time points from initial seeding (1, 3, 7, and 14 days), the MTT assay was used to measure reduction of 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) into formazan. 0.5 mg/ml MTT solution (Sigma-Aldrich; St. Louis, MO, USA) was added to each scaffold for 3 h at 37°C and 5% CO2. Isopropanol (Merck, Darmstadt, Germany) containing 0.01 N HCl (Merck, Darmstadt, Germany) was added for 30 min at 37°C in the dark with moderate shaking to solubilize formazan. The intensity of color was determined using a photometer at 560 nm (BioPhotometer, Eppendorf, Germany). Three repeats were performed and all measurements were done in triplicate.
Scanning electron microscopy (SEM) of scaffolds
Seeded scaffolds were fixed in 2.5% glutaraldehyde for 24 h, then washed twice in PBS for 20 min. Incubation in ascending concentrations of ethanol was done for 20 min for each. After two dehydrations in absolute ethanol, they were dried in air for at least 24 h. They were mounted on aluminum stubs, sputter-coated with gold-palladium (AuPd), and examined under SEM at 20 kV (Tescan Vega II). Scaffolds with no seeded cells were analyzed without fixation and dehydration.
Histology and immunohistochemistry of scaffolds
Scaffolds were fixed in 4% formaldehyde and embedded in paraffin at each point of analysis. Five µm sections were either stained with hematoxylin and eosin (H + E) or immunolabeled against expression of pan-cytokeratin and uroplakin III. In brief, section were dewaxed with xylene (Merck, Darmstadt, Germany) and rehydrated through descending concentrations of ethanol. Following permeabilization with 0.2% Triton X-100 (Sigma-Aldrich; St. Louis, MO, USA) and citrate-based antigen retrieval, nonspecific binding sites were blocked with 1% normal horse serum in PBS for 30 min. Sections were incubated with rabbit polyclonal uroplakin III or pan-cytokeratin antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) overnight at 4°C. Primary antibody binding sites were probed using biotinylated universal secondary antibody included in Vectastain Universal Elite ABC Kit (Vector Laboratories, Inc., Burlingame, CA). Biotinylated horseradish peroxidase was added; then, the reaction was revealed by brown color after 3,3’-diaminobenzidine substrate (Vector Laboratories, Inc., Burlingame, CA). Slides were counterstained with hematoxylin and examined under a microscope (Olympus BX41, Japan).
Statistical analysis
Data were presented as the mean ± standard error. Friedman statistical analysis was used to compare cell proliferation during 14 days of culture using SPSS software (Statistical Package for the Social Sciences, version 18.0, SPSS Inc, Chicago, Illinois, USA). Values of p less than 0.05 were considered statistically significant.
Results
Chemical and mechanical properties of electrospun scaffolds
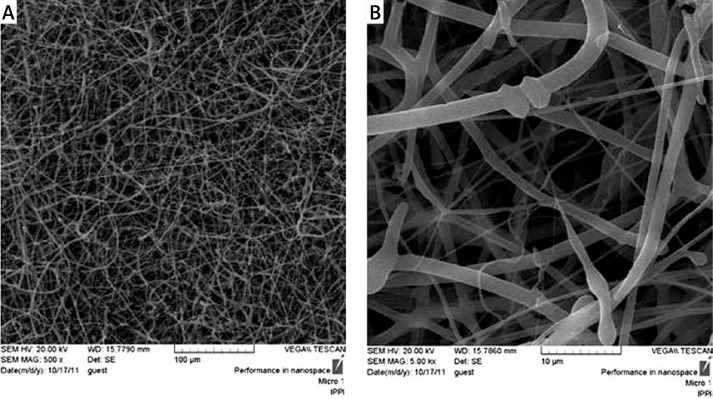
Scanning electron microscopy micrographs of electrospun nanofibrous scaffolds revealed randomly oriented PCL/PLLA nanofibers free of beads with fiber diameter in the range of 120 to 1500 nm, forming a porous structure (Figure 1). No change in the surface morphology of PCL/PLLA nanofibers after plasma treatment was considered using SEM images (data not shown). To compare surface hydrophilic properties of PCL/PLLA and plasma-treated PCL/PLLA scaffolds, contact angle studies were performed. The contact angles obtained were about 134° and 80°, respectively, which indicates that PCL/PLLA was highly hydrophobic and nonabsorbent to water, whereas plasma-treated PCL/PLLA scaffolds were extremely hydrophilic, with their water contact angle being less than 80°, showing 100% wettability by the water droplet, suggesting the presence of hydrophilic groups on the surface of the scaffold.
Figure 1.
Scanning electron micrographs of PCL/PLLA nanofibers at magnification of 500× (A), 5000× (B). The scaffold is composed of nonwoven and randomly oriented nanofibers which formed a 3D structure. The diameters of the nanofibers were distributed in the range of 110 to 1500 nm
Urothelium cell culture and characterization
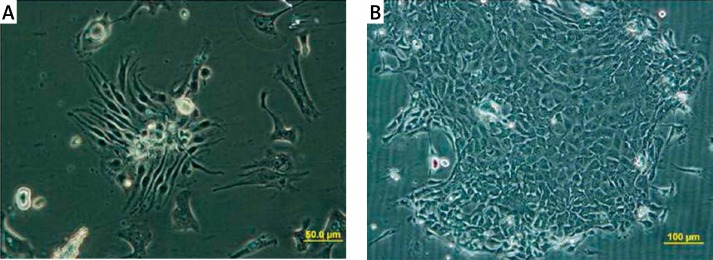
After initial plating, typical colonies of cultured urothelium can be observed, which tend to propagate rapidly, forming colonies with an indistinguishable border of cells (Figure 2). Cells in colonies upon reaching a large size differentiate into a non-proliferative and flattened phenotype. To avoid terminal differentiation of cultured cells after primary culture, cells were transferred to passage 1 in a medium containing 50% 3T3-conditioned medium.
Figure 2.
Morphological features of cultured urothelial cells. A – Dog urothelial cells formed small colonies 24 h after culture. B – 5 days after plating a typical colony of urothelial cells emerged
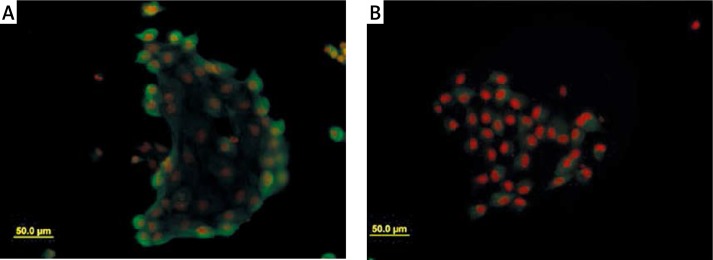
Cells were examined for expression of markers. Cells in colonies were highly positive for expression of pan-cytokeratin, which verified the epithelial origin of cultured cells (Figure 3A). Furthermore, a positive signal for the expression of uroplakin III was observed (Figure 3B).
Figure 3.
Immunocytochemical expression of urothelial markers. A – Diffuse expression of pan-cytokeratin was observed in colonies. B – Cells in colonies expressed uroplakin III (UPIII) as urothelium specific marker. Nuclei of cell are red because of PI
Cells proliferation and expansion on scaffold
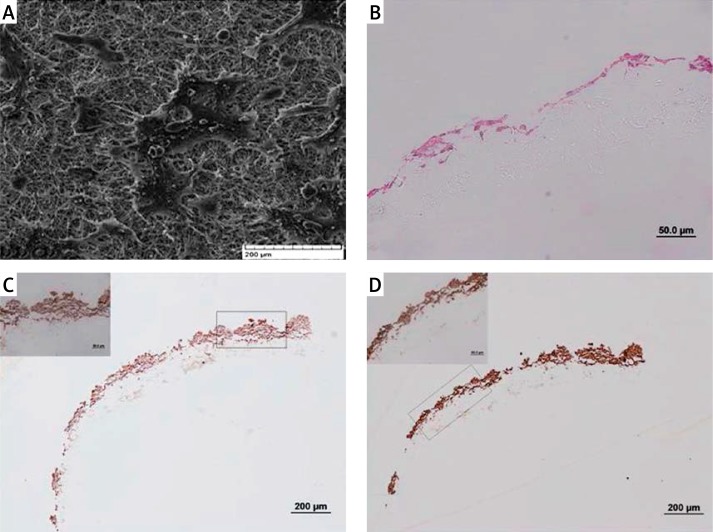
Scanning electron micrographs of scaffolds after 7 days revealed their normal distribution all over the scaffold surface and colonization as they propagate on the surface of culture flasks (Figure 4A). H + E staining on scaffolds after sectioning apparently showed cells on the surface of scaffolds forming a continuous layer after 7 days of culture (Figure 4B). Immunohistochemistry was used to assess the expression of urothelial markers in cells which seeded on the scaffold. Uroplakin III and pan-cytokeratin were expressed evidently in cells after growth on the scaffold (Figures 4C, D).
Figure 4.
Cells on the surface of PCL/PLLA were examined for their distribution, cyto-architecture of layering and expression of markers by SEM, H + E staining and immunohistochemistry, respectively. A – Scanning electron micrograph of scaffolds revealed distribution of urothelial cells all over the surface of the scaffold after 5 days of seeding, forming their characteristic colonies. B – 7 days after growth on scaffold, a layer of urothelial cells composed of different numbers of cells in different parts of the scaffold can be seen. C – Expression of UPIII and (D) pan-cytokeratin in urothelial cells while expanding on scaffolds after 7 days of culture showed suitability of the scaffold for urothelial cell culture concerning maintenance of marker expression
Cell viability and cytotoxicity
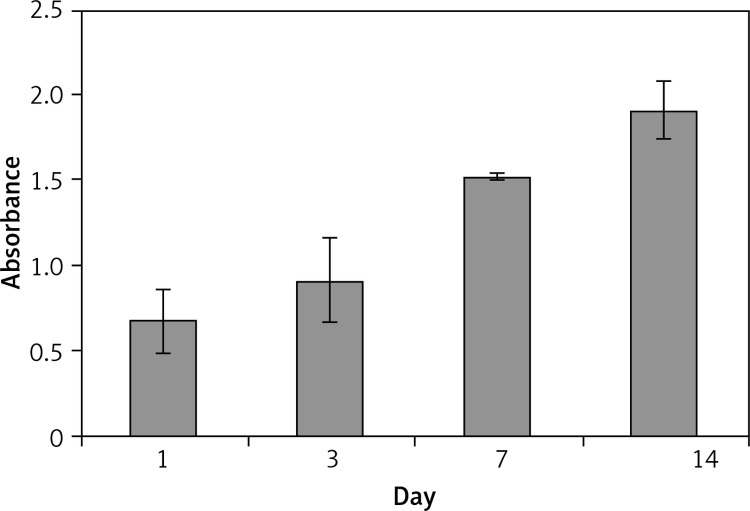
MTT assay, which determines mitochondrial dehydrogenase activity of cells, was utilized to study cell viability and the possible cytotoxic influence of the scaffold, as the enzyme activity would be compromised after exposure to toxic components. No cytotoxic effect was observed after 14 days of culture on scaffolds, and the MTT signal, which also correlates with cell viability, significantly increased during the experiment (p < 0.05). It can be inferred that the cells were proliferating during this period, because the MTT signal is directly proportional to the number of live cells at each time point of the experiment, which was enhanced from day 1 to 14 (Figure 5).
Figure 5.
MTT assay of urothelial cells on scaffolds over 14 days of culture revealed supportive nature of PCL/PLLA for this type of cells. The significant increase (p < 0.05) of MTT signal during the experiment is an indicator of cell proliferation and growth on the scaffold. Number of cells increased from day 1 to day 14 of culture
Discussion
In the present study, we cultured primary urothelial cells in a specific serum-free medium to propagate urothelial cells and prevent stromal cell proliferation. To maintain the cells in a proliferating state after primary culture that would otherwise turn into flat and non-proliferating morphology, 50% 3T3-conditioned medium was added to the culture medium. 3T3 cells as a feeder layer have been used to resolve this problem previously [23, 24], but here we used 3T3-conditioned medium successfully. Cells in this medium preserved their capability of proliferation, and the expression of pan-cytokeratin and uroplakin III continued. Contrary to some earlier reports [25, 26], we detected uroplakin III expression in our culture system. In accordance with our finding, some other groups have reported uroplakin III expression [10, 24] and even verified its expression by western blotting [27]. Uroplakin III is probably not the ultimate marker of urothelium differentiation, and the cells can maintain their proliferative capacity while expressing uroplakin III. Furthermore, the contradictory results observed in the present study may be due to differences between species, isolating conditions, culture media, and detection level or sensitivity.
Sectioning and staining of scaffolds is a straightforward method to study cell attachment, distribution, and layering. Cells were located all over the scaffold surface, but owing to the harsh process of sectioning, superficial layers detached; hence, it is not rational to count cell layers. Uroplakin III and pan-cytokeratin were also expressed after seeding and propagation of cells on the scaffold, which is a very critical criterion for construction of engineered tissues, because cells should not change their phenotype after growing on the scaffolds. Scanning electron micrograph analysis revealed normal appearing colonies of urothelial cells on the surface of scaffolds with even distribution. These colonies can grow and create a continuous layer (data not shown), which is a vital point in urothelium function [4]. Scanning electron micrograph images also demonstrated nano-scale properties of the PCL/PLLA scaffold. MTT assay is a well-documented technique for cytotoxicity evaluation of biomaterials, and cell proliferation can also be inferred indirectly from its result [6, 28]. PCL/PLLA not only exerted no toxic effect after 14 days, but also supported cell growth in this period.
Current knowledge of nanotechnology was recruited in construction of new scaffolds with nano-scale dimensions to mimic extracellular matrix structure. Nanometer surface features facilitate adsorption of extracellular matrix proteins, such as fibronectin, which promote cell adhesion and increase their functions [17]. Furthermore, Chun et al. [29] showed that nanometer-scale surface roughness inhibits calcium oxalate stone formation. Superior supportive effects of nano-sized poly lactic-co-glycolic acid, PCL, and polyether urethane versus their micron counterparts in bladder tissue engineering have been proved [30, 31].
Most nano-scaffolds have been prepared via chemical modifications of scaffolds, which were created using solvent casting and salt leaching processes [32], but we produced our scaffold with electrospun nanofibers of PCL and PLLA as a hybrid matrix. Fiber diameter of the synthetic PCL/PLLA scaffolds was in the range of 120 nm to 1500 nm, which allows slow diffusion of small molecules, metabolites, and macromolecules, such as gases, nutrients, and growth factors [33]. Our recent study to investigate compatibility and applicability of PCL/PLLA for surgery alongside with its reaction with the bladder tissue in an animal model demonstrated encouraging results [34]. Nevertheless, the scaffold was not preloaded with the bladder cells, suggesting that it could improve tissue regeneration compared to biomaterials with no cells [13]. In addition, our recent study [35], focused on the reaction of bladder smooth muscle cells on the same scaffold, revealed similar favorable results resembling the current study. Smooth muscle cells’ isolation and culture is much simpler than for urothelial cells. Also, their more robust nature compared to urothelial cells made their culture on scaffolds much less complicated. So, PCL/PLLA appropriateness for both main cell types of bladder received strong support from our studies, which propose the scaffold as a valuable matrix in this context. To the best of our knowledge, this is the first report that has studied PCL/PLLA biocompatibility and addressed proliferation, phenotype, and viability of urothelial cells on this nano-scaffold.
In conclusion, we revealed that the PCL/PLLA electrospun scaffold is a suitable matrix for urothelium engineering. Urothelial cells can expand on the surface of this nano-scaffold while preserving their phenotype. Biocompatibility of PCL/PLLA was confirmed for urothelial cells, as was previously shown for bladder smooth muscle cells on the same scaffold in our lab. In sum, biocompatibility of main cell types of bladder motivate future studies using animal models.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Turner AM, Subramanian R, Thomas DFM. Bladder tissue engineering. In: Boccaccini, Gough, editors. Tissue engineering using ceramics and polymers. Elsevier: Woodhead Publishing Limited; 2007. pp. 445–65. [Google Scholar]
- 2.Duel BP, Gonzalez R, Barthold JS. Alternative techniques for augmentation cystoplasty. J Urol. 1998;159:998–1005. [PubMed] [Google Scholar]
- 3.Kropp BP, Cheng EY. D.A. Bladder augmentation: current and future techniques. In: Docimo SGC, Khoury AE, editors. Clinical pediatric urology. London: Informa; 2007. [Google Scholar]
- 4.Bolland F, Southgate J. Bio-engineering urothelial cells for bladder tissue transplant. Exp Opin Biol Ther. 2008;8:1039–49. doi: 10.1517/14712598.8.8.1039. [DOI] [PubMed] [Google Scholar]
- 5.Yu DS, Lee CF, Chen HI, Chang SY. Bladder wall grafting in rats using salt-modified and collagen-coated polycaprolactone scaffolds: preliminary report. Int J Urol. 2007;14:939–44. doi: 10.1111/j.1442-2042.2007.01871.x. [DOI] [PubMed] [Google Scholar]
- 6.Pariente JL, Kim BS, Atala A. In vitro biocompatibility evaluation of naturally derived and synthetic biomaterials using normal human bladder smooth muscle cells. J Urol. 2002;167:1867–71. [PubMed] [Google Scholar]
- 7.Kim BS, Baez CE, Atala A. Biomaterials for tissue engineering. World J Urol. 2000;18:2–9. doi: 10.1007/s003450050002. [DOI] [PubMed] [Google Scholar]
- 8.Wunsch L, Ehlers EM, Russlies M. Matrix testing for urothelial tissue engineering. Eur J Pediatr Surg. 2005;15:164–9. doi: 10.1055/s-2004-830356. [DOI] [PubMed] [Google Scholar]
- 9.Park KD, Kwon IK, Kim YH. Tissue engineering of urinary organs. Yonsei Med J. 2000;41:780–8. doi: 10.3349/ymj.2000.41.6.780. [DOI] [PubMed] [Google Scholar]
- 10.Sharifiaghdas F, Hamzehiesfahani N, Moghadasali R, Ghaemimanesh F, Baharvand H. Human amniotic membrane as a suitable matrix for growth of mouse urothelial cells in comparison with human peritoneal and omentum membranes. Urol J. 2007;4:71–8. [PubMed] [Google Scholar]
- 11.Liu Y, Bharadwaj S, Lee SJ, Atala A, Zhang Y. Optimization of a natural collagen scaffold to aid cell-matrix penetration for urologic tissue engineering. Biomaterials. 2009;30:3865–73. doi: 10.1016/j.biomaterials.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Chen MK, Badylak SF. Small bowel tissue engineering using small intestinal submucosa as a scaffold. J Surg Res. 2001;99:352–8. doi: 10.1006/jsre.2001.6199. [DOI] [PubMed] [Google Scholar]
- 13.Yoo JJ, Meng J, Oberpenning F, Atala A. Bladder augmentation using allogenic bladder submucosa seeded with cells. Urology. 1998;51:221–5. doi: 10.1016/s0090-4295(97)00644-4. [DOI] [PubMed] [Google Scholar]
- 14.Turner A, Subramanian R, Thomas DF, et al. Transplantation of autologous differentiated urothelium in an experimental model of composite cystoplasty. Eur Urol. 2011;59:447–54. doi: 10.1016/j.eururo.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Z, Kotaki M, Inai R, Ramakrishna S. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Engineering. 2005;11:101–9. doi: 10.1089/ten.2005.11.101. [DOI] [PubMed] [Google Scholar]
- 16.Courtney T, Sacks MS, Stankus J, Guan J, Wagner WR. Design and analysis of tissue engineering scaffolds that mimic soft tissue mechanical anisotropy. Biomaterials. 2006;27:3631–8. doi: 10.1016/j.biomaterials.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 17.Chun YW, Lim H, Webster TJ, Haberstroh KM. Nanostructured bladder tissue replacements. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3:134–45. doi: 10.1002/wnan.89. [DOI] [PubMed] [Google Scholar]
- 18.Shor L, Guceri S, Wen X, Gandhi M, Sun W. Fabrication of three-dimensional polycaprolactone/hydroxyapatite tissue scaffolds and osteoblast-scaffold interactions in vitro. Biomaterials. 2007;28:5291–7. doi: 10.1016/j.biomaterials.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 19.Bakhshandeh H, Soleimani M, Hosseini SS, et al. Poly (epsilon-caprolactone) nanofibrous ring surrounding a polyvinyl alcohol hydrogel for the development of a biocompatible two-part artificial cornea. Int J Nanomed. 2011;6:1509–15. doi: 10.2147/IJN.S19011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu J, Sun X, Ma H, Xie C, Chen YE, Ma PX. Porous nanofibrous PLLA scaffolds for vascular tissue engineering. Biomaterials. 2010;31:7971–7. doi: 10.1016/j.biomaterials.2010.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang F, Murugan R, Ramakrishna S, Wang X, Ma YX, Wang S. Fabrication of nano-structured porous PLLA scaffold intended for nerve tissue engineering. Biomaterials. 2004;25:1891–900. doi: 10.1016/j.biomaterials.2003.08.062. [DOI] [PubMed] [Google Scholar]
- 22.Ma Z, Gao C, Gong Y, Shen J. Cartilage tissue engineering PLLA scaffold with surface immobilized collagen and basic fibroblast growth factor. Biomaterials. 2005;26:1253–9. doi: 10.1016/j.biomaterials.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen MM, Lieu DK, deGraffenried LA, Isseroff RR, Kurzrock EA. Urothelial progenitor cells: regional differences in the rat bladder. Cell Prolif. 2007;40:157–65. doi: 10.1111/j.1365-2184.2007.00424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thangappan R, Kurzrock EA. Three clonal types of urothelium with different capacities for replication. Cell Prolif. 2009;42:770–9. doi: 10.1111/j.1365-2184.2009.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurzrock EA, Lieu DK, deGraffenried LA, Isseroff RR. Rat urothelium: improved techniques for serial cultivation, expansion, freezing and reconstitution onto acellular matrix. J Urol. 2005;173:281–5. doi: 10.1097/01.ju.0000141585.17953.fa. [DOI] [PubMed] [Google Scholar]
- 26.Freshney RIF, Mary G. In: Culture of epithelial cells. 2nd ed. Freshney RIFaMG., editor. New York: Wiley-Liss; 2002. [Google Scholar]
- 27.Oottamasathien S, Williams K, Franco OE, et al. Bladder tissue formation from cultured bladder urothelium. Dev Dyn. 2006;235:2795–801. doi: 10.1002/dvdy.20886. [DOI] [PubMed] [Google Scholar]
- 28.Seyedjafari E, Soleimani M, Ghaemi N, Sarbolouki MN. Enhanced osteogenic differentiation of cord blood-derived unrestricted somatic stem cells on electrospun nanofibers. J Mater Sci Mater Med. 2011;22:165–74. doi: 10.1007/s10856-010-4174-6. [DOI] [PubMed] [Google Scholar]
- 29.Chun YW, Khang D, Haberstroh KM, Webster TJ. The role of polymer nanosurface roughness and submicron pores in improving bladder urothelial cell density and inhibiting calcium oxalate stone formation. Nanotechnology. 2009;20:085104. doi: 10.1088/0957-4484/20/8/085104. [DOI] [PubMed] [Google Scholar]
- 30.Thapa A, Webster TJ, Haberstroh KM. Polymers with nano-dimensional surface features enhance bladder smooth muscle cell adhesion. J Biomed Mater Res A. 2003;67:1374–83. doi: 10.1002/jbm.a.20037. [DOI] [PubMed] [Google Scholar]
- 31.Thapa A, Miller DC, Webster TJ, Haberstroh KM. Nano-structured polymers enhance bladder smooth muscle cell function. Biomaterials. 2003;24:2915–26. doi: 10.1016/s0142-9612(03)00123-6. [DOI] [PubMed] [Google Scholar]
- 32.Pattison MA, Wurster S, Webster TJ, Haberstroh KM. Three-dimensional, nano-structured PLGA scaffolds for bladder tissue replacement applications. Biomaterials. 2005;26:2491–500. doi: 10.1016/j.biomaterials.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Tian F, Hosseinkhani H, Hosseinkhani M, et al. Quantitative analysis of cell adhesion on aligned micro- and nanofibers. J Biomed Mater Res A. 2008;84:291–9. doi: 10.1002/jbm.a.31304. [DOI] [PubMed] [Google Scholar]
- 34.Shakhssalim N, Dehghan MM, Moghadasali R, Soltani MH, Shabani I, Soleimani M. Bladder tissue engineering using biocompatible nanofibrous electrospun constructs: feasibility and safety investigation. Urol J. 2012;9:410–9. [PubMed] [Google Scholar]
- 35.Shakhssalim NRJ, Moghadasali R, Aghdas FS, Naji M, Soleimani M. Bladder smooth muscle cells interaction and proliferation on PCL/PLLA electrospun nanofibrous scaffold. Int J Artif Organs. 2013;36:113–20. doi: 10.5301/ijao.5000175. [DOI] [PubMed] [Google Scholar]