Abstract
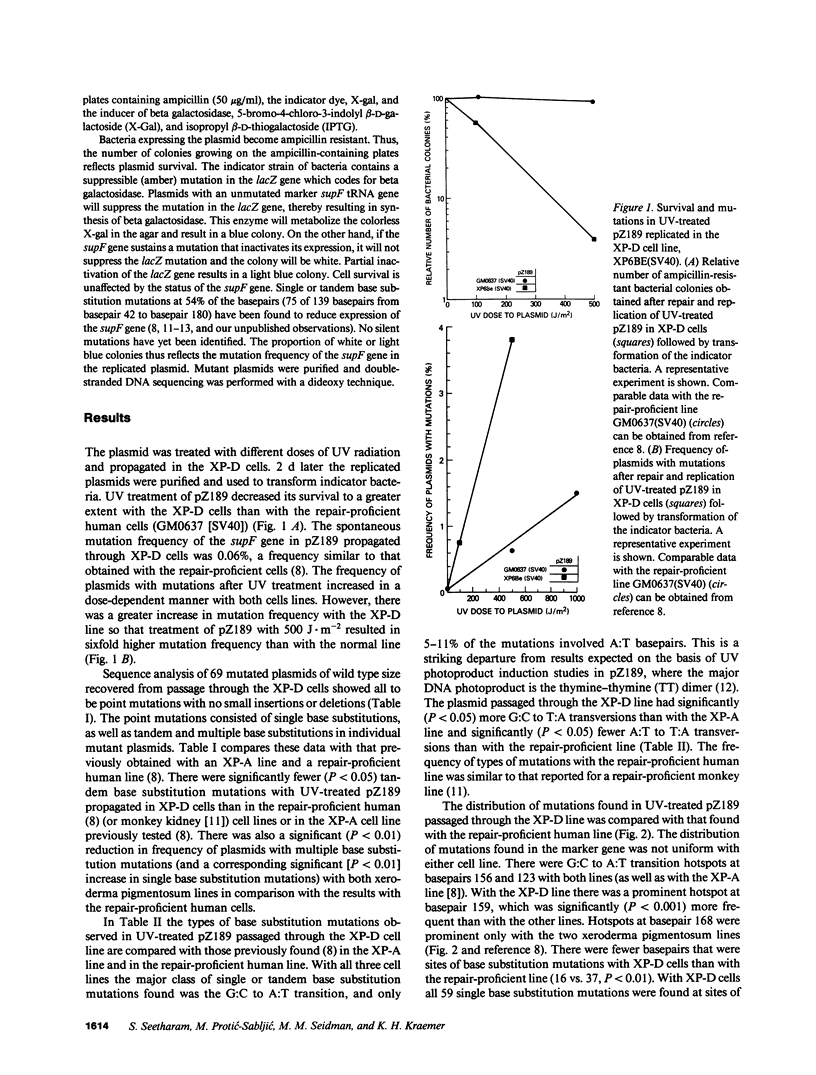
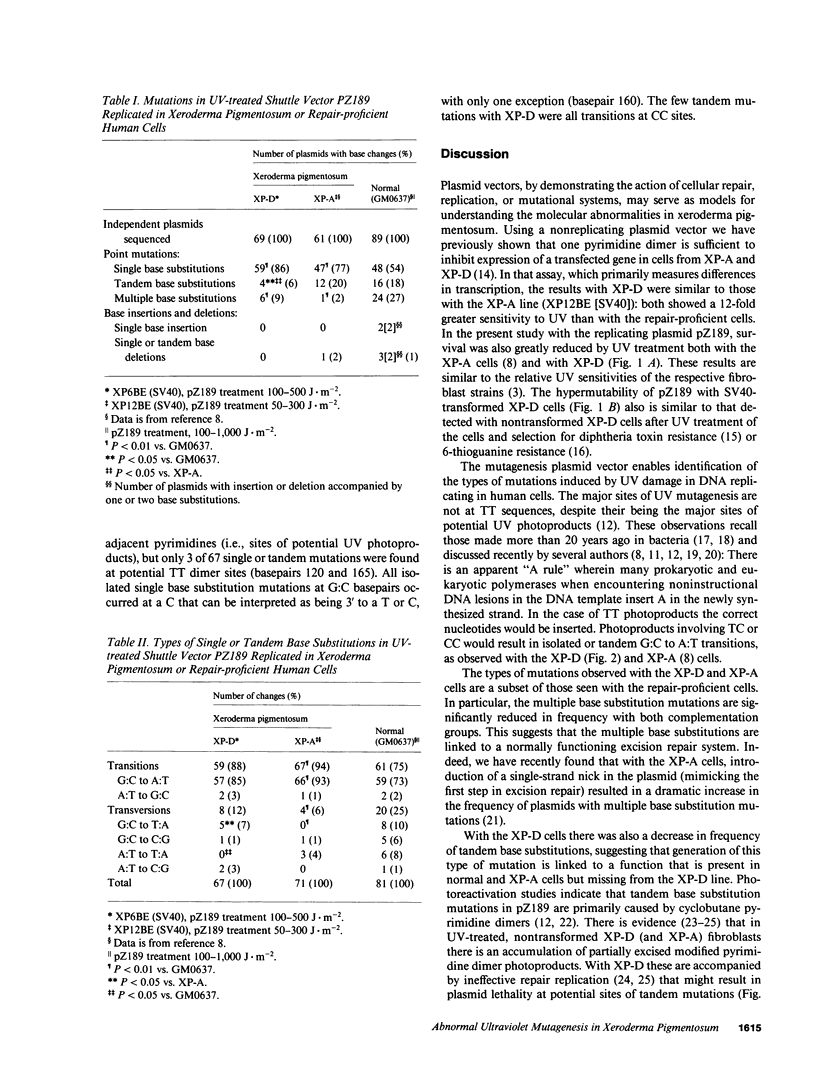
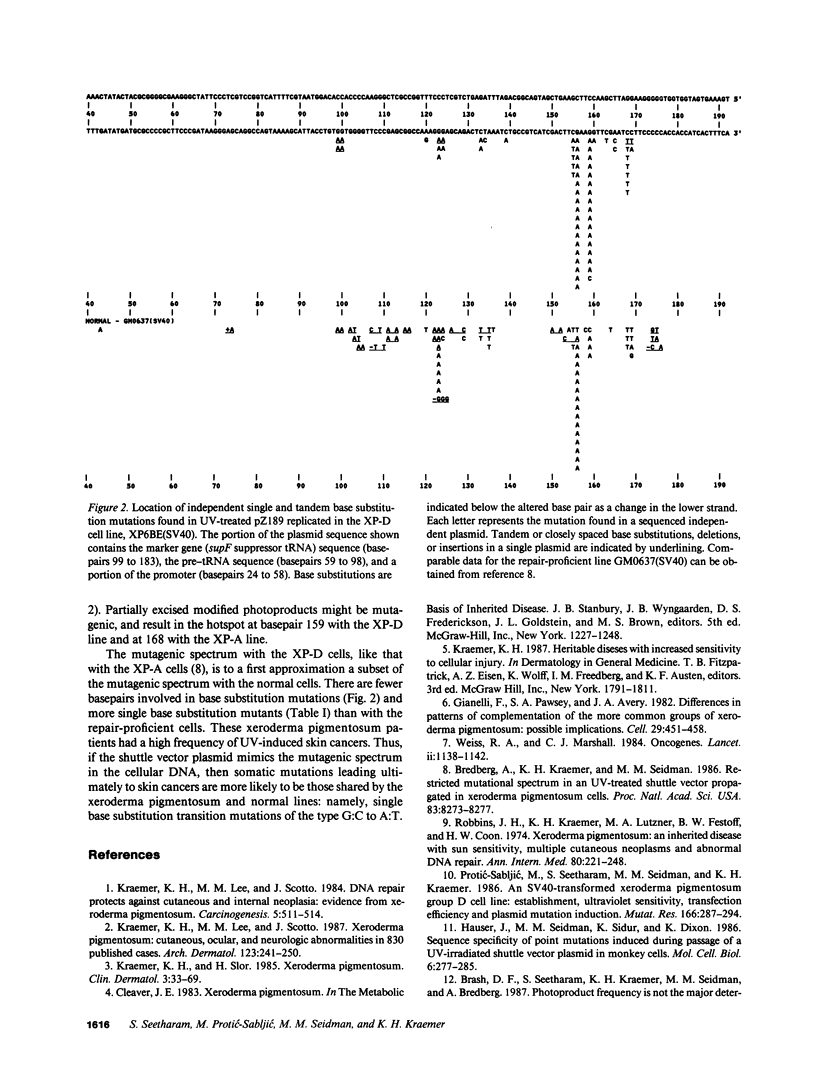
A shuttle vector plasmid, pZ189, was utilized to assess the types of mutations that cells from a patient with xeroderma pigmentosum, complementation group D, introduce into ultraviolet (UV) damaged, replicating DNA. Patients with xeroderma pigmentosum have clinical and cellular UV hypersensitivity, increased frequency of sun-induced skin cancer, and deficient DNA repair. In comparison to UV-treated pZ189 replicated in DNA repair-proficient cells, there were fewer surviving plasmids, a higher frequency of plasmids with mutations, fewer plasmids with two or more mutations in the marker gene, and a new mutagenic hotspot. The major type of base substitution mutation was the G:C to A:T transition with both cell lines. These results, together with similar findings published earlier with cells from a xeroderma pigmentosum patient in complementation group A, suggest that isolated G:C to A:T somatic mutations may be particularly important in generation of human skin cancer by UV radiation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brash D. E., Seetharam S., Kraemer K. H., Seidman M. M., Bredberg A. Photoproduct frequency is not the major determinant of UV base substitution hot spots or cold spots in human cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3782–3786. doi: 10.1073/pnas.84.11.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredberg A., Kraemer K. H., Seidman M. M. Restricted ultraviolet mutational spectrum in a shuttle vector propagated in xeroderma pigmentosum cells. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8273–8277. doi: 10.1073/pnas.83.21.8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannelli F., Pawsey S. A., Avery J. A. Differences in patterns of complementation of the more common groups of xeroderma pigmentosum: possible implications. Cell. 1982 Jun;29(2):451–458. doi: 10.1016/0092-8674(82)90161-1. [DOI] [PubMed] [Google Scholar]
- Glover T. W., Chang C. C., Trosko J. E., Li S. S. Ultraviolet light induction of diphtheria toxin-resistant mutants of normal and xeroderma pigmentosum human fibroblasts. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3982–3986. doi: 10.1073/pnas.76.8.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD B. D., TESSMAN I. IDENTIFICATION OF THE ALTERED BASES IN MUTATED SINGLE-STRANDED DNA. 3. MUTAGENESIS BY ULTRAVIOLET LIGHT. J Mol Biol. 1964 Aug;9:372–375. doi: 10.1016/s0022-2836(64)80214-x. [DOI] [PubMed] [Google Scholar]
- Hauser J., Seidman M. M., Sidur K., Dixon K. Sequence specificity of point mutations induced during passage of a UV-irradiated shuttle vector plasmid in monkey cells. Mol Cell Biol. 1986 Jan;6(1):277–285. doi: 10.1128/mcb.6.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer K. H., Lee M. M., Scotto J. DNA repair protects against cutaneous and internal neoplasia: evidence from xeroderma pigmentosum. Carcinogenesis. 1984 Apr;5(4):511–514. doi: 10.1093/carcin/5.4.511. [DOI] [PubMed] [Google Scholar]
- Kraemer K. H., Lee M. M., Scotto J. Xeroderma pigmentosum. Cutaneous, ocular, and neurologic abnormalities in 830 published cases. Arch Dermatol. 1987 Feb;123(2):241–250. doi: 10.1001/archderm.123.2.241. [DOI] [PubMed] [Google Scholar]
- Kraemer K. H., Slor H. Xeroderma pigmentosum. Clin Dermatol. 1985 Jan-Mar;3(1):33–69. doi: 10.1016/0738-081x(85)90096-3. [DOI] [PubMed] [Google Scholar]
- Paterson M. C., Gentner N. E., Middlestadt M. V., Weinfeld M. Cancer predisposition, carcinogen hypersensitivity, and aberrant DNA metabolism. J Cell Physiol Suppl. 1984;3:45–62. doi: 10.1002/jcp.1041210408. [DOI] [PubMed] [Google Scholar]
- Protić-Sabljić M., Kraemer K. H. One pyrimidine dimer inactivates expression of a transfected gene in xeroderma pigmentosum cells. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6622–6626. doi: 10.1073/pnas.82.19.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protić-Sabljić M., Seetharam S., Seidman M. M., Kraemer K. H. An SV40-transformed xeroderma pigmentosum group D cell line: establishment, ultraviolet sensitivity, transfection efficiency and plasmid mutation induction. Mutat Res. 1986 Nov;166(3):287–294. doi: 10.1016/0167-8817(86)90028-3. [DOI] [PubMed] [Google Scholar]
- Protić-Sabljić M., Tuteja N., Munson P. J., Hauser J., Kraemer K. H., Dixon K. UV light-induced cyclobutane pyrimidine dimers are mutagenic in mammalian cells. Mol Cell Biol. 1986 Oct;6(10):3349–3356. doi: 10.1128/mcb.6.10.3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. H., Kraemer K. H., Lutzner M. A., Festoff B. W., Coon H. G. Xeroderma pigmentosum. An inherited diseases with sun sensitivity, multiple cutaneous neoplasms, and abnormal DNA repair. Ann Intern Med. 1974 Feb;80(2):221–248. doi: 10.7326/0003-4819-80-2-221. [DOI] [PubMed] [Google Scholar]
- Schaaper R. M., Kunkel T. A., Loeb L. A. Infidelity of DNA synthesis associated with bypass of apurinic sites. Proc Natl Acad Sci U S A. 1983 Jan;80(2):487–491. doi: 10.1073/pnas.80.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman M. M., Bredberg A., Seetharam S., Kraemer K. H. Multiple point mutations in a shuttle vector propagated in human cells: evidence for an error-prone DNA polymerase activity. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4944–4948. doi: 10.1073/pnas.84.14.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons J. W. Development of a liquid-holding technique for the study of DNA-repair in human diploid fibroblasts. Mutat Res. 1979 Feb;59(2):273–283. doi: 10.1016/0027-5107(79)90165-9. [DOI] [PubMed] [Google Scholar]
- Strauss B., Rabkin S., Sagher D., Moore P. The role of DNA polymerase in base substitution mutagenesis on non-instructional templates. Biochimie. 1982 Aug-Sep;64(8-9):829–838. doi: 10.1016/s0300-9084(82)80138-7. [DOI] [PubMed] [Google Scholar]
- Weinfeld M., Gentner N. E., Johnson L. D., Paterson M. C. Photoreversal-dependent release of thymidine and thymidine monophosphate from pyrimidine dimer-containing DNA excision fragments isolated from ultraviolet-damaged human fibroblasts. Biochemistry. 1986 May 6;25(9):2656–2664. doi: 10.1021/bi00357a055. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Marshall C. J. DNA in medicine. Oncogenes. Lancet. 1984 Nov 17;2(8412):1138–1142. doi: 10.1016/s0140-6736(84)91566-6. [DOI] [PubMed] [Google Scholar]
- Yang J. L., Maher V. M., McCormick J. J. Kinds of mutations formed when a shuttle vector containing adducts of (+/-)-7 beta, 8 alpha-dihydroxy-9 alpha, 10 alpha-epoxy-7,8,9, 10-tetrahydrobenzo[a]pyrene replicates in human cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3787–3791. doi: 10.1073/pnas.84.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]


