Background: The mechanism underlying tadalafil regulation of diabetes-induced matrix synthesis in the kidney is unknown.
Results: In podocytes, tadalafil stimulated inducible nitric-oxide synthase to generate hydrogen sulfide and inhibit high glucose-induced matrix protein synthesis.
Conclusion: Tadalafil recruits nitric oxide and hydrogen sulfide to inhibit high glucose-induced matrix protein synthesis.
Significance: Tadalafil may be tested for treating diabetic kidney disease.
Keywords: AMP-activated kinase (AMPK), diabetes, fibrosis, kidney, mammalian target of rapamycin (mTOR)
Abstract
Diabetes-induced kidney cell injury involves an increase in matrix protein expression that is only partly alleviated by current treatment, prompting a search for new modalities. We have previously shown that hydrogen sulfide (H2S) inhibits high glucose-induced protein synthesis in kidney podocytes. We tested whether tadalafil, a phosphodiesterase 5 inhibitor used to treat erectile dysfunction, ameliorates high glucose stimulation of matrix proteins by generating H2S in podocytes. Tadalafil abrogated high glucose stimulation of global protein synthesis and matrix protein laminin γ1. Tadalafil inhibited high glucose-induced activation of mechanistic target of rapamycin complex 1 and laminin γ1 accumulation in an AMP-activated protein kinase (AMPK)-dependent manner. Tadalafil increased AMPK phosphorylation by stimulating calcium-calmodulin kinase kinase β. Tadalafil rapidly increased the expression and activity of the H2S-generating enzyme cystathionine γ-lyase (CSE) by promoting its translation. dl-Propargylglycine, a CSE inhibitor, and siRNA against CSE inhibited tadalafil-induced AMPK phosphorylation and abrogated the tadalafil effect on high glucose stimulation of laminin γ1. In tadalafil-treated podocytes, we examined the interaction between H2S and nitric oxide (NO). Nω-Nitro-l-arginine methyl ester and 1H-[1,2,4]-oxadiazolo-[4,3-a]-quinoxalin-1-one, inhibitors of NO synthase (NOS) and soluble guanylyl cyclase, respectively, abolished tadalafil induction of H2S and AMPK phosphorylation. Tadalafil rapidly augmented inducible NOS (iNOS) expression by increasing its mRNA, and siRNA for iNOS and 1400W, an iNOS blocker, inhibited tadalafil stimulation of CSE expression and AMPK phosphorylation. We conclude that tadalafil amelioration of high glucose stimulation of synthesis of proteins including matrix proteins in podocytes requires integration of the NO-H2S-AMPK axis leading to the inhibition of high glucose-induced mechanistic target of rapamycin complex 1 activity and mRNA translation.
Introduction
Diabetes-associated kidney injury is characterized by hypertrophy and accumulation of matrix proteins culminating in kidney fibrosis. The mechanisms leading to increment in matrix protein content include an increase in synthesis and inhibition of degradation. High glucose-induced synthesis of matrix proteins can be independently regulated at the levels of transcription (1, 2) and mRNA translation (3, 4). Elaborate signaling pathways regulate both transcription and translation in the kidney in diabetic mice (2, 5, 6). These signaling pathways feature kinases that serve to stimulate protein synthesis, e.g. phosphatidylinositol 3-kinase, Akt, mechanistic target of rapamycin complex 1 (mTORC1),3 and ERK. In addition, recent work has shown that high glucose suppresses kinases that normally inhibit protein synthesis, e.g. AMP-activated protein kinase (AMPK) (7–10) and glycogen synthase kinase 3β (11). These observations have suggested that the control of pathologically increased protein synthesis could be achieved by the activation of inhibitory kinases. Thus, metformin, 5-aminoimidazole-4-carboxamide ribonucleotide, and adiponectin, agents that augment AMPK activity, inhibit oxidative stress, renal hypertrophy, matrix increment, and albuminuria in diabetic rodents (7, 8, 10). There is a growing interest in other agents that stimulate AMPK. Recent investigations have shown that hydrogen sulfide (H2S) activates AMPK in kidney cells (12).
H2S is constitutively synthesized in several tissues in mammals. It serves as a gasotransmitter and regulates neuronal functions and contraction of blood vessels (13, 14). Mice lacking cystathionine γ-lyase (CSE), an enzyme that synthesizes H2S, have high blood pressure that is ameliorated by sodium hydrosulfide, an H2S donor (14). We have recently reported that H2S inhibits high glucose-induced synthesis of proteins including extracellular matrix proteins in kidney epithelial cells (12). The mechanism involves activation of AMPK followed by inhibition of mTORC1 and events in mRNA translation culminating in inhibition of high glucose-induced matrix protein synthesis. H2S is generated in the kidney by cystathionine β-synthase, CSE, and, 3-mercaptopyruvate sulfurtransferase (12, 15–17). The content of cystathionine β-synthase and CSE is decreased in the kidney cortex of mice with type 1 or type 2 diabetes, and sodium hydrosulfide, an H2S donor, ameliorates diabetes-induced kidney injury (12, 18, 19). These data suggest that diabetes-induced renal injury is associated with H2S deficiency. Pharmacologic agents that promote H2S generation are being identified. The beneficial effect of phosphodiesterase 5 (PDE5) inhibitors such as tadalafil on ischemic injury of the heart is mediated by H2S (20). Thus, regulators of H2S could include the nitric oxide (NO) pathway. Recent reports suggest that the two gasotransmitters interact in a cell-specific manner (21). In addition to NO synthases and H2S-generating enzymes, PDE5 has been localized to the kidney including the glomerulus (22–24), indicating that cell machinery exists for the interaction of pathways involving H2S and NO in renal cells. However, to our knowledge, this interaction has not been investigated in the kidney. Our objective was to explore whether the PDE5 inhibitor tadalafil affects high glucose-induced synthesis of proteins including matrix proteins in podocytes and whether this regulation involves the NO-H2S-AMPK pathway.
EXPERIMENTAL PROCEDURES
Cell Culture
Mouse podocytes were kindly provided by Dr. P. Mundel, Harvard University, Boston, MA (25). In brief, Mundel et al. (25) isolated the glomeruli obtained from 10-week-old transgenic H-2kb-tsA58 mice. The glomeruli were plated on collagen I-coated dishes in RPMI 1640 medium containing γ-interferon. The parent glomeruli were removed by sieving, and primary cell outgrowths were replated in the presence of γ-interferon to permit cell growth. WT-1-positive (a podocyte marker) clonal cell lines were obtained by the limited dilution method and propagated (25). For this study, podocytes were grown in RPMI 1640 medium containing 7% FBS, 5 mm glucose, 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mm glutamine, and 50 units/ml recombinant mouse γ-interferon (Gibco) on collagen I (BD Biosciences)-coated plates at 33 °C, and differentiation was induced as described previously (12, 25). To study the effects of high glucose, medium glucose was increased to 30 mm; equimolar 5 mm glucose + 25 mm mannitol served as an osmotic control. Preincubation with tadalafil (provided by Eli Lilly, Indianapolis, IN) was chosen at 8 h based on the initial data on AMPK phosphorylation. Rat glomerular epithelial cells (podocytes) that express nephrin and podocin similar to mouse podocytes (12) are more amenable for transfection; these cells were transfected with siRNA or scrambled RNA using Lipofectamine RNAiMAX (Invitrogen).
Protein Synthesis and Cell Hypertrophy Measurement
These assays were performed as described (12).
Protein Detection
Immunoblotting was performed as described (11, 12). All primary antibodies were from Cell Signaling Technology (Danvers, MA) except for those against fibronectin, CSE, laminin γ1 (Santa Cruz Biotechnology), and cystathionine β-synthase (Abgent).
Live Cellular Calcium Imaging
Quiescent cells were incubated with 5 μm Fura2-AM (Life Technologies) for 30 min in Hanks' balanced salt solution without calcium (Cellgro, Manassas, VA). Cells were washed with Hanks' balanced salt solution without calcium three times and treated with or without 10 μm tadalafil for 1 h in Hanks' balanced salt solution with calcium (Gibco). Live cell calcium imaging with Fura2 was conducted on a Nikon Eclipse Ti inverted microscope with a CFI Super Fluor 40×/numerical aperture 1.3 oil immersion objective and a Semrock Fura2-C-NTE set, which includes 340- and 380-nm dual excitation filters and a 510/84-nm bandpass emission filter. Fluorescence images were collected by a Photometrics CoolSnap HQ2 charge-coupled device camera. Cells were kept in a stage chamber at 37 °C and 5% CO2 during imaging. The built-in Perfect Focus System device in the microscope was enabled to prevent the focus from drifting during the time course data collection.
Quantitative RT-PCR
Quantitative RT-PCR was performed in a MasterCycler RealPlex4 (Eppendorf) using the SYBR Green RT2 qPCR Primer Assay (Qiagen) with specific gene primers for CSE, iNOS, and GAPDH (Qiagen/SAB science) as described previously (11, 26).
Polysome Assay
The polysome assay was performed as described (26). Briefly, postnuclear supernatants were separated on a 15–40% sucrose gradient by centrifugation at 200,000 × g and divided into 10 fractions. Total RNA was isolated by the TRIzol method and used for quantitative RT-PCR.
Assay for H2S Generation
The assay was performed as described previously with some modification (27). Briefly, cells were lysed in ice-cold 100 mm potassium phosphate buffer (pH 7.4) using a sonicator. 250 μg of cell lysate was incubated with 20 μl of l-cysteine (10 mm) and 20 μl of pyridoxal 5′-phosphate (2 mm) in a 500-μl reaction volume for 3 h at 37 °C. 250 μl of zinc acetate (1%, w/v) was added to the reaction tube to trap H2S in solution followed by addition of 10% TCA. Next, 133 μl of N,N-dimethyl-p-phenylenediamine sulfate (20 μm) in 7.2 m HCl was added followed by incubation with 133 μl of FeCl3 in 1.2 m HCl for 2 h. Total H2S was determined by a 96-well microplate reader (Magellan 6, Tecan Systems Inc.) with 200-μl aliquots at 670 nm. The enzymatic activity was calculated as total H2S synthesis/unit of protein/unit of time with NaHS standard in 100 mm potassium phosphate buffer (0.1–50 μm).
Griess Reaction (28)
Mouse podocytes were incubated with serum-free RPMI 1640 medium for 24 h, and then the medium was changed to Hanks' balanced salt solution. After a 30-min incubation, cells were incubated with or without 10 μm tadalafil for up to 2 h. Aliquots of medium were used for measuring nitrate and nitrite by a colorimetric assay kit (Sigma-Aldrich).
Statistical Analysis
Data were expressed as mean ± S.E. Statistical comparisons between multiple groups were performed by one-way analysis of variance, and post hoc analysis was done using the Student-Newman-Keuls multiple comparison test using GraphPad Prism 4 software. A p value of <0.05 was considered statistically significant.
RESULTS
Tadalafil Inhibits High Glucose-induced Protein Synthesis, Cellular Hypertrophy, and Extracellular Matrix Protein Expression
In differentiated mouse podocytes, high glucose increased protein synthesis at 16 h, and this was inhibited by preincubation with tadalafil (Fig. 1A); equimolar mannitol, used as an osmotic control for high glucose, did not affect de novo protein synthesis (data not shown). High glucose increased the protein content per unit cell number in podocytes demonstrating hypertrophy, and this was abolished by tadalafil (Fig. 1B). Podocytes synthesize matrix proteins laminin and fibronectin that are deposited in the glomerular basement membrane (29). Expansion of glomerular basement membrane is a common feature of kidney injury in diabetes (30). High glucose, but not equimolar mannitol, increased the expression of laminin γ1 and fibronectin in the podocytes, and this was abolished by tadalafil (Fig. 1, C and D).
FIGURE 1.
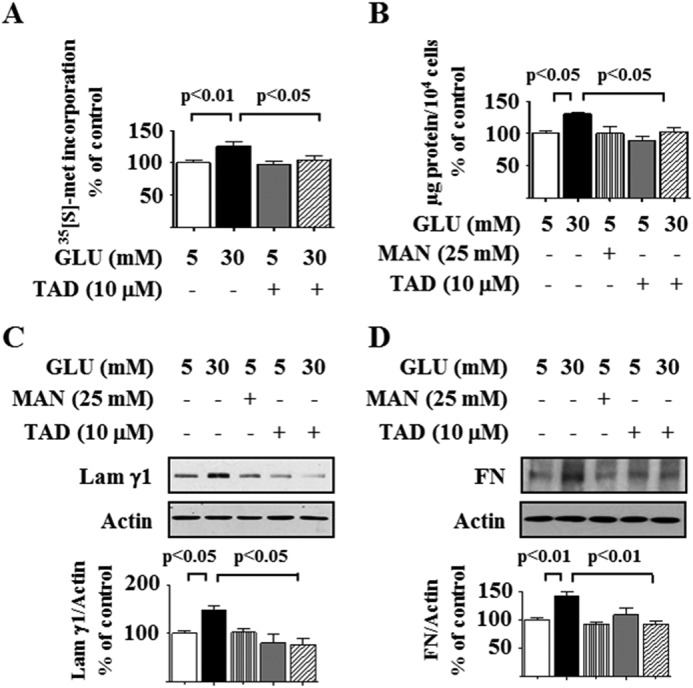
Tadalafil inhibits high glucose-stimulated protein synthesis, cellular hypertrophy, and matrix protein expression in podocytes. Quiescent podocytes were incubated with 5 or 30 mm glucose (GLU) for 16 h with or without preincubation for 8 h with tadalafil (TAD). A, de novo protein synthesis was measured by [35S]methionine incorporation into TCA-precipitable protein. B, cellular hypertrophy was estimated as total cellular protein per unit cell number. C and D, cell lysate protein was immunoblotted with laminin γ1 (Lam γ1) and fibronectin (FN) antibodies. In A–D, composite data from three to six experiments are shown in histograms; error bars represent S.E.
Tadalafil Regulates mRNA Translation by Inhibiting mTORC1
mTORC1 is a major regulator of mRNA translation, a rate-limiting step in protein synthesis (31). An increase in the phosphorylation of 4E-BP1 and p70S6 kinase is a direct readout of mTORC1 activation (32). In the resting state, 4E-BP1 binds to eukaryotic initiation factor 4E (eIF4E), the mRNA cap-binding protein, and keeps it inactive, and mTORC1-induced phosphorylation of 4E-BP1 facilitates the initiation phase of translation by releasing eIF4E. In addition to phosphorylating ribosomal proteins, p70S6 kinase stimulates the elongation phase by phosphorylating eukaryotic elongation factor 2 (eEF2) kinase on Ser-366 and inhibiting its activity; reduced activity of eEF2 kinase contributes to dephosphorylation of eEF2 on Thr-56, which facilitates the elongation phase of translation (33, 34). High glucose significantly stimulated phosphorylation of 4E-BP1, p70S6 kinase, and eEF2 kinase by 5 min, whereas reduction in eEF2 phosphorylation, evident at 5 min, reached significance at 30 min (Fig. 2, A–D). High glucose-induced changes in phosphorylation of 4E-BP1, p70S6 kinase, eEF2 kinase, and eEF2 were inhibited by tadalafil without significant changes in their basal status (Fig. 2, A–D). These data show that tadalafil abolishes both the initiation and elongation phases of mRNA translation induced by high glucose by inhibiting mTORC1 activity.
FIGURE 2.
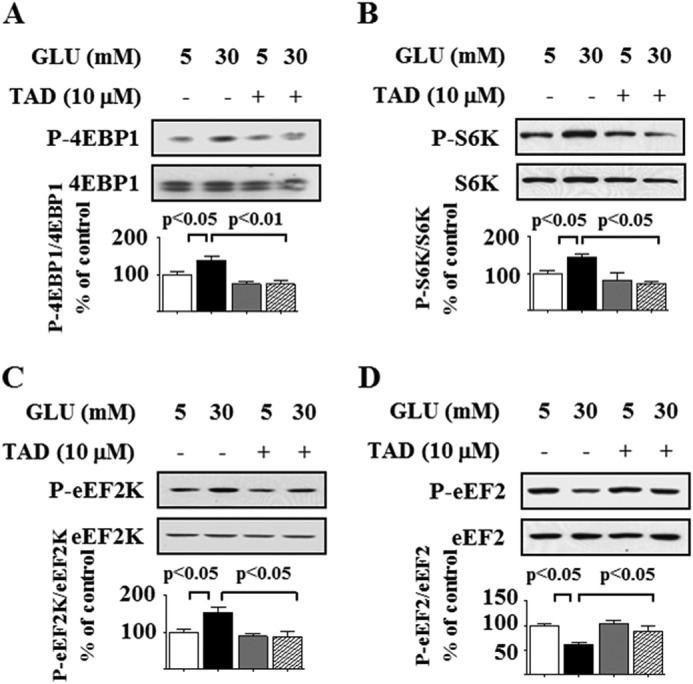
Tadalafil inhibits high glucose-stimulated events in mRNA translation. Podocytes were incubated with 5 or 30 mm glucose (GLU) for 5 (A–C) and 30 min (D) with or without preincubation with 10 μm tadalafil (TAD) for 8 h. Cell lysate protein was immunoblotted with specific antibodies. A, antibody against 4E-BP1 phosphorylated on Thr-36/47 (P-4E-BP1) or 4E-BP1. B, antibody against p70S6 kinase phosphorylated on Thr-389 (P-S6K) or p70S6 kinase (S6K). C, antibody against eEF2 kinase phosphorylated on Ser-366 (P-eEF2K) and eEF2 kinase (eEF2K). D, antibody against eEF2 phosphorylated on Thr-56 (P-eEF2) and eEF2. In A–D, composite data from three to four experiments are shown in histograms; error bars represent S.E.
Tadalafil Increases AMPK Phosphorylation
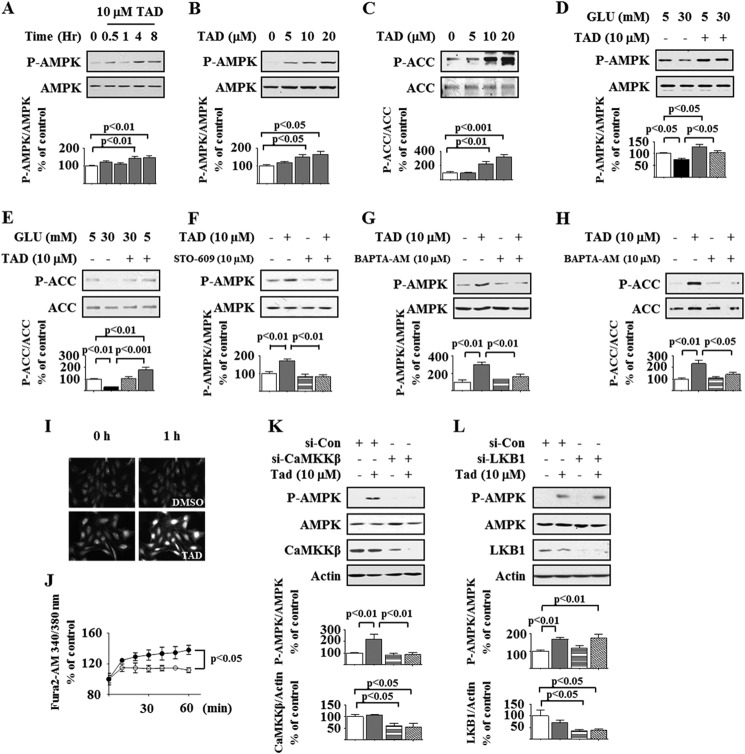
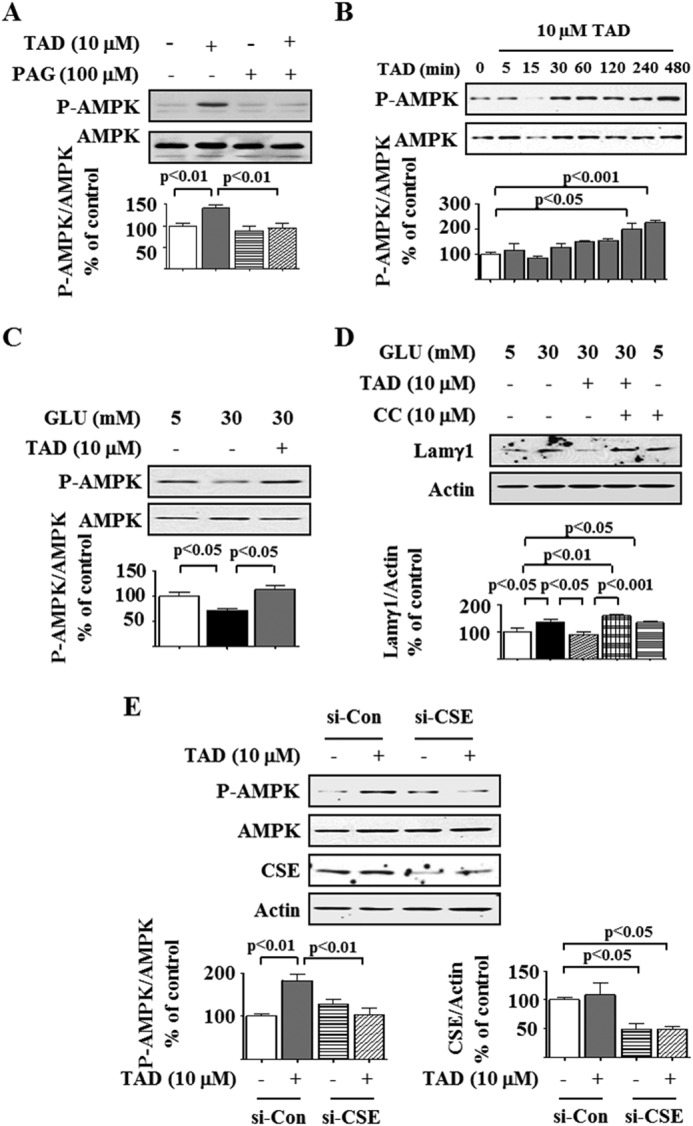
AMPK activation by Thr-172 phosphorylation of the catalytic α subunit blocks high glucose-induced protein synthesis by inhibiting mTORC1 in podocytes (7, 8, 10, 12). We explored whether tadalafil inhibition of mTORC1 involves stimulation of AMPK. Tadalafil increased AMPK phosphorylation in a time- and concentration-dependent manner, peaking at 8 h and at 10–20 μm, respectively (Fig. 3, A and B). Tadalafil also stimulated phosphorylation of acetyl-CoA carboxylase, an AMPK substrate, providing evidence for AMPK activation by tadalafil (Fig. 3C). High glucose reduced the phosphorylation of AMPK and acetyl-CoA carboxylase at 5 min, and this was restored to baseline by preincubation with tadalafil (Fig. 3, D and E). Calcium-calmodulin kinase kinase β and LKB1 phosphorylate AMPK on Thr-172; the former is inhibited by STO-609. Tadalafil-induced AMPK phosphorylation could be abolished by STO-609 (Fig. 3F). Additionally, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester), a membrane-permeable calcium chelator, blocked tadalafil stimulation of phosphorylation of AMPK and acetyl-CoA carboxylase (Fig. 3, G and H). Tadalafil augmented intracellular Ca2+ flow in Fura2-AM-loaded cells in a live cell imaging assay (Fig. 3, I and J). Finally, whereas siRNA for LKB1 did not affect AMPK phosphorylation by tadalafil, it was abrogated by siRNA for calcium-calmodulin kinase kinase β in rat podocytes (Fig. 3, K and L), demonstrating that tadalafil stimulates calcium-calmodulin kinase kinase β to phosphorylate AMPK.
FIGURE 3.
Tadalafil increases AMPK phosphorylation. Cell lysate protein was immunoblotted with the antibody against the α subunit of AMPK phosphorylated on Thr-172 (P-AMPK), AMPK (A, B, D, F, G, K, and L), phosphorylated acetyl-CoA carboxylase (P-ACC) or acetyl-CoA carboxylase (ACC) (C, E, and H), calcium-calmodulin kinase kinase β (CaMKKβ) (K), LKB1 (L), and actin (K and L). A, podocytes were treated with tadalafil (TAD) for the indicated times. B and C, cells were treated with the indicated concentration of tadalafil for 8 h. D and E, cells were incubated with 5 or 30 mm glucose (GLU) for 5 min with or without preincubation with tadalafil. F, tadalafil increased AMPK phosphorylation at 8 h, and this was abolished by STO-609. G and H, cells were treated with or without tadalafil for 8 h followed by incubation with 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester) (BAPTA-AM), a calcium chelator, for 30 min. I and J, in a live cell imaging assay using Fura2-AM, tadalafil promoted Ca2+ transients in podocytes up to 1 h (closed circles) relative to DMSO-treated control cells (open circles). K and L, tadalafil-induced AMPK phosphorylation was inhibited in rat podocytes by siRNA against calcium-calmodulin kinase kinase β (si-CaMKKβ) but not that against LKB1 (si-LKB1). Si-Con, siRNA control. In A–H, K, and L, composite data from three to five experiments are shown in graphs; error bars represent S.E.
Tadalafil Inhibition of High Glucose-stimulated Protein Synthesis Requires AMPK Activation
Stimulation of AMPK inhibits renal hypertrophy induced by hyperglycemia both in vitro and in vivo (7, 10, 12). We tested whether tadalafil inhibition of high glucose-induced protein synthesis requires AMPK activation by using Compound C, a selective inhibitor of the kinase (12, 35). Compound C abolished tadalafil inhibition of high glucose stimulation of de novo protein synthesis and laminin γ1 expression (Fig. 4, A and B). Compound C significantly increased basal protein synthesis and laminin γ1 expression (Fig. 4, A and B), suggesting that AMPK maintains a tonal inhibition on protein synthesis in the podocyte. These data suggest that AMPK activation is required for tadalafil inhibition of high glucose-induced protein synthesis and laminin γ1 expression.
FIGURE 4.
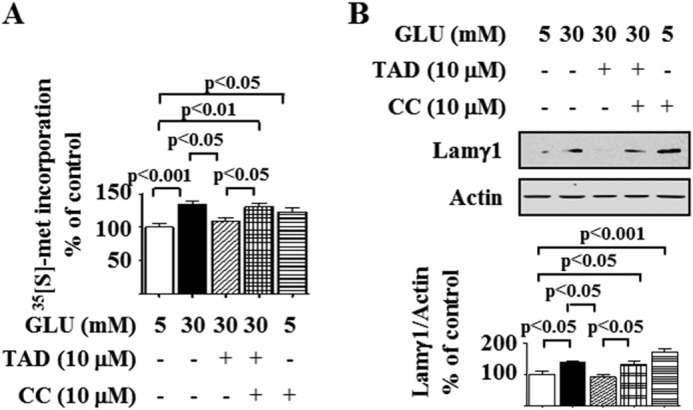
AMPK mediates tadalafil inhibition of high glucose-induced protein synthesis and matrix laminin γ1 increment. Podocytes were preincubated with or without Compound C (CC) for 30 min. Cells were then incubated with 30 mm glucose (GLU) for 16 h with or without preincubation with tadalafil (TAD). A, de novo protein synthesis was measured as described in Fig. 1A. B, laminin γ1 (Lamγ1) content was detected by immunoblotting against laminin γ1 antibody. In A and B, composite data from four to five experiments are shown in graphs; error bars represent S.E.
Tadalafil Inhibits High Glucose-induced mTORC1 Activation and mRNA Translation by Stimulating AMPK
Compound C prevented tadalafil-induced reversal of high glucose-stimulated phosphorylation of 4E-BP1 and p70S6 kinase, indices of mTORC1 activation (Fig. 5, A and B). Similarly, Compound C abolished tadalafil-induced modulation of phosphorylation changes in eEF2 kinase and eEF2 caused by high glucose (Fig. 5, C and D). In podocytes incubated with normal glucose, Compound C significantly increased 4E-BP1 phosphorylation and decreased eEF2 phosphorylation in 5 mm glucose-treated cells, suggesting that AMPK serves to inhibit important reactions in the initiation and elongation phases of mRNA translation in the basal state. These data suggest that AMPK activation is a prerequisite for tadalafil inhibition of high glucose-stimulated mTORC1 and mRNA translation.
FIGURE 5.
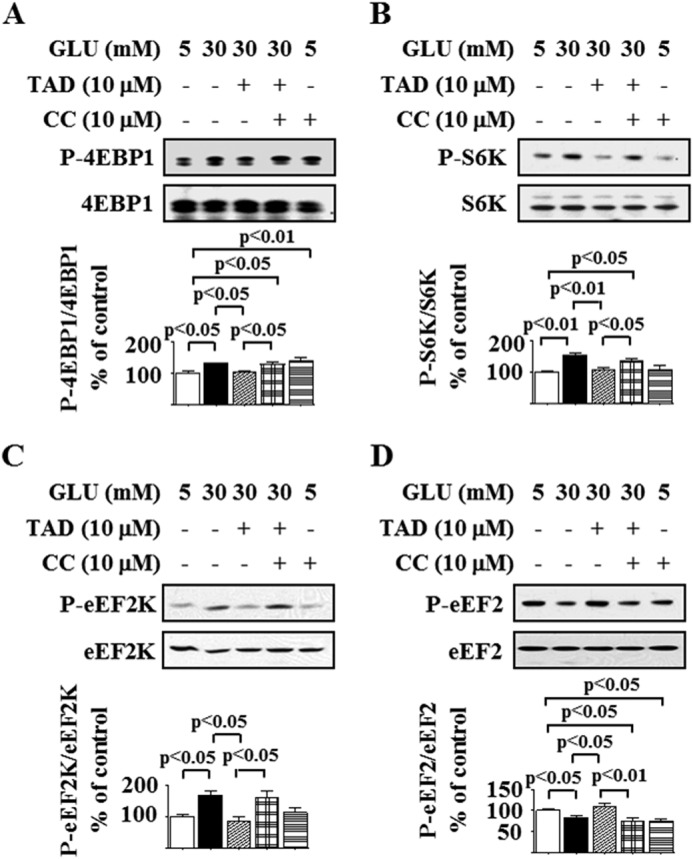
AMPK mediates tadalafil inhibition of high glucose-stimulated mTOR complex 1 activation. Podocytes were preincubated with Compound C (CC) for 30 min. Cells were incubated with 5 or 30 mm glucose (GLU) for 5 (A–C) or 30 min (D) with or without preincubation with tadalafil (TAD) for 8 h. Cell lysate proteins were immunoblotted with specific antibodies. A, antibody against phospo-4E-BP1 (Thr-37/46) (P-4E-BP1) or 4E-BP1. B, antibody against phospho-p70S6 kinase (Thr-389) (P-S6K) and p70S6 kinase (S6K). C, antibody against phosphorylated eEF2 kinase (Ser-366) (P-eEF2K) and eEF2 kinase (eEF2K). D, antibody against phospho-eEF2 (Thr-56) (P-eEF2) or eEF2. In A–D, composite data from three to five experiments are shown in histograms; error bars represent S.E.
Tadalafil Induction of AMPK Phosphorylation Requires Activation of CSE, an H2S-generating Enzyme
H2S inhibits high glucose-stimulated protein synthesis by activating AMPK (12). CSE and cystathionine β-synthase, enzymes that generate H2S, are highly expressed in the kidney (12, 15). We tested whether H2S mediates the aforementioned tadalafil effects. Tadalafil increased H2S generation at 1 h, and the level returned to control levels over the next 4–24 h (Fig. 6A). Tadalafil-induced H2S generation was abolished by preincubation with dl-propargylglycine (PAG), an irreversible CSE inhibitor (Fig. 6B) (36, 37), suggesting that CSE was the major H2S-generating enzyme in the podocyte. We explored whether tadalafil regulated the expression of CSE. Tadalafil increased CSE protein expression in cells incubated in normal glucose medium; the level peaked at 1 h but returned to baseline between 4 and 24 h (Fig. 6C) without changes in its mRNA content (Fig. 6D), suggesting a non-transcriptional mechanism. Preincubation with cycloheximide, a translation inhibitor, but not actinomycin D, a transcription inhibitor, abolished the tadalafil-induced increase in CSE expression (Fig. 6E), supporting regulation at the level of mRNA translation. This was further tested by the polysome assay. Whereas in untreated podocytes 16% of the CSE mRNA was associated with polysomal fractions, the proportion was increased to 64% in tadalafil-treated cells (Fig. 6F). Given the tendency of tadalafil not to affect general protein synthesis in normal glucose-treated cells (Fig. 1A), selective stimulation of CSE synthesis by tadalafil suggests that CSE is somehow targeted. Modeling of the 5′-untranslated region of CSE (16) showed that it contains two stem loop structures. Such secondary stem loop structures render mRNAs to be regulated by translation, e.g. ribosomal proteins RPL23, RPL34, cyclin D1, baculoviral inhibitor of apoptosis protein (IAP) repeat-containing 5, and osteopontin (38). Additionally, the short 5′-UTR of CSE does not seem to make it less of a candidate for regulation by translation as recent reports suggest that mRNAs with short and less complex UTRs could also be regulated by translation (39). These data demonstrated that tadalafil rapidly augments CSE expression in the podocyte by increasing the efficiency of translation and not transcription. We next explored whether CSE activity was required for tadalafil-induced AMPK phosphorylation. PAG abrogated tadalafil-induced AMPK phosphorylation (Fig. 7A). To genetically reduce CSE expression, we used rat podocytes that express nephrin and podocin (12) and are more amenable for transfection. Similar to mouse podocytes, tadalafil augmented AMPK phosphorylation in these cells in a time-dependent manner and abolished the high glucose-induced reduction in AMPK phosphorylation (Fig. 7, B and C). As was observed in mouse podocytes, tadalafil abrogated the high glucose-induced increase in laminin γ1 content in an AMPK-dependent manner (Fig. 7D), supporting the use of rat podocytes to evaluate the requirement of CSE in tadalafil actions. Expression of siRNA against CSE reduced the CSE expression by nearly 50% and abolished tadalafil-induced AMPK activation (Fig. 7E). Thus, tadalafil activates CSE by promoting its expression by mRNA translation, leading to H2S generation and downstream stimulation of AMPK phosphorylation.
FIGURE 6.
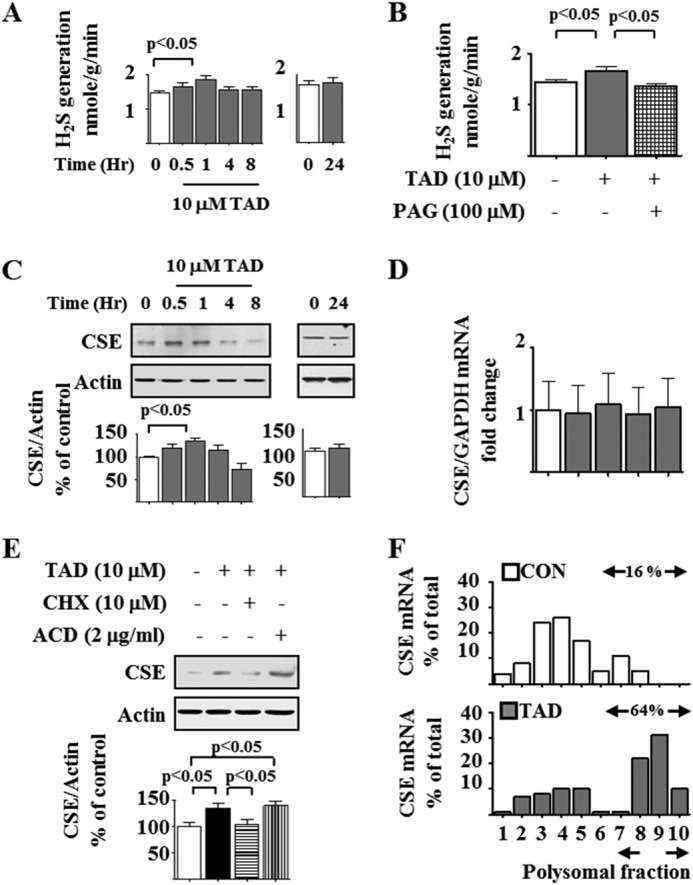
Tadalafil increases CSE expression by promoting its mRNA translation. A, tadalafil (TAD) augmented H2S generation at 1 h but not at later time points. B, PAG abolished tadalafil stimulation of H2S generation. C, immunoblotting showed that tadalafil stimulated CSE expression at 1 h but not at later time points. D, cells were incubated with tadalafil for the indicated duration. Quantitative RT-PCR was performed with primers for CSE and GAPDH. E, cells were incubated with tadalafil for 1 h with or without preincubation with cycloheximide (CHX) or actinomycin D (ACD) for 30 min. Cell lysate protein was immunoblotted with antibodies against the indicated proteins. In A–E, data from four to 11 experiments are shown in graphs; error bars represent S.E. F, polysome assay. Cells were incubated with or without tadalafil for 1 h. Postnuclear supernatants were centrifuged through a 15–40% sucrose gradient. CSE mRNA was estimated in each fraction of the gradient. CON, control.
FIGURE 7.
Tadalafil increases AMPK phosphorylation through CSE activation. A, cells were incubated with tadalafil (TAD) for 8 h with or without PAG. Cell lysate protein was immunoblotted with antibodies against phospho-AMPK (P-AMPK) or AMPK. B, rat podocytes were treated with tadalafil for the indicated times. C, rat podocytes were incubated with 5 or 30 mm glucose (GLU) for 5 min with or without preincubation with tadalafil. Cell lysate protein was immunoblotted with antibody against the α subunit of AMPK phosphorylated on Thr-172 or AMPK antibody. D, rat podocytes were preincubated with or without Compound C (CC) for 30 min. Cells were then incubated with 30 mm glucose for 16 h with or without preincubation with tadalafil. Laminin γ1 (Lamγ1) content was detected by immunoblotting with laminin γ1 antibody. E, rat podocytes were transfected with siRNA against CSE (si-CSE) or control siRNA (Si-Con) and incubated with or without tadalafil. Immunoblotting was done with antibodies against the indicated proteins. In A–E, composite data from three to five experiments are shown in histograms; error bars represent S.E.
Tadalafil Inhibition of High Glucose-stimulated Protein Synthesis Depends on H2S
We investigated whether tadalafil inhibition of high glucose-induced protein synthesis required CSE activity and H2S generation. Tadalafil inhibition of high glucose-induced de novo protein synthesis and laminin γ1 expression was abrogated by PAG, suggesting that the tadalafil effect requires CSE activity and H2S generation (Fig. 8, A and B), and PAG augmented de novo protein synthesis and laminin γ1 content in cells incubated with 5 mm glucose, suggesting that H2S controls constitutive expression of proteins including laminin γ1. Inhibition of the high glucose-induced increase in p70S6 kinase phosphorylation by tadalafil was abrogated by PAG (Fig. 8C). A reduction in CSE expression by siRNA in rat podocytes also abolished tadalafil inhibition of high glucose-induced stimulation of laminin γ1, confirming the data with PAG (Fig. 8D). Taken together, these data show that tadalafil activation of CSE and generation of H2S are required for the inhibition of high glucose-induced AMPK suppression, mTORC1 activation, and protein synthesis.
FIGURE 8.
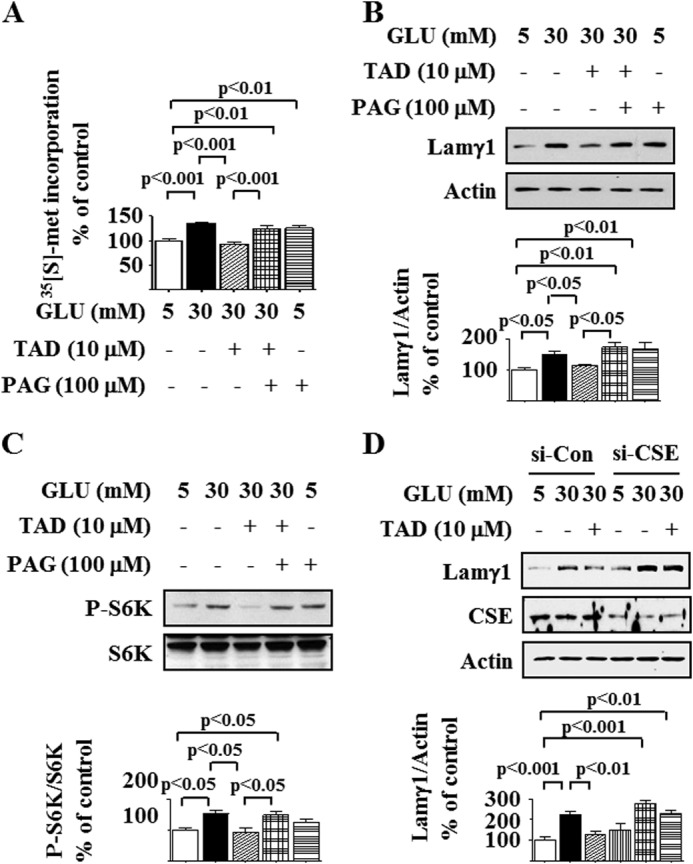
Cystathionine γ-lyase mediates tadalafil inhibition of high glucose-stimulated protein synthesis and mTOR complex 1 activation. A, de novo protein synthesis was measured as described in Fig. 1A with or without preincubation with PAG for 30 min prior to incubation with tadalafil (TAD) and high glucose (GLU). B, cells were preincubated with PAG for 30 min followed by incubation with tadalafil and glucose as described in Fig. 1C. Cell lysates were immunoblotted with antibody against laminin γ1 (Lamγ1) antibody and actin. C, cells were preincubated with PAG for 30 min followed by incubation with tadalafil and glucose as described in Fig. 2B. Immunoblotting was performed with antibody against phospho-p70S6 kinase (Thr-389) (P-S6K) and p70S6 kinase (S6K). D, rat podocytes were treated with siRNA against CSE (si-CSE) or control siRNA (Si-Con) and incubated with or without tadalafil. Immunoblotting was done with antibodies against laminin γ1, CSE, or actin. In A–D, composite data from four to five experiments are shown in graphs; error bars represent S.E.
Generation of cGMP Is Required for Tadalafil Effect on AMPK and CSE
Because tadalafil affects the nitric oxide signaling pathway by inhibiting PDE5 and increasing cGMP (40) and our data show that it stimulates H2S generation as well, we explored the interaction between the two gasotransmitters. We first examined whether tadalafil stimulation of CSE and AMPK phosphorylation required cGMP increment by using 1H-[1,2,4]-oxadiazolo-[4,3-a]-quinoxalin-1-one (ODQ), an inhibitor of soluble guanylyl cyclase. Tadalafil significantly augmented the cGMP content in podocytes that was inhibited by ODQ (Fig. 9A). Tadalafil-induced AMPK phosphorylation was abolished by ODQ (Fig. 9B). Because tadalafil-induced AMPK phosphorylation was dependent on CSE activity (Fig. 7, A and E), we tested whether guanylyl cyclase was upstream of CSE. ODQ abolished tadalafil stimulation of CSE generation of H2S by blocking its augmented expression (Fig. 9, C and D), suggesting that intact soluble guanylyl cyclase activity was required for tadalafil generation of H2S. The ability of tadalafil to inhibit the high glucose-induced increase in laminin γ1 content was also abrogated by ODQ (Fig. 9E); ODQ by itself increased laminin γ1 expression in 5 mm glucose-treated cells. Furthermore, 8-bromo-cGMP augmented CSE expression and stimulated AMPK phosphorylation (Fig. 9, F and G), confirming the data with ODQ. These data show that in podocytes cGMP generation by soluble guanylyl cyclase is required for tadalafil induction of CSE expression/activity and reversal of high glucose-induced changes in AMPK phosphorylation and laminin content.
FIGURE 9.
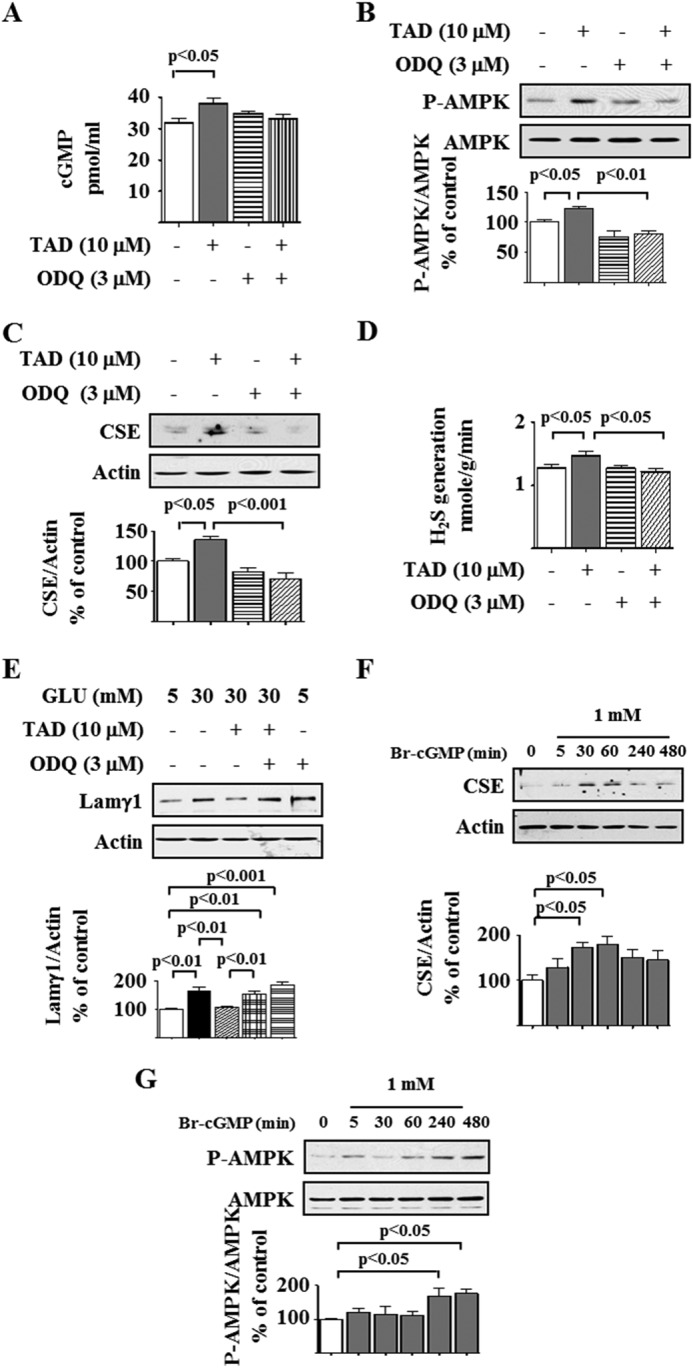
Tadalafil induction of AMPK and inhibition of high glucose-induced laminin γ1 increment requires guanylyl cyclase activation. A, cyclic GMP (cGMP) content was measured by a commercial kit. B, podocytes were preincubated with or without ODQ for 30 min followed by incubation with or without tadalafil (TAD) for 8 h. Cell lysates were immunoblotted with antibody against the indicated proteins. C, cells were preincubated with or without ODQ for 30 min followed by incubation with or without tadalafil for 1 h. Cell lysates were immunoblotted with antibodies against the indicated proteins. D, under conditions described in C, hydrogen sulfide generation was measured as described in Fig. 6A. E, cells were preincubated with ODQ for 30 min followed by incubation with tadalafil and glucose as described in Fig. 1C. Cell lysates were immunoblotted with antibody against laminin γ1 (Lamγ1) antibody and actin. F, 8-bromo-cyclic GMP (Br-cGMP) was added to the cells for the indicated duration, and immunoblotting was done with antibodies against CSE and actin. G, 8-bromo-cyclic GMP was added to the cells for the indicated duration, and immunoblotting was done with antibodies against phospho-AMPK (P-AMPK) and AMPK. In A–G, composite data from three to six experiments are shown in graphs; error bars represent S.E.
NO Generation by Inducible NOS (iNOS) Is Required for Tadalafil Effect on AMPK and CSE
We tested whether tadalafil affected events upstream of PDE5 inhibition in the NO pathway. Tadalafil has been reported to increase the expression of endothelial NOS (eNOS) in the lung (41). Preincubation with Nω-nitro-l-arginine methyl ester, a global NOS inhibitor, abrogated tadalafil stimulation of AMPK phosphorylation (Fig. 10A) and the increase in CSE expression and activity (Fig. 10, B and C). We screened for the type of NOS regulated by tadalafil. Podocytes expressed eNOS, which was not affected by tadalafil, but neuronal NOS was not detected in these cells (Fig. 10, D and E). However, tadalafil rapidly augmented the expression of iNOS at both the mRNA and protein levels (Fig. 10, F and G). Tadalafil induction of iNOS was blocked by both actinomycin D and cycloheximide, suggesting regulation at the level of iNOS transcription (Fig. 10H). The iNOS inhibitor 1400W and siRNA against iNOS significantly inhibited the tadalafil-induced increase in CSE expression and AMPK phosphorylation (Fig. 11, A–D), showing a requirement for iNOS for the tadalafil effects. The tadalafil-induced increase in iNOS expression was associated with increased NO production as assessed by the Griess assay (Fig. 11E). We evaluated the effect of H2O2, a source of free radicals, on tadalafil stimulation of AMPK; H2O2 did not affect tadalafil-induced AMPK phosphorylation (Fig. 11F). We also tested the effect of an NO scavenger, 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (42); tadalafil-induced AMPK phosphorylation was significantly reduced by 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide, showing that NO mediates tadalafil stimulation of AMPK phosphorylation (Fig. 11G). Finally, we measured H2S generation by tadalafil in podocytes and found that tadalafil significantly augmented H2S production by nearly 2-fold in cells incubated in 5 mm and high glucose media (Fig. 11H).
FIGURE 10.
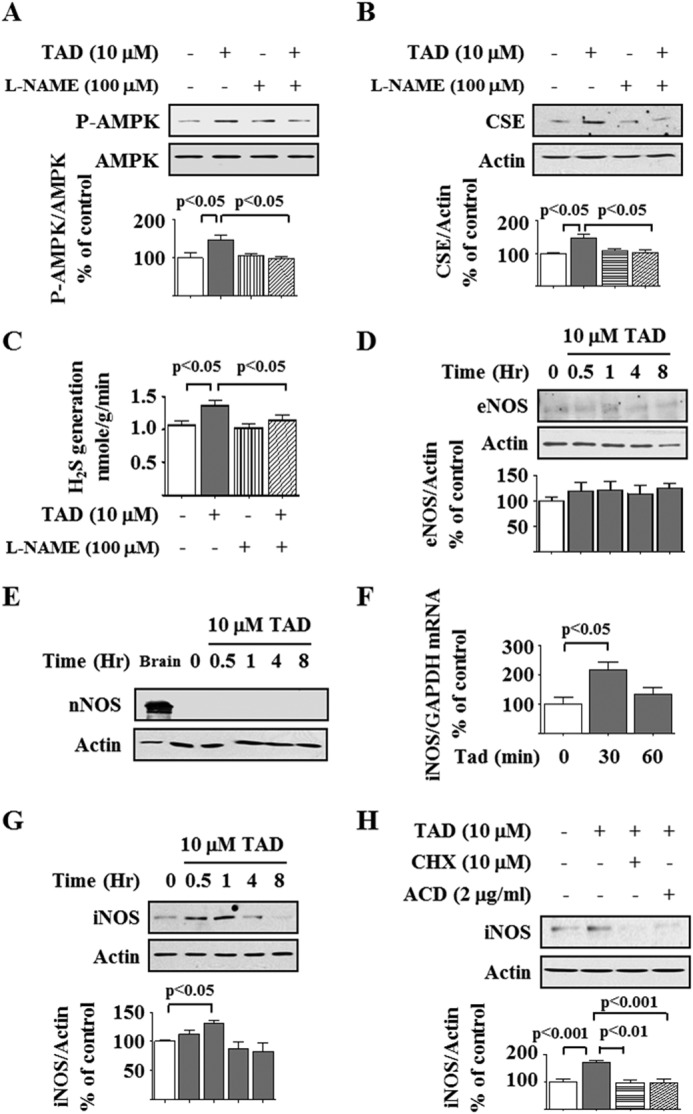
Tadalafil induction of AMPK and CSE expression requires nitric oxide generation. A and B, experimental conditions were similar to those described in Fig. 9 except preincubation with Nω-nitro-l-arginine methyl ester (L-NAME) was for 30 min. Immunoblotting was done with antibodies against phospho-AMPK (P-AMPK) and AMPK, CSE, and actin. C, under conditions described in B, H2S generation was measured. D, tadalafil (TAD) was added to the cells for the indicated duration, and immunoblotting was done with antibodies against eNOS and actin. In A–D, composite data from three to six experiments are shown in graphs; error bars represent S.E. E, immunoblotting was done with antibodies against neuronal NOS (nNOS) and actin with mouse brain as a positive control. F, following treatment with or without tadalafil, iNOS mRNA was estimated by quantitative RT-PCR. Error bars represent S.E. G, cells were treated with or without tadalafil, and iNOS protein expression was tested by immunoblotting. H, cells were incubated with tadalafil for 1 h with or without preincubation with cycloheximide (CHX) or actinomycin D (ACD) for 30 min. Cell lysate protein was immunoblotted with antibodies against the indicated proteins. In G and H, composite data from four to six experiments are shown in graphs; error bars represent S.E.
FIGURE 11.
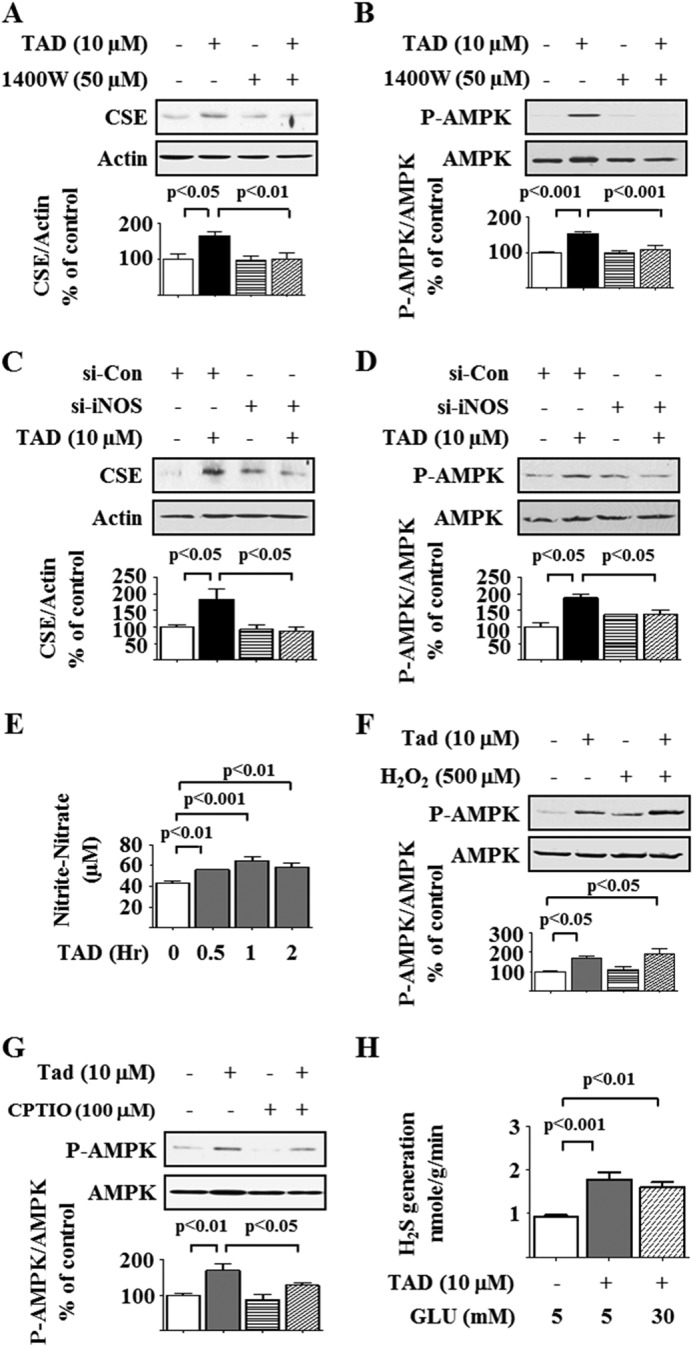
Tadalafil induction of AMPK and CSE expression depends on iNOS. A and B, mouse podocytes were incubated with tadalafil with or without 1400W, a selective iNOS inhibitor. Cell lysate protein was immunoblotted with antibodies against CSE, actin, phospho-AMPK (P-AMPK), and AMPK. C and D, rat podocytes were transfected with siRNA against iNOS (si-iNOS) or control siRNA (Si-Con) and incubated with or without tadalafil (TAD) for 4–8 h. Immunoblotting was done with antibodies against CSE, actin, phospho-AMPK, and AMPK. E, Griess reaction was performed to estimate nitrite + nitrate content in the medium following incubation with tadalafil. F, cells were incubated with tadalafil with or without H2O2 for 8 h. Cell lysate protein was immunoblotted with antibodies against phospho-AMPK and AMPK. G, cells were incubated with tadalafil for 8 h with or without preincubation for 30 min with 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (C-PTIO), an NO scavenger. Cell lysate protein was immunoblotted with antibodies against phospho-AMPK and AMPK. H, tadalafil augmented H2S generation in cells incubated in 5 or 30 mm glucose (GLU) for 1 h. In A–H, composite data from three to six experiments are shown in graphs; error bars represent S.E.
DISCUSSION
Our data show that tadalafil inhibits high glucose-induced synthesis of proteins including matrix laminin by coordinate regulation of the NO-H2S-AMPK-mTORC1 pathway (Fig. 12). Conversely, high glucose reduces AMPK activity by inhibiting H2S generation in addition to reducing AMP content as reported previously (7). Thus, tadalafil recruits and integrates signaling by two gasotransmitters to ameliorate injurious effects of high glucose in podocytes.
FIGURE 12.
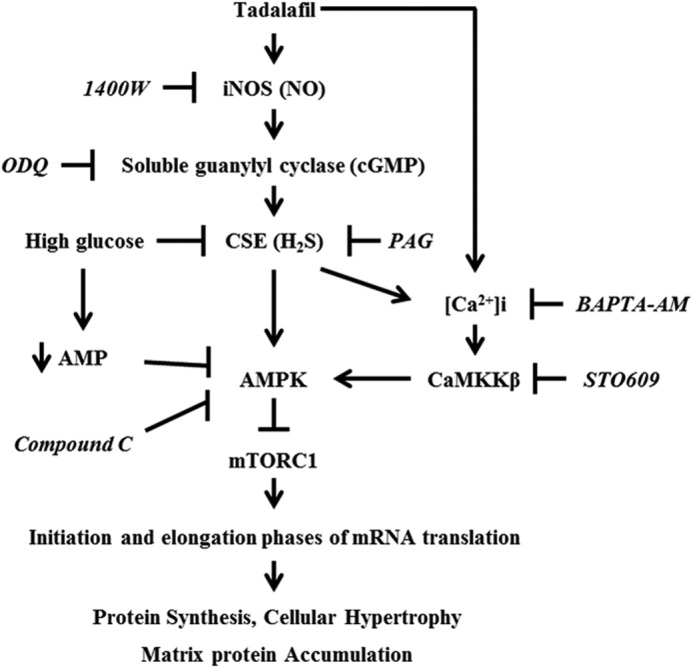
A schematic shows pathways of interaction between NO and H2S signaling pathways in podocytes incubated with tadalafil. Tadalafil stimulates NO generation by iNOS and activates soluble guanylyl cyclase to augment cGMP, which leads to an increase in CSE expression and H2S generation; H2S generation by CSE is required for tadalafil to promote phosphorylation of AMPK by calcium-calmodulin kinase kinase β (CaMKKβ), a Ca2+-dependent enzyme, and inhibit successively mTORC1 activity and events in mRNA translation, culminating in amelioration of high glucose-induced cellular hypertrophy and increase in matrix protein expression. Additionally, previous reports have shown that high glucose reduces AMP content in podocytes (7), which may also contribute to reduction in AMPK phosphorylation. BAPTA-AM, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester).
The widespread tissue distribution of PDE5 has permitted investigation of PDE5 inhibitors in diverse clinical conditions including erectile dysfunction, pulmonary hypertension, and cardiovascular diseases (43). PDE5 is expressed in the kidney and the glomerulus (22–24), suggesting that renal cells would be responsive to tadalafil. As expected, tadalafil increased cGMP content in the renal glomerular podocyte in this study. Inhibition of tadalafil regulation of CSE expression by Nω-nitro-l-arginine methyl ester in the podocyte confirmed an important regulatory role for NO in tadalafil actions. Recent reports suggest that PDE5 inhibitors act at other sites in the NO pathway. For instance, tadalafil augments the expression of eNOS in the lung (41). Sildenafil stimulates iNOS expression in cardiac myocytes and cytokine-primed vascular smooth muscle cells (44–46). PDE5 inhibitors do not promote NOS content in all cells; for example, sildenafil inhibits iNOS expression in synovial sarcoma cells and microglial cells (47, 48), suggesting that regulation of NOS by PDE5 inhibitors is cell-specific. In the current study, tadalafil stimulated iNOS expression in the podocyte. Activation of iNOS in the kidney has been implicated in inflammation and matrix protein accumulation (renal fibrosis) (49). In contrast, the novel finding in the current study is that iNOS activation by tadalafil resulted in the amelioration of high glucose-induced podocyte injury via CSE activation and AMPK phosphorylation. There are conflicting data on the role of iNOS in kidney injury in diabetes. Diabetes in iNOS knock-out mice was associated with worse renal injury as indicated by greater glomerular basement membrane thickening and worse tubule interstitial fibrosis, suggesting that iNOS is protective against diabetes-induced kidney injury (50). In contrast, kidney injury in streptozotocin-induced type 1 diabetes in rat was associated with an increase in iNOS expression, and sildenafil inhibited the iNOS increment and ameliorated kidney injury (51). However, streptozotocin may increase iNOS expression in the kidney (52), and sildenafil may reduce kidney injury by streptozotocin. iNOS appears to have a role in astragaloside-induced protection of the heart in ischemia reperfusion injury as astragaloside stimulates HIF-1α, which in turn augments iNOS expression coinciding with cardiac protection (53). Other studies in cardiac myocytes highlight the complex role of iNOS in myocardial injury in lipopolysaccharide-induced endotoxemia. Whereas iNOS activation in the infiltrating leukocytes inhibits cardiomyocyte contraction via oxidative stress, the activity of iNOS in the cardiomyocyte is important for its adaptive increased contraction in response to adrenergic stimulation in endotoxemia (54). These data highlight the importance of context and cellular source of iNOS in assessing whether it plays an injurious or beneficial role in tissue injury. eNOS is thought to play a protective role in diabetes because mice that lack eNOS manifest greater kidney injury in both type 1 and type 2 diabetes (55, 56); however, tadalafil did not alter eNOS expression in our study. Because our studies were limited to cultured cells, the role of iNOS needs to be critically tested in tadalafil-treated animals with spontaneous diabetes. It is important to evaluate whether kidney-specific overexpression of iNOS protects against diabetes-induced kidney injury. Nitric oxide binds to the heme moiety of soluble guanylyl cyclase, resulting in an increase in its activity to generate cGMP (57). Studies with ODQ suggested the requirement of soluble guanylyl cyclase activity for tadalafil induction of CSE. 8-Bromo-cGMP directly augmented CSE expression, suggesting that cGMP mediates tadalafil action on CSE.
The current study in the podocyte showed that tadalafil integrates the pathways of two gasotransmitters, NO and H2S, to inhibit high glucose-induced protein synthesis. The interaction between H2S and NO has drawn considerable attention recently (21). In some instances, NO is upstream of H2S generation as was the case in our study. Similarly, in the heart, tadalafil protection against ischemia reperfusion injury was abolished by the absence of CSE, suggesting mediation by H2S (20). Conversely, H2S has been shown to activate eNOS via the VEGF-Akt axis in the failing heart (58). Angiogenesis and promotion of wound healing by H2S requires eNOS activation (59). H2S can post-translationally modify eNOS by S-sulfhydration of Cys-443, leading to stabilization of its dimers, thus facilitating NO production (60). H2S increased cGMP and activated PKG in endothelial cells (59), leading the authors to suggest that NO and H2S converge at cGMP in endothelial cells. It is evident from the above that the interaction between NO and H2S varies with the cell type and context.
An important observation to emerge from this study is that tadalafil augments H2S generation by increasing CSE expression by augmenting translation of its mRNA. There is a limited understanding of factors regulating CSE transcription. The CSE gene is 1.8 kb in size with 12 exons with rich expression in the kidney (16). Dietary restriction of cysteine augments CSE expression in the liver; because this is blocked in the liver-specific tuberous sclerosis knock-out mice that have constitutive mTORC1 activation, the latter appears to inhibit CSE gene expression in the dietary restriction model (61). The transcription factor Sp1 regulates CSE gene expression in vascular smooth muscle cells (62). miR-21 is an important negative regulator of Sp1 expression and thus can indirectly affect CSE transcription (63). In colon carcinoma cells, β-catenin binds to the promoter of CSE to enhance its expression, which in turn augments cell proliferation (64). In the current study, CSE-specific inhibition showed that nearly all the increment in H2S generation by tadalafil in the podocyte could be accounted for by the increase in CSE. Because CSE content of the kidney is reduced in diabetic nephropathy (12, 18), H2S deficiency likely contributes to kidney injury in diabetes. NaHS, an H2S donor, ameliorated albuminuria and the increase in matrix collagen protein in rats with type 1 diabetes (18); however, the signaling mechanisms involved were not explored. From a clinical translation perspective, NaHS is not suitable for human consumption. Our studies suggest that PDE5 inhibitors may serve as H2S donors. The effect of PDE5 inhibitors has been investigated in animal models of diabetic nephropathy. The PDE5 inhibitors sildenafil and vardenafil ameliorated matrix accumulation and albuminuria in rats with streptozotocin-induced type 1 or spontaneous type 2 diabetes (51, 65, 66). However, these studies did not explore whether H2S was involved in the salutary effects of PDE5 inhibitors.
Our study demonstrates that tadalafil activates AMPK by recruiting H2S. Tadalafil-induced AMPK inhibited high glucose-induced mTORC1 activation and events in mRNA translation leading to matrix protein synthesis similarly to our previous report on H2S (12). We now add PDE5 inhibitors to the list of agents stimulating AMPK that includes adiponectin, metformin, 5-aminoimidazole-4-carboxamide ribonucleotide, and resveratrol (7–10, 67). A limitation of our study is the lack of in vivo data to assess the need for H2S generation in tadalafil amelioration of diabetic kidney injury. Most of the studies on diabetic kidney injury using H2S donors including PDE5 inhibitors have been of short duration. It remains to be seen whether these interventions will result in long term amelioration of kidney injury in diabetes. For instance, early diabetes is associated with AMPK inhibition and activation of Akt and mTOR in the kidney (6, 7), whereas at a longer duration of diabetes these signaling pathways may not be activated (68, 69); thus, agents that activate AMPK and inhibit mTOR may be effective in the early but not in the late phase of diabetes. Additionally, H2S is not beneficial in all models of renal injury as its effect can vary with the context (70). Whereas H2S ameliorates renin-induced hypertension (71), ischemia-reperfusion injury in the kidney and heart (17, 20), obstructive kidney injury (72), preeclampsia (73), and hyperhomocysteinemia-induced chronic kidney disease (74) and protects endothelium against high glucose (75), it assumes a pathologic role as a mediator in cisplatinum-induced kidney cell injury (76), streptozotocin-induced pancreatic β cell injury (77), and colon carcinoma (78). These considerations suggest that a critical evaluation of PDE5 inhibitors and H2S donors should be done in long term models of kidney injury in diabetes. If found beneficial in animal models, PDE5 inhibitors can be rapidly evaluated in clinical trials in diabetic kidney disease because they are already approved for use in other disorders such as erectile dysfunction.
Acknowledgments
We thank Dr. Dan Riley for critical reading of the manuscript and Dr. Doug Yoon Lee for helpful suggestions. Images were generated in the Core Optical Imaging Facility, which is supported by the University of Texas Health Science Center at San Antonio (UTHSCSA) and National Institutes of Health Grants P30 CA54174 from the National Cancer Institute (to the Cancer Therapy and Research Center at UTHSCSA) and P01AG19316 from the National Institute on Aging.
This work was supported, in whole or in part, by National Institutes of Health Grants DK077295 (to B. S. K.), RC2AG036613 (to B. S. K.), DK050190 (to G. G. C.), and DK079996 (to Y. G.). This work was also supported by the Veterans Affairs Research Service (to B. S. K. and G. G. C.) and the Juvenile Diabetes Research Foundation (to D. F., G. G. C., and Y. G.).
We dedicate this work to the memory of Dr. Hanna E. Abboud, Director, Division of Nephrology, University of Texas Health Science Center at San Antonio. He unexpectedly passed away on January 7, 2015. His encouragement was vital for the completion of this work.
- mTORC1
- mechanistic target of rapamycin complex 1
- PDE5
- phosphodiesterase 5
- AMPK
- AMP-activated protein kinase
- CSE
- cystathionine γ-lyase
- ODQ
- 1H-[1,2,4]-oxadiazolo-[4,3-a]-quinoxalin-1-one
- iNOS
- inducible NOS
- 4E-BP1
- eukaryotic translation initiation factor 4E-binding protein 1
- eEF2
- eukaryotic elongation factor 2
- PAG
- dl-propargylglycine
- eNOS
- endothelial NOS
- mTOR
- mechanistic target of rapamycin.
REFERENCES
- 1. Kanwar Y. S., Sun L., Xie P., Liu F. Y., Chen S. (2011) A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu. Rev. Pathol. 6, 395–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sanchez A. P., Sharma K. (2009) Transcription factors in the pathogenesis of diabetic nephropathy. Expert Rev. Mol. Med. 11, e13. [DOI] [PubMed] [Google Scholar]
- 3. Kasinath B. S., Mariappan M. M., Sataranatarajan K., Lee M. J., Feliers D. (2006) mRNA translation: unexplored territory in renal science. J. Am. Soc. Nephrol. 17, 3281–3292 [DOI] [PubMed] [Google Scholar]
- 4. Mariappan M. M., Feliers D., Mummidi S., Choudhury G. G., Kasinath B. S. (2007) High glucose, high insulin, and their combination rapidly induce laminin-β1 synthesis by regulation of mRNA translation in renal epithelial cells. Diabetes 56, 476–485 [DOI] [PubMed] [Google Scholar]
- 5. Mariappan M. M. (2012) Signaling mechanisms in the regulation of renal matrix metabolism in diabetes. Exp. Diabetes Res. 2012, 749812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feliers D., Duraisamy S., Faulkner J. L., Duch J., Lee A. V., Abboud H. E., Choudhury G. G., Kasinath B. S. (2001) Activation of renal signaling pathways in db/db mice with type 2 diabetes. Kidney Int. 60, 495–504 [DOI] [PubMed] [Google Scholar]
- 7. Lee M. J., Feliers D., Mariappan M. M., Sataranatarajan K., Mahimainathan L., Musi N., Foretz M., Viollet B., Weinberg J. M., Choudhury G. G., Kasinath B. S. (2007) A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am. J. Physiol. Renal Physiol. 292, F617–F627 [DOI] [PubMed] [Google Scholar]
- 8. Sharma K., Ramachandrarao S., Qiu G., Usui H. K., Zhu Y., Dunn S. R., Ouedraogo R., Hough K., McCue P., Chan L., Falkner B., Goldstein B. J. (2008) Adiponectin regulates albuminuria and podocyte function in mice. J. Clin. Investig. 118, 1645–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dugan L. L., You Y. H., Ali S. S., Diamond-Stanic M., Miyamoto S., DeCleves A. E., Andreyev A., Quach T., Ly S., Shekhtman G., Nguyen W., Chepetan A., Le T. P., Wang L., Xu M., Paik K. P., Fogo A., Viollet B., Murphy A., Brosius F., Naviaux R. K., Sharma K. (2013) AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J. Clin. Investig. 123, 4888–4899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eid A. A., Ford B. M., Block K., Kasinath B. S., Gorin Y., Ghosh-Choudhury G., Barnes J. L., Abboud H. E. (2010) AMP-activated protein kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. J. Biol. Chem. 285, 37503–37512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mariappan M. M., Shetty M., Sataranatarajan K., Choudhury G. G., Kasinath B. S. (2008) Glycogen synthase kinase 3β is a novel regulator of high glucose- and high insulin-induced extracellular matrix protein synthesis in renal proximal tubular epithelial cells. J. Biol. Chem. 283, 30566–30575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee H. J., Mariappan M. M., Feliers D., Cavaglieri R. C., Sataranatarajan K., Abboud H. E., Choudhury G. G., Kasinath B. S. (2012) Hydrogen sulfide inhibits high glucose-induced matrix protein synthesis by activating AMP-activated protein kinase in renal epithelial cells. J. Biol. Chem. 287, 4451–4461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abe K., Kimura H. (1996) The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 16, 1066–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang G., Wu L., Jiang B., Yang W., Qi J., Cao K., Meng Q., Mustafa A. K., Mu W., Zhang S., Snyder S. H., Wang R. (2008) H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine γ-lyase. Science 322, 587–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stipanuk M. H., Beck P. W. (1982) Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem. J. 206, 267–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ishii I., Akahoshi N., Yu X. N., Kobayashi Y., Namekata K., Komaki G., Kimura H. (2004) Murine cystathionine γ-lyase: complete cDNA and genomic sequences, promoter activity, tissue distribution and developmental expression. Biochem. J. 381, 113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bos E. M., Wang R., Snijder P. M., Boersema M., Damman J., Fu M., Moser J., Hillebrands J. L., Ploeg R. J., Yang G., Leuvenink H. G., van Goor H. (2013) Cystathionine γ-lyase protects against renal ischemia/reperfusion by modulating oxidative stress. J. Am. Soc. Nephrol. 24, 759–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yuan P., Xue H., Zhou L., Qu L., Li C., Wang Z., Ni J., Yu C., Yao T., Huang Y., Wang R., Lu L. (2011) Rescue of mesangial cells from high glucose-induced over-proliferation and extracellular matrix secretion by hydrogen sulfide. Nephrol. Dial. Transplant. 26, 2119–2126 [DOI] [PubMed] [Google Scholar]
- 19. Yamamoto J., Sato W., Kosugi T., Yamamoto T., Kimura T., Taniguchi S., Kojima H., Maruyama S., Imai E., Matsuo S., Yuzawa Y., Niki I. (2013) Distribution of hydrogen sulfide (H2S)-producing enzymes and the roles of the H2S donor sodium hydrosulfide in diabetic nephropathy. Clin. Exp. Nephrol. 17, 32–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Salloum F. N., Chau V. Q., Hoke N. N., Abbate A., Varma A., Ockaili R. A., Toldo S., Kukreja R. C. (2009) Phosphodiesterase-5 inhibitor, tadalafil, protects against myocardial ischemia/reperfusion through protein-kinase G-dependent generation of hydrogen sulfide. Circulation 120, S31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang R. (2012) Shared signaling pathways among gasotransmitters. Proc. Natl. Acad. Sci. U.S.A. 109, 8801–8802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matousovic K., Tsuboi Y., Walker H., Grande J. P., Dousa T. P. (1997) Inhibitors of cyclic nucleotide phosphodiesterase isozymes block renal tubular cell proliferation induced by folic acid. J. Lab. Clin. Med. 130, 487–495 [DOI] [PubMed] [Google Scholar]
- 23. Kotera J., Fujishige K., Omori K. (2000) Immunohistochemical localization of cGMP-binding cGMP-specific phosphodiesterase (PDE5) in rat tissues. J. Histochem. Cytochem. 48, 685–693 [DOI] [PubMed] [Google Scholar]
- 24. Hohenstein B., Daniel C., Wittmann S., Hugo C. (2008) PDE-5 inhibition impedes TSP-1 expression, TGF-β activation and matrix accumulation in experimental glomerulonephritis. Nephrol. Dial. Transplant. 23, 3427–3436 [DOI] [PubMed] [Google Scholar]
- 25. Mundel P., Reiser J., Zúñiga Mejía Borja A., Pavenstädt H., Davidson G. R., Kriz W., Zeller R. (1997) Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp. Cell Res. 236, 248–258 [DOI] [PubMed] [Google Scholar]
- 26. Sataranatarajan K., Feliers D., Mariappan M. M., Lee H. J., Lee M. J., Day R. T., Yalamanchili H. B., Choudhury G. G., Barnes J. L., Van Remmen H., Richardson A., Kasinath B. S. (2012) Molecular events in matrix protein metabolism in the aging kidney. Aging Cell 11, 1065–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mok Y. Y., Atan M. S., Yoke Ping C., Zhong Jing W., Bhatia M., Moochhala S., Moore P. K. (2004) Role of hydrogen sulphide in haemorrhagic shock in the rat: protective effect of inhibitors of hydrogen sulphide biosynthesis. Br. J. Pharmacol. 143, 881–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gharavi N., El-Kadi A. O. (2003) Measurement of nitric oxide in murine Hepatoma Hepa1c1c7 cells by reversed phase HPLC with fluorescence detection. J. Pharm. Pharm. Sci. 6, 302–307 [PubMed] [Google Scholar]
- 29. Courtoy P. J., Timpl R., Farquhar M. G. (1982) Comparative distribution of laminin, type IV collagen, and fibronectin in the rat glomerulus. J. Histochem. Cytochem. 30, 874–886 [DOI] [PubMed] [Google Scholar]
- 30. Jerums G., Panagiotopoulos S., MacIsaac R. (2006) in The Diabetic Kidney (Cortes P., Mogensen E., eds) pp. 59–81, Humana Press, Totowa, NJ [Google Scholar]
- 31. Kasinath B. S., Feliers D., Sataranatarajan K., Ghosh Choudhury G., Lee M. J., Mariappan M. M. (2009) Regulation of mRNA translation in renal physiology and disease. Am. J. Physiol. Renal Physiol. 297, F1153–F1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Holz M. K., Ballif B. A., Gygi S. P., Blenis J. (2005) mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell 123, 569–580 [DOI] [PubMed] [Google Scholar]
- 33. Redpath N. T., Foulstone E. J., Proud C. G. (1996) Regulation of translation elongation factor-2 by insulin via a rapamycin-sensitive signalling pathway. EMBO J. 15, 2291–2297 [PMC free article] [PubMed] [Google Scholar]
- 34. Nairn A. C., Palfrey H. C. (1987) Identification of the major Mr 100,000 substrate for calmodulin-dependent protein kinase III in mammalian cells as elongation factor-2. J. Biol. Chem. 262, 17299–17303 [PubMed] [Google Scholar]
- 35. Blättler S. M., Rencurel F., Kaufmann M. R., Meyer U. A. (2007) In the regulation of cytochrome P450 genes, phenobarbital targets LKB1 for necessary activation of AMP-activated protein kinase. Proc. Natl. Acad. Sci. U.S.A. 104, 1045–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ang S. F., Sio S. W., Moochhala S. M., MacAry P. A., Bhatia M. (2011) Hydrogen sulfide upregulates cyclooxygenase-2 and prostaglandin E metabolite in sepsis-evoked acute lung injury via transient receptor potential vanilloid type 1 channel activation. J. Immunol. 187, 4778–4787 [DOI] [PubMed] [Google Scholar]
- 37. Tripatara P., Patel N. S., Collino M., Gallicchio M., Kieswich J., Castiglia S., Benetti E., Stewart K. N., Brown P. A., Yaqoob M. M., Fantozzi R., Thiemermann C. (2008) Generation of endogenous hydrogen sulfide by cystathionine γ-lyase limits renal ischemia/reperfusion injury and dysfunction. Lab. Invest. 88, 1038–1048 [DOI] [PubMed] [Google Scholar]
- 38. Fonseca B. D., Smith E. M., Yelle N., Alain T., Bushell M., Pause A. (2014) The ever-evolving role of mTOR in translation. Semin. Cell Dev. Biol. 36, 102–112 [DOI] [PubMed] [Google Scholar]
- 39. Thoreen C. C., Chantranupong L., Keys H. R., Wang T., Gray N. S., Sabatini D. M. (2012) A unifying model for mTORC1-mediated regulation of mRNA translation. Nature 485, 109–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Blount M. A., Beasley A., Zoraghi R., Sekhar K. R., Bessay E. P., Francis S. H., Corbin J. D. (2004) Binding of tritiated sildenafil, tadalafil, or vardenafil to the phosphodiesterase-5 catalytic site displays potency, specificity, heterogeneity, and cGMP stimulation. Mol. Pharmacol. 66, 144–152 [DOI] [PubMed] [Google Scholar]
- 41. Shue E. H., Schecter S. C., Gong W., Etemadi M., Johengen M., Iqbal C., Derderian S. C., Oishi P., Fineman J. R., Miniati D. (2014) Antenatal maternally-administered phosphodiesterase type 5 inhibitors normalize eNOS expression in the fetal lamb model of congenital diaphragmatic hernia. J. Pediatr. Surg. 49, 39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wangsiripaisan A., Gengaro P. E., Nemenoff R. A., Ling H., Edelstein C. L., Schrier R. W. (1999) Effect of nitric oxide donors on renal epithelial cell-matrix adhesion. Kidney Int. 55, 2281–2288 [DOI] [PubMed] [Google Scholar]
- 43. Chrysant S. G. (2013) Effectiveness and safety of phosphodiesterase 5 inhibitors in patients with cardiovascular disease and hypertension. Curr. Hypertens. Rep. 15, 475–483 [DOI] [PubMed] [Google Scholar]
- 44. Salloum F., Yin C., Xi L., Kukreja R. C. (2003) Sildenafil induces delayed preconditioning through inducible nitric oxide synthase-dependent pathway in mouse heart. Circ. Res. 92, 595–597 [DOI] [PubMed] [Google Scholar]
- 45. Das A., Xi L., Kukreja R. C. (2005) Phosphodiesterase-5 inhibitor sildenafil preconditions adult cardiac myocytes against necrosis and apoptosis. Essential role of nitric oxide signaling. J. Biol. Chem. 280, 12944–12955 [DOI] [PubMed] [Google Scholar]
- 46. Liu X. M., Peyton K. J., Wang X., Durante W. (2012) Sildenafil stimulates the expression of gaseous monoxide-generating enzymes in vascular smooth muscle cells via distinct signaling pathways. Biochem. Pharmacol. 84, 1045–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim K. O., Park S. Y., Han C. W., Chung H. K., Yoo D. H., Ryu D. H., Han J. S. (2008) Effect of sildenafil citrate on interleukin-1β-induced nitric oxide synthesis and iNOS expression in SW982 cells. Exp. Mol. Med. 40, 286–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhao S., Zhang L., Lian G., Wang X., Zhang H., Yao X., Yang J., Wu C. (2011) Sildenafil attenuates LPS-induced pro-inflammatory responses through down-regulation of intracellular ROS-related MAPK/NF-κB signaling pathways in N9 microglia. Int. Immunopharmacol. 11, 468–474 [DOI] [PubMed] [Google Scholar]
- 49. Fanton d'Andon M., Quellard N., Fernandez B., Ratet G., Lacroix-Lamandé S., Vandewalle A., Boneca I. G., Goujon J. M., Werts C. (2014) Leptospira interrogans induces fibrosis in the mouse kidney through iNOS-dependent, TLR- and NLR-independent signaling pathways. PLoS Negl. Trop. Dis. 8, e2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Trachtman H., Futterweit S., Pine E., Mann J., Valderrama E. (2002) Chronic diabetic nephropathy: role of inducible nitric oxide synthase. Pediatr. Nephrol. 17, 20–29 [DOI] [PubMed] [Google Scholar]
- 51. Jeong K. H., Lee T. W., Ihm C. G., Lee S. H., Moon J. Y., Lim S. J. (2009) Effects of sildenafil on oxidative and inflammatory injuries of the kidney in streptozotocin-induced diabetic rats. Am. J. Nephrol. 29, 274–282 [DOI] [PubMed] [Google Scholar]
- 52. Aktug H., Cetintas V. B., Kosova B., Oltulu F., Baktidemiray S., Cavusoglu T., Akarca S. O., Yavasoglu A. (2012) Dysregulation of nitric oxide synthase activity and Bcl-2 and caspase-3 gene expression in renal tissue of streptozotocin-induced diabetic rats. Turk. J. Med. Sci. 42, 830–838 [Google Scholar]
- 53. Si J., Wang N., Wang H., Xie J., Yang J., Yi H., Shi Z., Ma J., Wang W., Yang L., Yu S., Li J. (2014) HIF-1α signaling activation by post-ischemia treatment with astragaloside IV attenuates myocardial ischemia-reperfusion injury. PLoS One 9, e107832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Poon B. Y., Raharjo E., Patel K. D., Tavener S., Kubes P. (2003) Complexity of inducible nitric oxide synthase: cellular source determines benefit versus toxicity. Circulation 108, 1107–1112 [DOI] [PubMed] [Google Scholar]
- 55. Kanetsuna Y., Takahashi K., Nagata M., Gannon M. A., Breyer M. D., Harris R. C., Takahashi T. (2007) Deficiency of endothelial nitric-oxide synthase confers susceptibility to diabetic nephropathy in nephropathy-resistant inbred mice. Am. J. Pathol. 170, 1473–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhao H. J., Wang S., Cheng H., Zhang M. Z., Takahashi T., Fogo A. B., Breyer M. D., Harris R. C. (2006) Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. J. Am. Soc. Nephrol. 17, 2664–2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stone J. R., Marletta M. A. (1994) Soluble guanylate cyclase from bovine lung: activation with nitric oxide and carbon monoxide and spectral characterization of the ferrous and ferric states. Biochemistry 33, 5636–5640 [DOI] [PubMed] [Google Scholar]
- 58. Kondo K., Bhushan S., King A. L., Prabhu S. D., Hamid T., Koenig S., Murohara T., Predmore B. L., Gojon G., Sr., Gojon G., Jr., Wang R., Karusula N., Nicholson C. K., Calvert J. W., Lefer D. J. (2013) H2S protects against pressure overload-induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation 127, 1116–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Coletta C., Papapetropoulos A., Erdelyi K., Olah G., Módis K., Panopoulos P., Asimakopoulou A., Gerö D., Sharina I., Martin E., Szabo C. (2012) Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc. Natl. Acad. Sci. U.S.A. 109, 9161–9166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Altaany Z., Ju Y., Yang G., Wang R. (2014) The coordination of S-sulfhydration, S-nitrosylation, and phosphorylation of endothelial nitric oxide synthase by hydrogen sulfide. Sci. Signal. 7, ra87. [DOI] [PubMed] [Google Scholar]
- 61. Hine C., Harputlugil E., Zhang Y., Ruckenstuhl C., Lee B. C., Brace L., Longchamp A., Treviño-Villarreal J. H., Mejia P., Ozaki C. K., Wang R., Gladyshev V. N., Madeo F., Mair W. B., Mitchell J. R. (2015) Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell 160, 132–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yang G., Pei Y., Teng H., Cao Q., Wang R. (2011) Specificity protein-1 as a critical regulator of human cystathionine gamma-lyase in smooth muscle cells. J. Biol. Chem. 286, 26450–26460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yang G., Pei Y., Cao Q., Wang R. (2012) MicroRNA-21 represses human cystathionine γ-lyase expression by targeting at specificity protein-1 in smooth muscle cells. J. Cell. Physiol. 227, 3192–3200 [DOI] [PubMed] [Google Scholar]
- 64. Fan K., Li N., Qi J., Yin P., Zhao C., Wang L., Li Z., Zha X. (2014) Wnt/β-catenin signaling induces the transcription of cystathionine-γ-lyase, a stimulator of tumor in colon cancer. Cell. Signal. 26, 2801–2808 [DOI] [PubMed] [Google Scholar]
- 65. Fang L., Radovits T., Szabó G., Mózes M. M., Rosivall L., Kökény G. (2013) Selective phosphodiesterase-5 (PDE-5) inhibitor vardenafil ameliorates renal damage in type 1 diabetic rats by restoring cyclic 3′,5′ guanosine monophosphate (cGMP) level in podocytes. Nephrol. Dial. Transplant. 28, 1751–1761 [DOI] [PubMed] [Google Scholar]
- 66. Kuno Y., Iyoda M., Shibata T., Hirai Y., Akizawa T. (2011) Sildenafil, a phosphodiesterase type 5 inhibitor, attenuates diabetic nephropathy in non-insulin-dependent Otsuka Long-Evans Tokushima Fatty rats. Br. J. Pharmacol. 162, 1389–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lee M. J., Feliers D., Sataranatarajan K., Mariappan M. M., Li M., Barnes J. L., Choudhury G. G., Kasinath B. S. (2010) Resveratrol ameliorates high glucose-induced protein synthesis in glomerular epithelial cells. Cell. Signal. 22, 65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zdychová J., Veselá J., Kazdová L., Komers R. (2008) Renal activity of Akt kinase in experimental type 1 diabetes. Physiol. Res. 57, 709–715 [DOI] [PubMed] [Google Scholar]
- 69. Sun W., Wang Y., Miao X., Wang Y., Zhang L., Xin Y., Zheng S., Epstein P. N., Fu Y., Cai L. (2014) Renal improvement by zinc in diabetic mice is associated with glucose metabolism signaling mediated by metallothionein and Akt, but not Akt2. Free Radic. Biol. Med. 68, 22–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kasinath B. S. (2014) Hydrogen sulfide to the rescue in obstructive kidney injury. Kidney Int. 85, 1255–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lu M., Liu Y. H., Goh H. S., Wang J. J., Yong Q. C., Wang R., Bian J. S. (2010) Hydrogen sulfide inhibits plasma renin activity. J. Am. Soc. Nephrol. 21, 993–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Song K., Wang F., Li Q., Shi Y. B., Zheng H. F., Peng H., Shen H. Y., Liu C. F., Hu L. F. (2014) Hydrogen sulfide inhibits the renal fibrosis of obstructive nephropathy. Kidney Int. 85, 1318–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Holwerda K. M., Burke S. D., Faas M. M., Zsengeller Z., Stillman I. E., Kang P. M., van Goor H., McCurley A., Jaffe I. Z., Karumanchi S. A., Lely A. T. (2014) Hydrogen sulfide attenuates sFlt1-induced hypertension and renal damage by upregulating vascular endothelial growth factor. J. Am. Soc. Nephrol. 25, 717–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sen U., Basu P., Abe O. A., Givvimani S., Tyagi N., Metreveli N., Shah K. S., Passmore J. C., Tyagi S. C. (2009) Hydrogen sulfide ameliorates hyperhomocysteinemia-associated chronic renal failure. Am. J. Physiol. Renal Physiol. 297, F410–F419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Suzuki K., Olah G., Modis K., Coletta C., Kulp G., Gerö D., Szoleczky P., Chang T., Zhou Z., Wu L., Wang R., Papapetropoulos A., Szabo C. (2011) Hydrogen sulfide replacement therapy protects the vascular endothelium in hyperglycemia by preserving mitochondrial function. Proc. Natl. Acad. Sci. U.S.A. 108, 13829–13834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Della Coletta Francescato H., Cunha F. Q., Costa R. S., Barbosa Júnior F., Boim M. A., Arnoni C. P., da Silva C. G., Coimbra T. M. (2011) Inhibition of hydrogen sulphide formation reduces cisplatin-induced renal damage. Nephrol Dial Transplant 26, 479–488 [DOI] [PubMed] [Google Scholar]
- 77. Yang G., Tang G., Zhang L., Wu L., Wang R. (2011) The pathogenic role of cystathionine γ-lyase/hydrogen sulfide in streptozotocin-induced diabetes in mice. Am. J. Pathol. 179, 869–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Szabo C., Coletta C., Chao C., Módis K., Szczesny B., Papapetropoulos A., Hellmich M. R. (2013) Tumor-derived hydrogen sulfide, produced by cystathionine-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc. Natl. Acad. Sci. U.S.A. 110, 12474–12479 [DOI] [PMC free article] [PubMed] [Google Scholar]