Abstract
Background
Although pneumonia is the leading cause of child mortality worldwide, little is known about the quality of routine pneumonia care in high burden settings like Malawi that utilize World Health Organization’s Integrated Management of Childhood Illnesses (IMCI) guidelines. Due to severe human resource constraints, the majority of clinical care in Malawi is delivered by non-physician clinicians called Clinical Officers (COs).
Aim
To assess the quality of child pneumonia care delivered by Malawian COs in routine care conditions.
Methods
At an outpatient district-level clinic in Lilongwe, Malawi, 10 COs caring for 695 children who presented with fever, cough, or difficulty breathing were compared to IMCI pneumonia diagnostic and treatment guidelines.
Results
Fewer than 1% of patients received an evaluation by COs that included all 16 elements of the history and physical examination. The respiratory rate was only determined in 16.1% of patients presenting with cough or difficulty breathing. Of the 274 children with IMCI-defined pneumonia, COs correctly diagnosed 30%, and administered correct pneumonia care in less than 25%. COs failed to hospitalize 40.8% of children with severe or very severe pneumonia.
Conclusions
IMCI pneumonia care quality at this Malawian government clinic is alarmingly low. Along with reassessing current pneumonia training and supervision approaches, novel quality improvement interventions are necessary to improve care.
Keywords: Pneumonia, Guidelines, Developing countries, Paediatrics
Introduction
The leading cause of child mortality globally is pneumonia, accounting for 1.6 million deaths annually, almost half of which occur in Africa.1 In Malawi, a southern African country, pneumonia is a primary cause of morbidity and mortality in young hospitalized children (0–59 months),2 accounting for over 13% of deaths annually.1
World Health Organization (WHO) Integrated Management of Childhood Illnesses (IMCI) guidelines are validated case-management strategies designed primarily for non-physician clinicians working in resource-poor settings. IMCI incorporates acute respiratory infection (ARI) patient care algorithms that can reduce paediatric mortality from pneumonia by up to 70%.3–6 In Malawi, the majority of patient care is provided by non-physician clinicians known as clinical officers (COs). In 2000, the Malawi ARI Programme introduced IMCI-based case management guidelines nationally, leading to significant improvements in case detection, government support, and reductions in case fatalities by 54.8% as of 2006.7
Despite the improved outcomes with IMCI implementation, pneumonia case mortality rates in Malawi remain substantially higher than in developed settings, suggesting that additional improvements in care are needed.1,7 To date, most evaluations of routine pneumonia care primarily use broader system indicators like case detection rates and mortality.7–11 These indicators assume that the pneumonia diagnosis and care are correct, whereas data generated at the individual healthcare provider level is not necessarily based on this assumption. Thus, assessing routine pneumonia care at the individual practitioner level, in addition to a broader system evaluation, may allow a more complete understanding of current respiratory care standards so that gaps can be more clearly identified and addressed.
For these reasons, the study aimed to evaluate the quality of routine child pneumonia care delivered by individual COs in Malawi.
Methods
Setting
The study site was a general paediatric government clinic in urban Malawi during the dry season (September-November, 2010). This clinic functions as the district-level facility for Lilongwe district and primarily serves non-referred patients; it has rates of pneumonia similar to those in other district-level facilities.12 Patient care is provided by full-time COs, CO interns, CO students, medical interns, nurses, and nursing students.
Participants
COs are the primary implementers of IMCI guidelines in Malawi. Full-time COs complete three years of classroom education including IMCI guidelines and one year of clinical internship before staffing a health care facility. At this government clinic, full-time COs are also responsible for supervising care provided by CO interns and students. A CO intern completes 3 years of classroom education and is currently performing clinical internship duties. Owing to limitations in human resource, CO interns routinely perform duties unsupervised, and temporary COs called ‘CO locums’ are also employed. At a minimum, CO locums finish 3 years of classroom training but might or might not complete an internship year.
Study design and sample size
This observational study was a cross-sectional assessment of ten COs and their ability to accurately care for (i.e. correctly diagnose, treat and manage) children with pneumonia. All COs (n=15) who routinely provide unsupervised patient care in the government paediatric clinic, excluding students, were eligible for participation, and ten COs were randomly selected.
With a modified version of a data-collection tool based on indicators used by WHO and in previous studies,13–17 investigators observed COs evaluating children presenting with cough, difficulty breathing, and/or fever. Investigators were trained in IMCI guidelines and use of the data collection tool; local translators were present for every patient encounter. Children complaining of fever without cough and difficulty breathing were included because caregivers do not always volunteer symptoms without direct questioning, and it was not assumed that COs asked all relevant questions.
Immediately after each observation, a paediatrician trained in IMCI pneumonia guidelines (adjusted for national guidelines) re-evaluated the patient using these criteria.18,19 The paediatrician’s assessment was considered the gold standard for physical examination findings of lung auscultation and chest indrawing. Children were re-evaluated by one of two available paediatricians. A complete assessment was defined as performing all 16 elements of a patient’s history and physical examination. These history and physical examination variables were the minimum number of tasks needed to accurately evaluate and manage a child with pneumonia. Chest auscultation, heart rate, assessing tuberculosis exposure risk, and determining HIV status are not required in the IMCI diagnostic and management criteria for pneumonia but were considered as necessary elements for achieving a complete respiratory evaluation in this setting. Chest auscultation was included because COs have stethoscopes and routinely listen to lung sounds, and bronchodilator-responsive wheezing is an auscultation finding that can re-classify a child as having asthma rather than pneumonia. Heart rate was included as it is both a core IMCI vital sign and a pulse oximeter that measures heart rate was available to COs. Chronic persistent cough is a key risk factor for Mycobacterium tuberculosis infection, so it was decided that any caregiver of a child with a cough for more than 2 weeks should be questioned regarding tuberculosis risk factors. Lastly, HIV status was included as on-site rapid HIV testing was available and it is an important criterion in the management decision-algorithm for treatment since HIV-infected children have greater risk for mixed viral and bacterial pneumonia, Pneumocystis jirovecii pneumonia, and intrapulmonary mycobacterium tuberculosis infection. For safety, the paediatrician intervened after the observation if the management plan was not sufficient or potentially harmful.
The study sought to determine the frequency with which COs performed all elements of the history, physical, diagnosis, and treatment plan correctly. To estimate sample size, it was assumed that COs would perform all 16 elements correctly on 80% of encounters; to measure this frequency within a confidence interval of ±10%, each CO would need to evaluate at least 62 patients. Each CO signed a consent form before being observed by the study team. Information leaflets in both English and Chichewa, the official Malawian languages, were provided to patients’ caregivers. Caregivers of patients were not required to sign a consent form since no additional risk was posed to the patient.
Measurements
The primary outcome was to determine the proportion of assessments in which COs correctly performed all 16 elements. Secondary outcomes were to determine which elements were deficient and how often correct pneumonia care was administered by COs per IMCI guidelines. Correct pneumonia care was defined as the appropriate pneumonia classification (no pneumonia, non-severe pneumonia, severe pneumonia, or very severe pneumonia) and the appropriate plan of care, including antibiotic choice, oxygen administration, and hospitalization, regardless of completion of the patient history and physical examination elements. For example, even if a CO did not complete all 16 history and examination elements, they could still achieve ‘correct pneumonia care’ by correctly classifying the child’s pneumonia status and making the right decision regarding antibiotics, oxygen supplementation, and hospitalization. Another secondary outcome was to assess the COs’ pneumonia care knowledge, including classification and treatment, using a multiple-choice questionnaire (Appendix 1).
Definitions
Pneumonia case definitions and management were based on IMCI guidelines, adapted to Malawian guidelines at the time of the study in 2010 (Appendix 2).18,19
Statistical analysis
Descriptive statistics are reported for all participating COs and patients meeting IMCI pneumonia criteria. Data was entered in Access and statistical analyses were performed using IBM SPSS Statistics version 19 (SPSS Inc., Chicago, IL). Ethical review boards at the University of North Carolina Chapel Hill and Malawi National Health Sciences Research Committee approved the study.
Results
All 10 COs completed at least 62 assessments (range 62–77, mean 69.5) with 695 total patient-provider encounters observed. Of all patients reviewed, 274 (39.4%) met the IMCI pneumonia criteria. Their presenting symptoms and signs are shown in Table 1. Lung auscultation findings were abnormal in over half of those with severe and very severe pneumonia (57/103, 55%). As expected, the majority of patients with severe (80/82, 97.6%) or very severe pneumonia (16/21, 76.2%) had chest indrawing. Two-thirds of the patients with very severe pneumonia (14/21) had altered mental status, convulsions and/or lethargy, either by history or on examination.
Table 1.
Symptoms and signs in patients with pneumonia observed by IMCI-trained paediatricians
| Non-severe pneumonia,* n=168 | Severe pneumonia, n=82 | Very severe pneumonia, n=21 | |
|---|---|---|---|
| Symptoms, n (%) | |||
| Cough | 150 (89.3) | 79 (96.3) | 18 (85.7) |
| Difficulty breathing | 34 (20.2) | 33 (40.2) | 9 (42.9) |
| Inability to drink or breastfeed | 0 (0.0) | 0 (0.0) | 1 (4.8) |
| Vomiting everything | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Signs, n (%) | |||
| Tachypnoea (adjusted for age)† | 168 (100.0) | 77 (93.9) | 18 (85.7) |
| Abnormal lung auscultation findings‡ | 38 (22.6) | 48 (58.5) | 9 (42.9) |
| Chest indrawing | 0 | 80 (97.6) | 16 (76.2) |
| Nasal flaring | 0 | 11 (13.4) | 3 (14.3) |
| Altered mental status, convulsions, or lethargy | 0 | 0 | 14 (66.7) |
| Severe respiratory distress | 0 | 0 | 7 (33.3) |
| Oxygen saturation <90% | 0 | 0 | 5 (23.8) |
| Grunting | 0 | 1 (1.2) | 0 |
| Central cyanosis | 0 | 0 | 0 |
3 with no symptoms/signs recorded by paediatrician;
tachypnoea defined as: ≥60 breaths/min for patients <2 months of age, ≥50 breaths/min for those ≥2 months and <11 months of age, and ≥40 breaths/min for those ≥11 and <60 months;
breakdown of abnormal lung auscultation findings were as follows (multiples included): crackles 71, transmitted upper respiratory sounds 17, wheezing 15, decreased breath sounds 10.
COs had a diverse level of training and experience (Table 2). Four full-time COs, two CO interns, and four CO locums participated in the study. The median age of COs was 27 years and two out of 10 were female. Years of post-training experience ranged from 3 months to 24 years. For the knowledge assessment, the median score among COs was 70.6% (12/17), with CO interns scoring the lowest of the three groups at 47.1% (8/17). However, the range in knowledge scores among all COs was substantive (35.3–82.4%, 6–14 of 17).
Table 2.
Observations of Individual Clinical Officers
| Ranking | Total | Individual clinical officers (COs)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO 1 | CO 2 | CO 3 | CO 4 | CO 5 | CO 6 | CO 7 | CO 8 | CO 9 | CO 10 | |||
| Age, y | – | 27.2 (median) | 23.5 | 57.8 | 25.4 | 30.0 | 32.4 | 28.0 | 26.3 | 50.5 | 22.3 | 26.1 |
| Level of training* | – | – | CO locum | Full-time CO | CO locum | CO intern | CO locum | Full-time CO | Full-time CO | Full-time CO | CO locum | CO intern |
| Years of experience | – | 1.3 (median) | 1 | 20 | 1 | 0.25 | 5 | 2 | 1.5 | 24 | 1 | 0.66 |
| Pneumonia knowledge assessment (highest score possible 17) | – | 12 (median) | 14 | 12 | 9 | 9 | 6 | 12 | 12 | 13 | 12 | 7 |
| Patient encounters observed, n | – | 695 | 72 | 70 | 62 | 68 | 77 | 64 | 69 | 70 | 73 | 70 |
| Elements of pneumonia evaluation, n (%): | ||||||||||||
| History | ||||||||||||
| Fever | Excellent | 634 (91.2) | 56 (77.8) | 55 (78.6) | 57 (91.9) | 66 (97.1) | 72 (93.5) | 55 (85.9) | 69 (100.0) | 68 (97.1) | 71 (97.3) | 65 (92.9) |
| Cough | Average | 553 (79.6) | 63 (87.5) | 54 (77.1) | 49 (79.0) | 49 (72.1) | 73 (94.8) | 47 (73.4) | 65 (94.2) | 44 (62.9) | 66 (90.4) | 43 (61.4) |
| Difficult breathing | Poor | 210 (30.2) | 48 (66.7) | 12 (17.1) | 36 (58.1) | 7 (10.3) | 5 (6.5) | 8 (12.5) | 40 (58.0) | 4 (5.7) | 39 (53.4) | 11 (15.7) |
| Duration of Illness | Average | 514 (74.0) | 64 (88.9) | 29 (41.4) | 53 (85.5) | 54 (79.4) | 48 (62.3) | 49 (76.6) | 59 (85.5) | 55 (78.6) | 47 (64.4) | 56 (80.0) |
| Inability to drink or breastfeed | Poor | 330 (47.5) | 39 (54.2) | 24 (34.3) | 46 (74.2) | 25 (36.8) | 25 (32.5) | 35 (54.7) | 38 (55.1) | 38 (54.3) | 48 (65.8) | 12 (17.1) |
| Vomiting everything | Poor | 390 (56.1) | 59 (81.9) | 30 (42.9) | 20 (32.3) | 50 (73.5) | 58 (75.3) | 34 (53.1) | 51 (73.9) | 19 (27.1) | 53 (72.6) | 16 (22.9) |
| Convulsions, lethargy, loss of consciousness | Poor | 49 (7.1) | 6 (8.3) | 5 (7.1) | 0 (0.0) | 1 (1.5) | 9 (11.7) | 4 (6.3) | 10 (14.5) | 4 (5.7) | 5 (6.8) | 5 (7.1) |
| TB exposure (if cough/fever ≥2 wks), n/N (%) † | Poor | 27/69 (39.1) | 7/9 (77.8) | 0/3 (0.0) | 2/6 (33.3) | 0/4 (0.0) | 0/2 (0.0) | 4/7 (57.1) | 3/9 (33.3) | 1/11 (9.1) | 3/11 (27.3) | 7/7 (100.0) |
| HIV status† | Poor | 88 (12.7) | 44 (61.1) | 0 (0.0) | 1 (1.6) | 1 (1.5) | 2 (2.6) | 8 (12.5) | 9 (13.0) | 10 (14.3) | 7 (9.6) | 6 (8.6) |
| Physical examination | ||||||||||||
| Temperature | Average | 439 (63.2) | 68 (94.4) | 14 (20.0) | 0 (0.0) | 54 (79.4) | 22 (28.6) | 62 (96.9) | 65 (94.2) | 39 (55.7) | 61 (83.6) | 54 (77.1) |
| Heart rate† | Poor | 173 (24.9) | 70 (97.2) | 0 (0.0) | 1 (1.6) | 1 (1.5) | 11 (14.3) | 41 (64.1) | 17 (24.6) | 1 (1.4) | 20 (27.4) | 11 (15.7) |
| Respiratory rate | Poor | 112 (16.1) | 33 (45.8) | 1 (1.4) | 0 (0.0) | 1 (1.5) | 11 (14.3) | 36 (56.3) | 21 (30.4) | 0 (0.0) | 9 (12.3) | 0 (0.0) |
| Oxygen saturation (measured by pulse oximetry) | Poor | 163 (23.5) | 70 (97.2) | 0 (0.0) | 1 (1.6) | 1 (1.5) | 9 (11.7) | 41 (64.1) | 16 (23.2) | 1 (1.4) | 13 (17.8) | 11 (15.7) |
| Chest auscultation performed† | Excellent | 556 (80.0) | 67 (93.1) | 68 (97.1) | 21 (33.9) | 67 (98.5) | 73 (94.8) | 55 (85.9) | 58 (84.1) | 43 (61.4) | 71 (97.3) | 33 (47.1) |
| Lung auscultation correct† | Average | 454 (65.3) | 55 (76.4) | 53 (75.7) | 14 (22.6) | 53 (77.9) | 60 (77.9) | 52 (81.3) | 51 (73.9) | 36 (51.4) | 59 (80.8) | 21 (30.0) |
| Chest indrawing assessment correct | Excellent | 608 (87.5) | 65 (90.3) | 62 (88.6) | 55 (88.7) | 63 (92.6) | 60 (77.9) | 59 (92.2) | 62 (89.9) | 61 (87.1) | 63 (86.3) | 58 (82.9) |
| 100% of elements completed‡ | Poor | 6 (0.9) | 1 (1.4) | 0 | 0 | 0 | 0 | 0 | 5 (7.2) | 0 | 0 | 0 (0.0) |
Excellent, elements completed and/or correct for ≥80% of patient encounters; average, elements completed and/or correct for ≥60% but <80% of patient encounters; Poor, elements completed and/or correct for <60% of patient encounters;
Level of training: full-time CO vs CO intern vs CO locum;
Criteria not required in IMCI guidelines for pneumonia diagnosis and management; N, number of patients with cough or fever ≥2 weeks; n, number of patients with cough or fever ≥2 wks in whom COs elicited a TB exposure history;
16 elements of pneumonia evaluation completed with assessment of chest indrawing and lung auscultation correct.
Sixteen history and physical examination elements essential for a complete respiratory evaluation were observed at each patient-provider encounter (Table 2). Among all 695 patients, COs performed all 16 elements on only six patients (0.9%). When considering each element individually, CO performance varied widely. However, all COs did similarly poorly at completing all 16 elements for each patient encounter (range 0.0–7.2%, 0–5/69). Of the 16 elements, COs collectively performed only three (enquiring about fever, performing chest auscultation, and assessing chest indrawing) on at least 80% of the patients. Providers obtained the respiratory rate in 16.1% (112/695) of children, and HIV status was determined in 12.7% (88/695) of patients. When evaluating CO performance excluding the four components not included in IMCI guidelines (chest auscultation, heart rate, tuberculosis exposure risk, and HIV status), only one additional patient encounter was performed correctly (7/695, 1.0%).
COs also did poorly with respect to diagnosis and classification of pneumonia. They correctly classified 61.2% (425/695) of all children with cough or difficult breathing (Table 3), the majority of whom did not have pneumonia. COs correctly diagnosed 30% (83/274) of patients with pneumonia of any severity. Less than one-third of patients meeting non-severe pneumonia (56/171, 32.7%) and severe pneumonia (25/82, 30.5%) criteria were correctly classified. Of the 21 children with very severe pneumonia, COs diagnosed two correctly and 13 were not given any diagnosis of pneumonia. The majority of misclassifications by COs tended to be the assignment of ‘no pneumonia’ to patients who in fact did meet pneumonia criteria (168/695, 24.2%).
Table 3.
Clinical officers’ performance evaluating patients on the basis of IMCI pneumonia case definitions
| IMCI Guidelines†
|
||||||
|---|---|---|---|---|---|---|
| No pneumonia (%) | Non-severe pneumonia (%) | Severe pneumonia (%) | Very severe pneumonia (%) | Total (%) | ||
| Clinical officers* | No pneumonia | 342 (81.2) | 112 (65.5) | 43 (52.4) | 13 (61.9) | 510 (73.4) |
| Non-severe pneumonia | 78 (18.5) | 56 (32.7) | 14 (17.1) | 3 (14.3) | 151 (21.7) | |
| Severe pneumonia | 1 (0.2) | 3 (1.8) | 25 (30.5) | 3 (14.3) | 32 (4.6) | |
| Very severe pneumonia | 0 | 0 | 0 (0.0) | 2 (9.5) | 2 (0.3) | |
| Total | 421 (60.6) | 171 (24.6) | 82 (11.8) | 21 (3.0) | 695 (100) | |
Note: Numbers in each cell indicate the diagnosis classification by the clinical officers (vertical left column) according to IMCI guidelines as confirmed by paediatrician (horizontal top row). Numbers in bold indicate clinical officers’ diagnoses confirmed to be correct per IMCI guidelines. Percentages reflect column %, except for bottom row which represents row %.
Clinical officers (COs) includes full-time COs (4), CO interns (2), and CO locums (4).
IMCI guidelines adjusted according to Malawian diagnostic guidelines for pneumonia.
As COs misdiagnosed and misclassified most children meeting pneumonia criteria, they not surprisingly mismanaged them as well (Table 4). The correct antibiotic, if any, was prescribed to 20.9% (53/254) of patients and oxygen supplementation was ordered for 22.7% of those eligible (5/22). COs failed to hospitalize 42 of the 103 patients (40.8%) meeting admission criteria based upon a diagnosis of either severe or very severe pneumonia. Irrespective of whether or not a CO completed all 16 history and physical examination elements, COs provided correct pneumonia care (i.e. correct diagnosis, antibiotic choice, oxygen supplementation, and hospitalization) to 22.3% (61/274) (range 4.0–64.9%) of patients with pneumonia of any severity. Although most children with very severe pneumonia were hospitalized correctly (18/21, 85.7%), the vast majority were prescribed incorrect antibiotics (14/15, 93.3%) and not prescribed oxygen (13/17, 76.5%).
Table 4.
Clinical officer care of patients meeting IMCI pneumonia criteria
| Ranking | Total | Individual clinical officers (COs)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO 1 | CO 2 | CO 3 | CO 4 | CO 5 | CO 6 | CO 7 | CO 8 | CO 9 | CO 10 | |||
| Level of training* | – | – | CO locum | Full-time CO | CO locum | CO intern | CO locum | Full-time CO | Full-time CO | Full-time CO | CO locum | CO intern |
| No. of patient encounters | – | 274 | 24 | 26 | 22 | 19 | 38 | 24 | 37 | 32 | 27 | 25 |
| Correct diagnosis | Poor | 83 (30.3) | 4 (16.7) | 13 (50.0) | 3 (13.6) | 2 (10.5) | 14 (36.8) | 6 (25.0) | 24 (64.9) | 5 (15.6) | 8 (29.6) | 4 (16.0) |
| Correct antibiotics | Poor | 53/254 (20.9) | 4/24 (16.7) | 4/26 (15.4) | 4/22 (18.2) | 2/18 (11.1) | 6/32 (18.8) | 4/24 (16.7) | 18/31 (58.1) | 4/32 (12.5) | 6/20 (30.0) | 1/25 (4.0) |
| Correct oxygen supplementation (if criteria met) † | Poor | 5/22 (22.7) | 0/1 (0.0) | 0/1 (0.0) | 0/3 (0.0) | 0/3 (0.0) | 0/2 (0.0) | 1/1 (100.0) | 2/6 (33.3) | 1/1 (100.0) | 0/2 (0.0) | 1/2 (50.0) |
| Correctly admitted patients with severe and very severe pneumonia | Poor | 61/103 (59.2) | 5/7 (71.4) | 4/8 (50.0) | 5/7 (71.4) | 2/6 (33.3) | 7/17 (41.2) | 5/5 (100.0) | 15/20 (75.0) | 2/10 (20.0) | 12/15 (80.0) | 4/8 (50.0) |
| 100% of pneumonia assessment correct‡ | Poor | 61 (22.3) | 4 (16.7) | 4 (15.4) | 3 (13.6) | 2 (10.5) | 7 (18.4) | 4 (16.7) | 24 (64.9) | 4 (12.5) | 8 (29.6) | 1 (4.0) |
Excellent, elements completed and/or correct for ≥80% of patient encounters; Average, elements completed and/or correct for ≥60% but <80% of patient encounters; Poor, elements completed and/or correct for <60% of patient encounters;
Level of training: full-time CO vs CO intern vs CO locum;
IMCI criteria for supplemental oxygen: pulse oximetry <90%, respiratory rate ≥70 breaths per minute, grunting in a young infant, head nodding, inability to drink, or severe chest indrawing.
CO correctly diagnosed and treated patient (antibiotics, oxygen, hospitalization) based on IMCI guidelines for pneumonia.
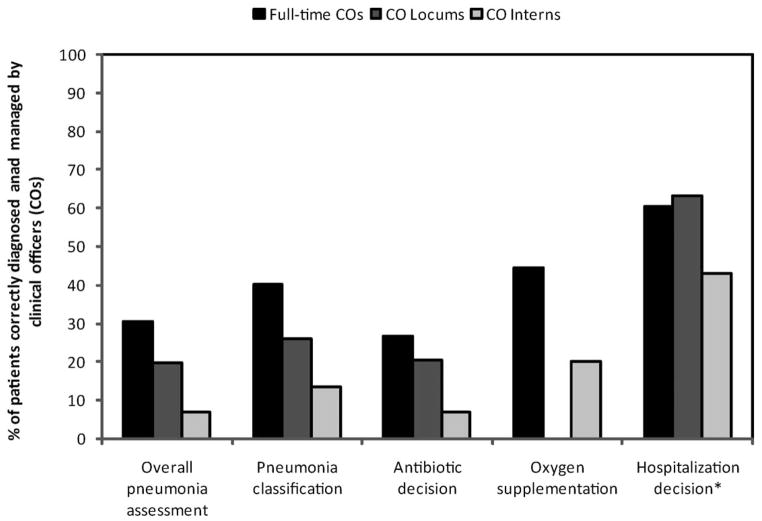
Management of children with pneumonia varied based on the CO’s experience level (Fig. 1). As expected, full-time COs delivered better overall pneumonia care than CO locums and CO interns, though all function as independent, unsupervised providers.
Figure 1.
Correct management of pneumonia by clinical officer training level
Discussion
Prior quality of care evaluations have largely focused on broader system-level assessments or individual patient-provider encounters associated with a training intervention intended to improve quality of care.9,20–24 This study uniquely focused on the quality of routine care at the individual practitioner level, rather than system-level indicators or care associated with a training-intervention. Routine care at the individual practitioner level provides a better understanding of actual patient-provider interactions and is more likely to represent actual day-to-day patient care in developing countries where comprehensive training exercises are not frequent or routine. These results demonstrate that more than 10 years after IMCI guideline implementation, the quality of paediatric pneumonia care at this Malawian government clinic is alarmingly inadequate. The vast majority of children with any severity of pneumonia were misdiagnosed and incorrectly treated. Given the high burden of paediatric mortality caused by pneumonia in Africa, the region’s heavy reliance on non-physician clinicians like COs for patient care, and the known clinical benefit of early, correct outpatient pneumonia treatment, this study strongly suggests that a critical reassessment of IMCI pneumonia training and supervision, including consideration of new innovative approaches, is warranted.
This study has identified key gaps in pneumonia care. For example, our results demonstrate that the minimum history and physical examination elements needed to correctly classify pneumonia were performed in less than 60% of patients with respiratory complaints. Determining a patient’s respiratory rate is the cornerstone of diagnosing non-severe pneumonia, but this was performed in only 16% of children. Other key gaps identified in this study show that clinicians need to both observe and ask about the child’s ability to drink or breastfeed, difficulty breathing, convulsions, lethargy, and loss of consciousness. These deficiencies could be addressed with a re-evaluation of pre-service CO education and implementing in-service clinical training sessions mandatory for COs to maintain their certification.
In a country with a high HIV prevalence, and an even higher one in children with severe pneumonia,25,26 COs largely ignored a child’s HIV status. Knowing a child’s HIV status is important for selecting the correct IMCI pneumonia treatment as an HIV-infected child often has different aetiologies of pneumonia than an uninfected child, and therefore requires different management.18,19 Given that very severe pneumonia is highly predictive of mortality in HIV-infected and HIV-exposed Malawian children, identifying children at risk of HIV prior to hospitalization could allow for more timely treatment and better outcomes.27 This study’s findings suggest that in HIV-endemic countries such as Malawi, IMCI training programmes currently in place are not effectively emphasizing the importance of considering HIV status in children with pneumonia or not training enough providers, or both.
In addition to targeting gaps in the history-gathering and physical examination skills of COs, training must also emphasize synthesizing this information to make correct treatment decisions. COs consistently prescribed incorrect antibiotics, or none at all, and failed to provide supplemental oxygen and hospitalization for eligible patients. These interventions are potentially life-saving.4,28 Although this study incorporated patient safety measures to address these mistakes, our results highlight the need for increased supervision during routine care, when no paediatricians are available to correct mistakes.
Supervision is a vital component of training COs. Supervision allows COs to apply skills learned in the classroom to direct patient care, and improves the quality of routine pneumonia care for those who have completed formal CO training. Not surprisingly, our study found that less experienced CO interns scored lower on their pneumonia knowledge questionnaire and were less proficient at providing quality pneumonia care to patients. Despite these findings, we observed that CO interns almost universally worked without supervision. Previous studies have demonstrated that supervision optimizes IMCI effectiveness and should be considered an essential component of IMCI programmes.8,20,29
Given the extreme human and material resource-constraints in Malawi, finding funds to more regularly provide pneumonia care trainings and to recruit and retain experienced practitioners to routinely supervise care may be difficult. Thus, novel, evidence-based approaches will be an important area of further investigation. Increased access to and the continued development of internet services and mobile phone technology may represent avenues to improve training and supervision. For example, in Tanzania, smart phone applications have been implemented to guide clinicians in the provision of IMCI guidelines.30 Mobile phone text-messaging has shown similar promise as a tool to improve IMCI malaria guideline adherence for Kenyan providers.31 Similarly, a supervision tool integrating provider-and systems-level data, known as the balanced scorecard model, was initially utilized to improve hospital care in high-income countries. A modified version of the balanced scorecard model has been similarly successful in Afghanistan.32,33 Continued study of these and other innovative supervision approaches will be important to address quality of care in resource-limited settings.
The study has several limitations. Our observational tool was adapted to focus on only pneumonia. While this may limit the generalizability of our findings to other conditions, we focused on pneumonia because it is the leading cause of child mortality in the African region. Although the pneumonia questionnaire was not formally validated, we thoroughly vetted the questions with local COs and senior physicians for fairness and appropriate phrasing of English, the language of higher education in Malawi. This study did not attempt to address every potential factor that may influence care quality. For example, difficult work conditions such as poor provider remuneration and high patient-to-provider ratios could increase healthcare provider burn-out and reduce care quality, but represent complex issues unlikely to be addressed in the near term. We instead attempted to identify immediately addressable factors that are more likely to optimize the quality of care delivered by existing healthcare personnel. While we also acknowledge that this study was conducted at only one facility, focusing on providers at one facility allowed us to collect more labour-intensive, detailed information regarding actual patient-provider interactions not possible with system-level assessments relying on broader care indicators. While additional study is needed for confirmation, it is likely representative of the quality of pneumonia care at other clinics in Malawi and perhaps the southern African region given that care is also primarily provided by non-physician clinicians at busy, poorly-resourced clinics with similar patient populations.31,34
Routine care at this Malawian government clinic demonstrated an alarmingly low quality of IMCI pneumonia care. In a country that implemented routine IMCI care guidelines over a decade ago, close scrutiny of existing IMCI and pneumonia training and supervision systems is needed. Given the similarly poor care quality observed in other countries, broader intervention-based research of pneumonia quality of care throughout the southern African region is necessary.
Supplementary Material
Acknowledgments
The authors wish to thank the translators, Edson Soko and Salama Itimu. This work was supported by a grant from the Doris Duke Charitable Foundation to University of North Carolina at Chapel Hill to fund clinical research fellow Erica (Chapin) Bjornstad. In addition, Eric D. McCollum and Dan Olson were supported by the National Institutes of Health (R24 TW007988) through the Fogarty International Center and International Clinical Research Fellows Program at Vanderbilt University. Eric D. McCollum also received support from the National Institutes of Health through the National Heart Lung and Blood Institute (T32HL072748-11).
References
- 1.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–87. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 2.Enarson P, La Vincente S, Gie R, Maganga E, Chokani C. Implementation of an oxygen concentrator system in district hospital paediatric wards throughout Malawi. Bull WHO. 2008;86:344–8. doi: 10.2471/BLT.07.048017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perkins BA, Zucker JR, Otieno J, Jafari HS, Paxton L, Redd SC, et al. Evaluation of an algorithm for integrated management of childhood illness in an area of Kenya with high malaria transmission. Bull WHO. 1997;75 (suppl 1):S33–42. [PMC free article] [PubMed] [Google Scholar]
- 4.Theodoratou E, Al-Jilaihawi S, Woodward F, Ferguson J, Jhass A, Balliet M, et al. The effect of case management on childhood pneumonia mortality in developing countries. Int J Epidemiol. 2010;39 (suppl 1):i155–71. doi: 10.1093/ije/dyq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber MW, Mulholland EK, Jaffar S, Troedsson H, Gove S, Greenwood BM. Evaluation of an algorithm for the integrated management of childhood illness in an area with seasonal malaria in the Gambia. Bull WHO. 1997;75 (suppl 1):S25–32. [PMC free article] [PubMed] [Google Scholar]
- 6.Bryce J, Gouws E, Adam T, Black RE, Schellenberg JA, Manzi F, et al. Improving quality and efficiency of facility-based child health care through Integrated Management of Childhood Illness in Tanzania. Health Policy Plan. 2005;20 (suppl 1):i69–76. doi: 10.1093/heapol/czi053. [DOI] [PubMed] [Google Scholar]
- 7.Enarson PM, Gie R, Enarson DA, Mwansambo C. Development and implementation of a national programme for the management of severe and very severe pneumonia in children in Malawi. PLoS Med. 2009;6:e1000137. doi: 10.1371/journal.pmed.1000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amaral J, Leite AJ, Cunha AJ, Victora CG. Impact of IMCI health worker training on routinely collected child health indicators in Northeast Brazil. Health Policy Plan. 2005;20 (suppl 1):i42–8. doi: 10.1093/heapol/czi058. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong Schellenberg JR, Adam T, Mshinda H, Masanja H, Kabadi G, Mukasa O, et al. Effectiveness and cost of facility-based Integrated Management of Childhood Illness (IMCI) in Tanzania. Lancet. 2004;364:1583–94. doi: 10.1016/S0140-6736(04)17311-X. [DOI] [PubMed] [Google Scholar]
- 10.Rowe AK, Onikpo F, Lama M, Osterholt DM, Deming MS. Impact of a malaria-control project in Benin that included the integrated management of childhood illness strategy. Am J Public Health. 2011;101:2333–41. doi: 10.2105/AJPH.2010.300068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mullins J. Malawi breathes new life into childhood pneumonia care. Lancet. 2012;380:717. doi: 10.1016/s0140-6736(12)61391-9. [DOI] [PubMed] [Google Scholar]
- 12.McCollum ED, Bjornstad E, Preidis GA, Hosseinipour MC, Lufesi N. Multicenter study of hypoxemia prevalence and quality of oxygen treatment for hospitalized Malawian children. Trans R Soc Trop Med Hyg. 2013;107:285–92. doi: 10.1093/trstmh/trt017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huicho L, Scherpbier RW, Nkowane AM, Victora CG. How much does quality of child care vary between health workers with differing durations of training? An observational multi-country study. Lancet. 2008;372:910–16. doi: 10.1016/S0140-6736(08)61401-4. [DOI] [PubMed] [Google Scholar]
- 14.Gouws E, Bryce J, Pariyo G, Armstrong Schellenberg J, Amaral J, Habicht JP. Measuring the quality of child health care at first-level facilities. Soc Sci Med. 2005;61:613–25. doi: 10.1016/j.socscimed.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 15.Hansen PM, Peters DH, Niayesh H, Singh LP, Dwivedi V, Burnham G. Measuring and managing progress in the establishment of basic health services: the Afghanistan health sector balanced scorecard. Int J Health Plan Manage. 2008;23:107–17. doi: 10.1002/hpm.931. [DOI] [PubMed] [Google Scholar]
- 16.Hansen PM, Peters DH, Edward A, Gupta S, Arur A, Niayesh H, et al. Determinants of primary care service quality in Afghanistan. Int J Qual Health Care. 2008;20:375–83. doi: 10.1093/intqhc/mzn039. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. Health Facility Survey: Tool to Evaluate the Quality of Care Delivered to Sick Children Attending Outpatient Facilities (using the Integrated Management of Childhood Illness clinical guidelines as best practices) [internet] 2003 [cited 1 May 2011]. Available from: http://www.who.int/maternal_child_adolescent/documents/9241545860/en/index.html.
- 18.World Health Organization. WHO Pocket Book of Hospital Care for Children: Guidelines for the Management of Common Illnesses with Limited Resources. Geneva: WHO; 2005. [PubMed] [Google Scholar]
- 19.Phillips JA, Kazembe PN, Nelson EAS, Fisher JAF, Grabosch E. A paediatric handbook for Malawi. 3. Limbe, Malawi: Montfort Press; 2008. [Google Scholar]
- 20.Rowe AK, Onikpo F, Lama M, Osterholt DM, Rowe SY, Deming MS. A multifaceted intervention to improve health worker adherence to integrated management of childhood illness guidelines in Benin. Am J Public Health. 2009;99:837–46. doi: 10.2105/AJPH.2008.134411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayieko P, Ntoburi S, Wagai J, Opondo C, Opiyo N, Migiro S, et al. A multifaceted intervention to implement guidelines and improve admission paediatric care in Kenyan district hospitals: a cluster randomised trial. PLoS Med. 2011;8:e1001018. doi: 10.1371/journal.pmed.1001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gouws E, Bryce J, Habicht JP, Amaral J, Pariyo G, Schellenberg JA, et al. Improving antimicrobial use among health workers in first-level facilities: results from the multi-country evaluation of the Integrated Management of Childhood Illness strategy. Bull WHO. 2004;82:509–15. [PMC free article] [PubMed] [Google Scholar]
- 23.Arifeen SE, Bryce J, Gouws E, Baqui AH, Black RE, Hoque DM, et al. Quality of care for under-fives in first-level health facilities in one district of Bangladesh. Bull WHO. 2005;83:260–7. [PMC free article] [PubMed] [Google Scholar]
- 24.Armstrong Schellenberg J, Bryce J, de Savigny D, Lambrechts T, Mbuya C, Mgalula L, et al. The effect of Integrated Management of Childhood Illness on observed quality of care of under-fives in rural Tanzania. Health Policy Plan. 2004;19:1–10. doi: 10.1093/heapol/czh001. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. Malawi: Summary Country Profile for HIV/AIDS Treatment Scale-up [internet] 2005 [updated December 2005, cited 10 Oct 2012]. Available from: http://www.who.int/hiv/HIVCP_MWI.pdf.
- 26.Graham SM, Mankhambo L, Phiri A, Kaunda S, Chikaonda T, Mukaka M, et al. Impact of human immunodeficiency virus infection on the etiology and outcome of severe pneumonia in Malawian children. Pediatr Infect Dis J. 2011;30:33–8. doi: 10.1097/INF.0b013e3181fcabe4. [DOI] [PubMed] [Google Scholar]
- 27.Preidis GA, McCollum ED, Mwansambo C, Kazembe PN, Schutze GE, Kline MW. Pneumonia and malnutrition are highly predictive of mortality among African children hospitalized with human immunodeficiency virus infection or exposure in the era of antiretroviral therapy. J Pediatr. 2011;159:484–9. doi: 10.1016/j.jpeds.2011.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duke T, Wandi F, Jonathan M, Matai S, Kaupa M, Saavu M, et al. Improved oxygen systems for childhood pneumonia: a multihospital effectiveness study in Papua New Guinea. Lancet. 2008;372:1328–33. doi: 10.1016/S0140-6736(08)61164-2. [DOI] [PubMed] [Google Scholar]
- 29.Osterholt DM, Onikpo F, Lama M, Deming MS, Rowe AK. Improving pneumonia case-management in Benin: a randomized trial of a multi-faceted intervention to support health worker adherence to Integrated Management of Childhood Illness guidelines. Hum Resour Health. 2009;7:77. doi: 10.1186/1478-4491-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell M, Getchell M, Nkaka M, Msellemu D, Van Esch J, Hedt-Gauthier B. Perceived improvement in integrated management of childhood illness implementation through use of mobile technology: qualitative evidence from a pilot study in Tanzania. J Health Commun. 2012;17 (suppl 1):S118–27. doi: 10.1080/10810730.2011.649105. [DOI] [PubMed] [Google Scholar]
- 31.Zurovac D, Sudoi RK, Akhwale WS, Ndiritu M, Hamer DH, Rowe AK, et al. The effect of mobile phone text-message reminders on Kenyan health workers’ adherence to malaria treatment guidelines: a cluster randomised trial. Lancet. 2011;378:795–803. doi: 10.1016/S0140-6736(11)60783-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peters DH, Noor AA, Singh LP, Kakar FK, Hansen PM, Burnham G. A balanced scorecard for health services in Afghanistan. Bull WHO. 2007;85:146–51. doi: 10.2471/BLT.06.033746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edward A, Kumar B, Kakar F, Salehi AS, Burnham G, Peters DH. Configuring balanced scorecards for measuring health system performance: evidence from 5 years’ evaluation in Afghanistan. PLoS Med. 2011;8:e1001066. doi: 10.1371/journal.pmed.1001066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nolan T, Angos P, Cunha AJ, Muhe L, Qazi S, Simoes EA, et al. Quality of hospital care for seriously ill children in less-developed countries. Lancet. 2001;357:106–10. doi: 10.1016/S0140-6736(00)03542-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.