Background: A structural module following the KMSKS catalytic loop is conserved in most class I synthetases.
Results: This module contributes to aminoacylation and editing of leucyl-tRNA synthetases (LeuRS).
Conclusion: This module affects the activities of LeuRS in both a structure- and sequence-dependent manner.
Significance: This work further extends the function of stem-contact fold in LeuRS.
Keywords: aminoacyl tRNA Synthetase, enzyme, evolution, protein synthesis, transfer RNA (tRNA), aminoacylation, editing, stem contact fold
Abstract
A conserved structural module following the KMSKS catalytic loop exhibits α-α-β-α topology in class Ia and Ib aminoacyl-tRNA synthetases. However, the function of this domain has received little attention. Here, we describe the effect this module has on the aminoacylation and editing capacities of leucyl-tRNA synthetases (LeuRSs) by characterizing the key residues from various species. Mutation of highly conserved basic residues on the third α-helix of this domain impairs the affinity of LeuRS for the anticodon stem of tRNALeu, which decreases both aminoacylation and editing activities. Two glycine residues on this α-helix contribute to flexibility, leucine activation, and editing of LeuRS from Escherichia coli (EcLeuRS). Acidic residues on the β-strand enhance the editing activity of EcLeuRS and sense the size of the tRNALeu D-loop. Incorporation of these residues stimulates the tRNA-dependent editing activity of the chimeric minimalist enzyme Mycoplasma mobile LeuRS fused to the connective polypeptide 1 editing domain and leucine-specific domain from EcLeuRS. Together, these results reveal the stem contact-fold to be a functional as well as a structural linker between the catalytic site and the tRNA binding domain. Sequence comparison of the EcLeuRS stem contact-fold domain with editing-deficient enzymes suggests that key residues of this module have evolved an adaptive strategy to follow the editing functions of LeuRS.
Introduction
Aminoacyl-tRNA synthetases (aaRSs)2 are a large and diverse family of enzymes that catalyze the attachment of amino acids to their cognate tRNAs in a two-step aminoacylation reaction as follows: 1) amino acid activation by ATP hydrolysis to form an aminoacyl-adenylate intermediate, and 2) transfer of the aminoacyl moiety from the intermediate to the cognate tRNA isoacceptor to form aminoacyl-tRNA (aa-tRNA) (1–3). Based on sequence homology and the structures of the catalytic active sites, aaRSs are divided into two classes of 10 members each. Class I synthetases are further divided into three subclasses, a, b, and c, according to sequence homology (4–6). Leucyl-tRNA synthetase (LeuRS) belongs to class I aaRSs that include a typical Rossmann dinucleotide-binding fold active site architecture with the signature sequence modules HIGH and KMSKS (6). According to evolutionary models, the primitive catalytic core is extended by the insertion and/or fusion of additional domains (also called modules) in LeuRSs (7), most of which have inserted a large connective polypeptide 1 (CP1) domain that is responsible for amino acid editing. To ensure translation accuracy, LeuRSs have evolved a mechanism to remove aminoacyl AMP (aa-AMP; pre-transfer editing) and aa-tRNA (post-transfer editing) (8). Although post-transfer editing is carried out by the CP1 domain in most LeuRSs, this domain has been naturally deleted in LeuRS from Mycoplasma mobile (MmLeuRS) (9), and MmLeuRS is therefore unable to maintain its catalytic fidelity in post-transfer editing functions. However, once the CP1 domain of LeuRS from Escherichia coli (EcLeuRS) is inserted into MmLeuRS, the engineered enzyme functions like a typical prokaryotic LeuRS with tRNA-dependent editing activity (10).
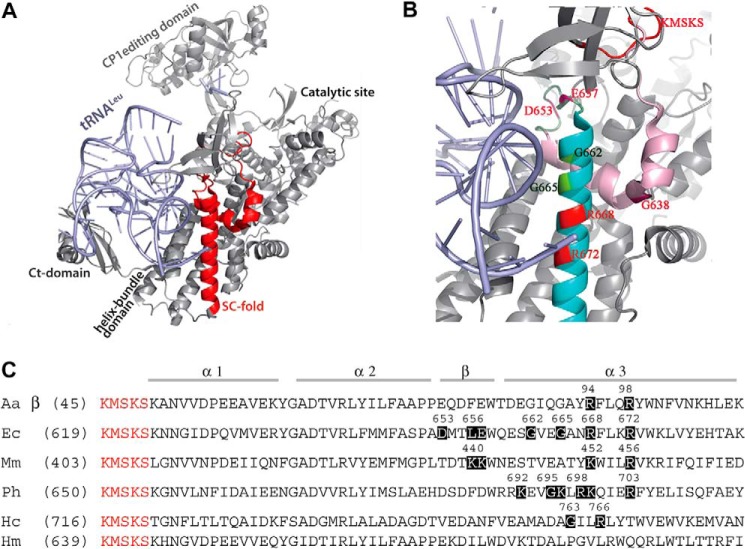
Additionally, LeuRSs recruit the tRNA anticodon binding domain to bind tRNALeu isoacceptors (11, 12). The refined crystal structure of the EcLeuRS and tRNALeu complex revealed a tRNA anticodon binding domain composed of a cylindrical α-helical bundle domain of five helices that is common to all class Ia synthetases. EcLeuRS establishes nonspecific backbone contacts between tRNALeu nucleotides 12, 13, 22–26, and 42 with amino acid residues 667–686 and 749–760 of enzyme (PDB entry 4AQ7 and 4ARC, see Ref. 11). Interestingly, a highly conserved structural module following the KMSKS loop exhibits the characteristic α-α-β-α topology in most class Ia and Ib aaRSs (Fig. 1A). Both the KMSKS loop and this structural module constitute the stem contact (SC)-fold (13–18). The last α-helix of the SC-fold is almost coaxial to the first α-helix of the helix bundle domain but is separated by a short linker segment in most class I synthetases (13). However, the SC-fold of LeuRS lacks this segment, and its last α-helix instead overlaps with the first α-helix of the helix bundle domain (Fig. 1A). A previous study suggested that the effect of this class Ia/b SC-fold domain on tRNA recognition is structure-dependent rather than sequence-dependent, because sequence is much less conserved than structure in this case (17). Furthermore, the SC-fold is likely to dock against the inner side of the L-shaped tRNA, thereby positioning the anticodon stem (13).
FIGURE 1.
Structure of the SC-fold and alignment of LeuRSs from different species. A, crystal structure of tRNAUAALeu (light blue) in complex with EcLeuRS (gray) in the editing conformation (PDB entry 4ARC). The major domains of LeuRS were labeled, and SC-fold is highlighted in red. B, residues of the third helix (α3; cyan) and β-strand (lime) investigated in this study are highlighted and numbered in red. C, sequence alignment based on structural elements of LeuRS enzymes from different species. Mutated residues are shown in dark boxes, and KMSKS residues are in red. Aa, A. aeolicus; Ec, E. coli; Mm, M. mobile; Ph, P. horikoshii; Hc, human cytoplasmic, and Hm, human mitochondria.
LeuRSs also employ other appended domains, such as the C-terminal domain and leucine-specific domain (LSD), to facilitate tRNALeu binding during the tRNALeu-charging reaction (7, 20, 21). Meanwhile, elements embedded in the sequence of each tRNA promote specific interactions with its cognate aaRS (22); for example, the so-called discriminator nucleotide A73 in tRNALeu is critical for both the aminoacylation and editing activities of LeuRS (23–25). The tertiary interactions between the D- and T-loops that form the elbow region of L-shaped tRNALeu are critical elements for leucine identification (26), and the nucleotide G42 in the anticodon arms of tRNALeu from Saccharomyces cerevisiae is crucial during the editing process (27, 28). All these results were consistent with the seminal studies performed on isoleucyl-tRNA synthetase showing that the editing activity was influenced by the D-loop size of tRNAIle (29).
Here, we systematically investigate the key residues of the conserved SC-fold structural module in prokaryotic and eukaryotic LeuRSs (Fig. 1B). Crucial basic and Gly residues in the third α-helix (α3) of the SC-fold were found to modulate the aminoacylation and editing activities of LeuRS. Additionally, several key residues of helix α3 interact with the tRNALeu anticodon stem, whereas acidic residues of the β-strand control the tRNA-dependent editing activity by sensing the size of the tRNALeu D-loop. These findings were further confirmed by experiments performed on the chimeric MmLeuRS-CP1/LSD enzyme, a minimalist LeuRS enzyme fused with the E. coli CP1 domain and LSD (21). The role of the β-strand in enhancing the editing activity of the chimera was further confirmed by substituting the crucial residues for the E. coli enzyme. Together, these results support an influential role for the SC-fold module in both aminoacylation and editing functions of LeuRSs.
EXPERIMENTAL PROCEDURES
Expression and Purification of LeuRSs and Their Mutants from Different Species
LeuRSs from human cytoplasm (hcLeuRS), Aquifex aeolicus (AaLeuRS), Pyrococcus horikoshii (PhLeuRS), E. coli (EcLeuRS), and M. mobile (MmLeuRS) along with their mutants were obtained and purified as reported previously by our laboratory (28, 30, 31). Final concentrations were determined using Bradford protein assays according to the manufacturer's protocol (Bio-Rad). Mutations were engineered using the KOD Plus mutagenesis kit and confirmed by DNA sequencing (BioSun Bioscience).
Preparation of RNA Substrates
E. coli tRNAGAGLeu (EctRNAGAGLeu), A. aeolicus tRNAGAGLeu (AatRNAGAGLeu), and human cytoplasmic tRNACAGLeu (hctRNACAGLeu) with accepting capabilities between 1400 and 1600 pmol/A260 units were prepared from overproducing strains constructed in our laboratory (30–33). In vitro transcription of P. horikoshii tRNAGAGLeu (PhtRNAGAGLeu), M. mobile tRNAUAALeu (MmtRNAUAALeu), M. mobile tRNACAALeu (MmtRNACAALeu), and their mutated derivatives were prepared using T7 RNA polymerase as described previously (20, 27). The MmtRNAUAALeu transcript and its mutated derivatives (-G12U, -G13U, -G24U, -C25U, -U39G, -C40U, -C41U, -C42U, -C41U/C42U) and the MmtRNACAALeu transcript and mutant (+C17a) all had accepting activities between 1200 and 1500 pmol/A260 units. [3H]Ile-EctRNAGAGLeu was obtained using the editing-deficient EcLeuRS-Y330D mutant as described previously (34).
ATP-Pyrophosphate (PPi) Exchange, tRNA Charging, and Deacylation
The ATP-PPi exchange of EcLeuRS (37 °C) and its mutants was performed as described previously (10, 34). Aminoacylation activities of MmLeuRS, EcLeuRS, and their mutants were measured in a reaction containing 100 mm Tris-HCl (pH 7.8), 30 mm KCl, 12 mm MgCl2, 0.5 mm dithiothreitol (DTT), 4 mm ATP, 10 μm tRNALeu, 40 μm [3H]Leu (11 Ci/mm), and enzyme (5 nm EcLeuRS or 20 nm MmLeuRS and their mutants). Aminoacylation activities of AaLeuRS (20 nm), PhLeuRS (100 nm), hcLeuRS (20 nm), and their mutants in the presence of their cognate tRNAs were measured as reported previously (30–33). All LeuRSs and derivatives were assayed at 37 °C with the exception of MmLeuRS (30 °C) and AaLeuRS (65 °C) and their mutants. Cognate tRNALeu concentrations ranging from 0.5 to 30 μm were used to determine the Km value of enzymes for their cognate tRNALeu. For MmLeuRS, MmLeuRS-CP1/LSD and their mutants, MmtRNAUAALeu were used. The deacylation reaction of EcLeuRS and its mutants was measured by determining hydrolytic rates at 37 °C in 100 mm Tris-HCl (pH 7.5), 30 mm KCl, 12 mm MgCl2, 0.5 mm DTT, and 1 μm [3H]Ile-EctRNALeu (300 μCi/μm). Reactions were initiated with enzyme diluted to 20 nm. Because radioactive Nva is commercially unavailable, [3H]Ile was used as a source to prepare mischarged tRNALeu.
AMP Formation
Because the net effect of the editing reaction is the consumption of ATP, editing can be measured by monitoring AMP formation in the presence of a noncognate amino acid. AMP formation rates for MmLeuRS-CP1/LSD, EcLeuRS, hcLeuRS, AaLeuRS, and their mutants were measured as described previously (20, 26, 28). MmtRNACAALeu and its mutants were used to study the editing ability of the chimeric MmLeuRS-CP1/LSD. The reaction mixture contained 100 mm Tris-HCl (pH 7.8), 30 mm KCl, 12 mm MgCl2, 5 mm DTT, 5 units/ml pyrophosphatase (Roche Applied Science), 3 mm ATP, 20 nm [α-32P]ATP (3000 Ci/mm; PerkinElmer Life Sciences), 15 mm Nva, and the presence or absence of 5 μm tRNALeu. Reactions were initiated by the addition of the following concentrations of LeuRSs and their mutants: 1 μm MmLeuRS-CP1/LSD (30 °C), 1 μm hcLeuRS (37 °C), 1 μm AaLeuRS (65 °C), and 0.2 μm EcLeuRS (37 °C). At regular time intervals, 1.5-μl aliquots were quenched in 6 μl of 200 mm sodium acetate (pH 5.0) and spotted in duplicate on polyethyleneimine cellulose plates (PEI, Merck) that had been pre-washed with water. TLC plates were developed in the presence of 0.1 m ammonium acetate and 5% acetic acid to separate [32P]aminoacyl-adenylate, [32P]AMP, and [32P]ATP. The plates were visualized by phosphorimaging and analyzed using MultiGauge version 3.0 software (Fujifilm). [32P]AMP spot gray densities were compared with those of known [32P]ATP concentrations, and rate constants (kobs) were obtained by plotting [32P]AMP formation versus time.
Measurement of tRNA Equilibrium Dissociation Constants by Filter Binding Assays
EcLeuRS-[32P]EctRNAGAGLeu, EcLeuRS-[32P]Mm tRNAUAALeu, and hcLeuRS-[32P]hctRNACAGLeu complex formations were monitored using the classical filter binding method of Berg and co-workers (35, 36). Radiolabeled M. mobile or E. coli [32P]tRNAGAGLeu (60 nm, 60,000 cpm) and human cytoplasmic [32P]tRNACAGLeu (30 nm, 30,000 cpm) were incubated in 50 μl of binding buffer (50 mm HEPES-KOH (pH 6.8), 30 mm KCl, 12 mm MgCl2) in the presence of increasing concentrations of LeuRS proteins for 15 min at 4 °C. Samples were filtered through nitrocellulose membranes (Millipore, 0.22 μm) previously equilibrated in washing buffer consisting of 50 mm potassium phosphate (pH 5.5) and 50 mm MgCl2. Filters were then washed twice with 0.3 ml of the washing buffer and air-dried before radioactive quantification. All data were analyzed using GraphPad PRISM software.
RESULTS
Crucial Basic Residues of the Connecting Structural Module Are Essential for the Aminoacylation Activity of LeuRSs
The refined structure of EcLeuRS revealed that the third α-helix (Ser661 to Lys683; helix α3; PDB entry 4ARC and 4AQ7; Fig. 1, B and C) is the only region of the SC-fold that interacts directly with tRNA in both aminoacylation and editing conformations (11, 12), indicating that this α-helix may be critical for both processes. To investigate the effect of the α-helix on enzymatic activity, we constructed a deletion mutant lacking the third α-helix. Despite the 22-amino acid deletion, CD spectroscopy data also indicated the deletion mutant was well expressed in a soluble form and had a definite stable secondary structure (Fig. 2A). However, this construct completely lacked both aminoacylation and editing activities, supporting the hypothesis that this helix is critical for these functions (Fig. 2B and Tables 1 and 2).
FIGURE 2.
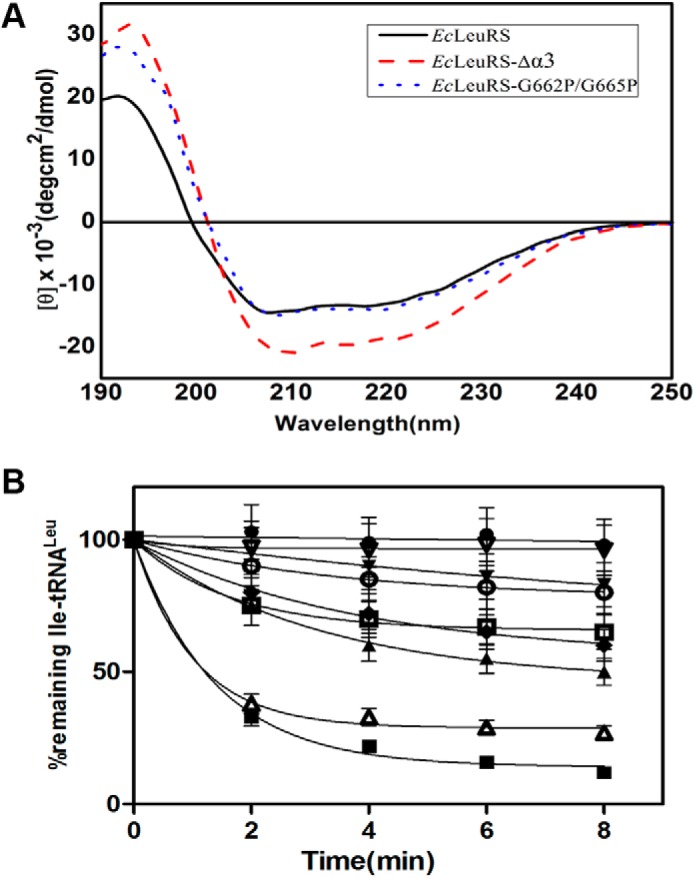
CD spectroscopic analysis of EcLeuRS-Δα3 and hydrolysis of EcLeuRS and its mutants. A, two enzymes were scanned by UV between 190 and 250 nm using CD spectroscopy. The concentration of each enzyme was 0.3 mg/ml in H2O. The CD signal in millidegrees was converted to mean residue ellipticity [θ]. B, deacylation curves of [3H]Ile-EctRNALeu (1 μm) induced by EcLeuRS enzymes (5 nm). No enzyme (●); EcLeuRS-R668E/R672E (▾); EcLeuRS (■); EcLeuRS-G662P/G665P (□); EcLeuRS-R668E (○); EcLeuRS-R672E (♦); EcLeuRS-G662P (▵); and EcLeuRS-G665P(▴).
TABLE 1.
Kinetic constants of LeuRSs and their mutants at basic residues from various species for their cognate tRNAs in aminoacylation reaction
Kinetic constants were determined using the tRNA charging assay described under “Experimental Procedures.” All parameters are the average of three repeats with the standard deviations indicated. NM means not measurable (too low to be measured).
| Enzyme | Mutant | Km | kcat | kcat/Km | Relative value |
|---|---|---|---|---|---|
| μm−1 | s−1 | s−1 μm−1 | |||
| EcLeuRS | WTa | 2.2 ± 0.1 | 4.9 ± 0.3 | 2.2 | 1.0 |
| -R668A | 2.8 ± 0.2 | 1.4 ± 0.1 | 0.50 | 0.23 | |
| -R668E | 5.4 ± 0.5 | 0.59 ± 0.05 | 0.11 | 0.050 | |
| -R672A | 2.4 ± 0.2 | 4.8 ± 0.4 | 2.0 | 0.91 | |
| -R672E | 2.8 ± 0.2 | 0.53 ± 0.05 | 0.19 | 0.086 | |
| -R668A/R672A | 2.2 ± 0.2 | 0.31 ± 0.03 | 0.14 | 0.064 | |
| -R668E/R672E | 5.8 ± 0.5 | 0.18 ± 0.01 | 0.030 | 0.013 | |
| -K671A | 2.4 ± 0.2 | 4.8 ± 0.5 | 2.0 | 0.91 | |
| -Δhelix α3 | NM | NM | NM | NM | |
| AaLeuRS | WT | 0.30 ± 0.02 | 1.5 ± 0.3 | 5.0 | 1.0 |
| β-R94A | 0.75 ± 0.06 | 1.3 ± 0.1 | 1.7 | 0.34 | |
| β-R94E | 0.81 ± 0.07 | 0.89 ± 0.09 | 1.1 | 0.22 | |
| β-R98A | 0.31 ± 0.03 | 1.5 ± 0.1 | 4.8 | 0.96 | |
| β-R98E | 0.39 ± 0.03 | 1.0 ± 0.1 | 2.6 | 0.52 | |
| β-R94A/R98A | 0.81 ± 0.08 | 0.67 ± 0.06 | 0.83 | 0.17 | |
| β-R94E/R98E | 0.88 ± 0.08 | 0.19 ± 0.02 | 0.22 | 0.044 | |
| MmLeuRS | WT | 1.5 ± 0.2 | 3.3 ± 0.2 | 2.2 | 1.0 |
| -K452A | 1.7 ± 0.2 | 3.2 ± 0.2 | 1.9 | 0.86 | |
| -K452E | 1.9 ± 0.2 | 2.8 ± 0.1 | 1.5 | 0.70 | |
| -R456A | 2.3 ± 0.2 | 1.3 ± 0.1 | 0.60 | 0.30 | |
| -R456E | 2.5 ± 0.2 | 0.85 ± 0.49 | 0.34 | 0.15 | |
| PhLeuRS | WTb | 0.20 ± 0.14 | 0.020 ± 0.003 | 0.11 | 1.0 |
| -K692A | 0.44 ± 0.04 | 0.041 ± 0.004 | 0.090 | 0.090 | |
| -K696A | 0.65 ± 0.10 | 0.017 ± 0.002 | 0.030 | 0.24 | |
| -R698A | 0.52 ± 0.05 | 0.067 ± 0.006 | 0.13 | 1.2 | |
| -K699A | 0.60 ± 0.05 | 0.050 ± 0.007 | 0.080 | 0.75 | |
| -R703A | 0.12 ± 0.04 | (5.5 ± 0.5) × 10−3 | 0.050 | 0.42 | |
| hcLeuRS | WTc | 0.74 ± 0.05 | 2.6 ± 0.2 | 3.5 | 1.0 |
| -R766A | 1.7 ± 0.2 | 0.56 ± 0.06 | 0.33 | 0.090 |
TABLE 2.
Observed rate constants of LeuRSs and their mutants at basic residues from various species for AMP synthesis in the presence of Nva and their cognate tRNAs
All rates are the average of three repeats with the standard deviations indicated. NM means not measurable (too low to be measured).
| LeuRS | Rate of AMP formation kobs | Relative value |
|---|---|---|
| s−1 | ||
| EcLeuRSa | 3.4 ± 0.5 | 1.0 |
| -R668A | 2.3 ± 0.3 | 0.67 |
| -R672A | 2.0 ± 0.2 | 0.59 |
| -R668E | 0.42 ± 0.02 | 0.12 |
| -R672E | 0.69 ± 0.05 | 0.20 |
| -R668A/R672A | 0.39 ± 0.05 | 0.11 |
| -R668E/R672E | 0.31 ± 0.03 | 0.091 |
| -Δhelix α3 | NM | NM |
| AaLeuRS | 1.4 ± 0.2 | 1.0 |
| -R94A | 0.23 ± 0.02 | 0.16 |
| -R98A | 0.21 ± 0.02 | 0.15 |
| -R94E | 0.19 ± 0.02 | 0.14 |
| -R98E | 0.19 ± 0.02 | 0.14 |
| -R94E/R98E | 0.13 ± 0.01 | 0.093 |
| hcLeuRS | 1.6 ± 0.1 | 1.0 |
| -R766A | 0.16 ± 0.02 | 0.10 |
a Data are from Ref. 10.
Considering the interaction between tRNA and aaRS is predominantly mediated by crucial basic residues (37), in EcLeuRS, we investigated the function of residues Arg668, Lys671, and Arg672 in the third α-helix of the SC-fold by site-directed mutagenesis (PDB codes 4ARC and 4AQ7; Fig. 1, B and C). EcLeuRS-K671A did not affect the catalytic efficiency (kcat/Km) at all (Table 1). However, the kcat/Km values of EcLeuRS-R668A and EcLeuRS-R668E had decreased to 23 and 5% of the kcat/Km values of wild-type (WT) EcLeuRS (Table 1). The kcat/Km values of the double mutant EcLeuRS-R668A/R672A was only 6.4% of that of the native enzyme, and the catalytic efficiency of the EcLeuRS-R668E/R672E double-site mutant was severely diminished, with only 1.4% of tRNA aminoacylation activity remaining (Table 1). Although mutations at Arg668 or Arg672 decreased the aminoacylation activity to different extents, all of these mutants, including the almost inactive EcLeuRS-R668E/R672E, exhibited intact Leu activation activity comparable with the WT enzyme (data not shown), suggesting these Arg residues are not involved in the amino acid activation step but rather the second tRNA transfer step. These results also suggest that the enzyme mutations alter interactions with the tRNALeu that are critical for the stabilization of the transition state of the aminoacylation reaction but not for the affinity of enzyme for tRNA.
We next investigated the two crucial basic residues of helix α3 of the SC-fold of LeuRS from A. aeolicus (AaLeuRS) and M. mobile (MmLeuRS). AaLeuRS forms a unique heterodimer with its SC-fold located in the β-subunit. The counterparts of EcLeuRS-Arg668 and EcLeuRS-Arg672 in AaLeuRS are βArg94 and βArg98, respectively, and mutating these residues in AaLeuRS had a similar effect on aminoacylation; AaLeuRS-βR98A did not alter the catalytic efficiency, whereas mutating βArg94 to either Ala or Glu decreased kcat/Km values to 34 and 22% of that of WT AaLeuRS (Table 1). When the Ala mutants were combined in βR94A/R98A, the aminoacylation activity dropped to only 17% of that of WT AaLeuRS, and the double Glu mutant βR94E/R98E exhibited an even lower kcat/Km value (only 4.4% of that of WT AaLeuRS; Table 1).
In MmLeuRS, Lys452 and Arg456 correspond to the Arg668 and Arg672 residues of EcLeuRS, respectively. Substitution of Lys452 with either Ala or Glu had only a minimal effect on aminoacylation activity. However the tRNA catalytic efficiency of these mutants was significantly decreased to 25% for MmLeuRS-R456A and to 21% for MmLeuRS-R456E of that of the WT MmLeuRS (Table 1). Notably, the Km values for the above MmLeuRS mutants were not significantly altered. These results suggest that helix α3 in the SC-fold of MmLeuRS interacts with tRNA in a different manner to that of EcLeuRS and AaLeuRS.
The crucial basic residues of helix α3 in the archaeal P. horikoshii LeuRS (PhLeuRS) (PDB entry 1WZ2) and eukaryotic human cytoplasmic LeuRS (hcLeuRS) enzymes were investigated. PhLeuRS contains five positively charged residues (Lys692, Lys696, Arg698, Lys699, and Arg703) in helix α3, and alanine-scanning mutagenesis revealed that the Km value for tRNA of PhLeuRS-K696A was 0.65 μm, an ∼3-fold increase compared with the WT value (0.20 μm). The kcat of PhLeuRS-R703A (0.0055 s−1) was much lower than that of native PhLeuRS (0.020 s−1; Table 1). The other PhLeuRS mutants had no effect on tRNA charging activity.
Arg766 is the only basic residue in helix α3 of hcLeuRS SC-fold (Fig. 1C). The mutant hcLeuRS-R766A decreased the kcat/Km value to less than 10% that of the native hcLeuRS (Table 1). These results demonstrate that basic residues on helix α3 of the SC-fold are critical for catalytic efficiency, which may explain their high sequence conservation in both prokaryotic and eukaryotic LeuRSs.
Crucial Basic Residues Contribute to the Fidelity of LeuRSs
TLC assays were performed to check whether the mutations of the basic residues affected the editing activity of LeuRS against Nva. AMP formation rates (kobs) of EcLeuRS-R668A/R672A and -R668E/R672E were ∼11 and 9.1% that of the WT EcLeuRS in the presence of EctRNAGAGLeu and Nva (Table 2). The kobs values of the single-site mutants EcLeuRS-R668A and EcLeuRS-R672A were 67 and 59% that of wild-type EcLeuRS in the presence of EctRNAGAGLeu and Nva (Table 2 and Fig. 3). Remarkably, mutating each basic residue to the acidic Glu severely impacted the AMP formation of LeuRS. The kobs values of EcLeuRS-R672E and EcLeuRS-R668E were decreased significantly to 20 and 12% that of WT EcLeuRS for EctRNAGAGLeu in the presence of Nva (Table 2 and Fig. 3). These results suggested that the basic residues contribute to both the AMP formation and catalytic efficiency of EcLeuRS.
FIGURE 3.
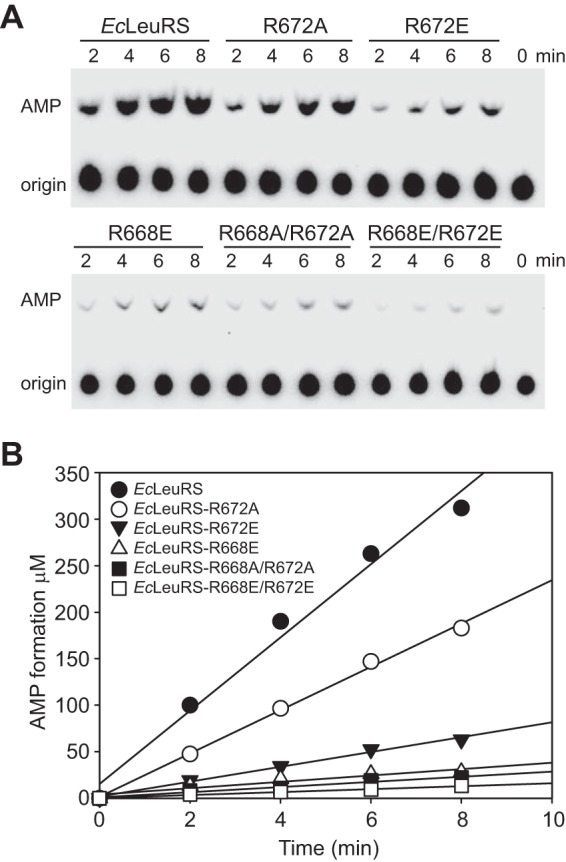
Effects of mutating the helix α3 Arg residues on editing activity in the presence of tRNA. A, AMP formation assays used to measure total editing capability with 0.2 μm EcLeuRS, EcLeuRS-R672A, EcLeuRS-R672E, EcLeuRS-R668E, EcLeuRS-R668A/R672A, and EcLeuRS-R668E/R672E in the presence of 5 μm EctRNAGAGLeu and 15 mm Nva. B, graphical representation of AMP formation by EcLeuRS and mutants. kobs values for AMP formation were calculated from the line gradients (Table 2).
To identify which editing pathway was affected by these residues, we measured the kobs value of the mutants in the presence of Nva but in the absence of tRNA. The kobs value of the editing-defective EcLeuRS-R672A mutant was 1.6-fold that of the native enzyme in the absence of tRNA (Table 3). In contrast, kobs values without tRNA for the other mutants were similar to the WT values (Table 3). These results reveal that mutation of the basic residues largely impacts the tRNA-dependent transfer editing capacity of EcLeuRS. Further deacylation results indicated the impaired tRNA-dependent transfer editing of the mutants resulted from their decreased hydrolysis ability (Fig. 2B).
TABLE 3.
Observed rate constants of LeuRS and its mutants at basic residues for AMP synthesis in the presence of Nva and absence of tRNALeu
All rates are the average of three repeats with the standard deviations indicated.
| LeuRS | Rate of AMP formation kobs | Relative value |
|---|---|---|
| s−1 | ||
| EcLeuRSa | 0.33 ± 0.04 | 1.0 |
| -R668A | 0.32 ± 0.04 | 0.67 |
| -R672A | 0.54 ± 0.05 | 1.6 |
| -R668E | 0.36 ± 0.03 | 1.1 |
| -R672E | 0.35 ± 0.05 | 1.1 |
| -R668A/R672A | 0.37 ± 0.08 | 1.1 |
| -R668E/R672E | 0.30 ± 0.02 | 0.91 |
a Data are from Ref. 10.
Finally, we probed the basic residues of AaLeuRS and hcLeuRS. Mutations of either Arg94 or Arg98 of AaLeuRS to Ala or Glu markedly decreased the editing activity of the enzyme, suggesting that both residues are essential for the editing process. The kobs values were ∼0.20 s−1 in the presence of AatRNAGAGLeu and Nva, which was around 10% that of WT AaLeuRS, and the double mutant βR94E/R98E was even lower (0.13 s−1; Table 2). Consistently, mutation of the unique basic residue Arg766 to Ala in hcLeuRS (hcLeuRS-R766A) led to a 90% decrease in AMP formation in the presence of hctRNACAGLeu (Table 2). Overall, our results show that both prokaryotic and eukaryotic LeuRSs employ crucial basic residues of helix α3 of the SC-fold to modulate the catalytic efficiency and fidelity of the enzyme.
Basic Residues Participate in the LeuRS Affinity for the Anticodon Stem of tRNALeu
Filter binding assays revealed that the lower catalytic efficiencies (kcat/Km) of the above mutants were due to a lower affinity for tRNALeu. Indeed, the dissociation constants (kd) of native EcLeuRS and hcLeuRS for their cognate tRNAs were 0.20 and 0.18 μm, respectively, and this was increased more than 10-fold for EcLeuRS-R668A/R672A and 27-fold for EcLeuRS-R668E/R672E (Table 4). The kd of mutant hcLeuRS-R766A was 15-fold higher than hcLeuRS for hctRNALeu (Table 4). As the kd value reflects the affinity of LeuRS for tRNALeu in the ground state before the aminoacylation catalysis, these results indicated that these residues were involved in the initial binding of enzyme with tRNALeu. Moreover, the unchanged Km values of mutants for tRNALeu suggested that the affinity of enzyme for tRNALeu in the transition state of the aminoacylation reaction was unaffected.
TABLE 4.
Dissociation constants of LeuRSs and their mutants from E. coli and human cytoplasm for their cognate tRNAs
The kd values were determined using filter binding assays in the presence of 0.01 to 15 μm LeuRS. Rates are the average of three assays with the standard deviations indicated.
| Enzyme | kd | Relative value |
|---|---|---|
| μm | ||
| EcLeuRS | 0.20 ± 0.04 | 1.0 |
| -R668A | 0.46 ± 0.04 | 2.3 |
| -R672A | 0.85 ± 0.07 | 4.3 |
| -R668A/R672A | 2.3 ± 0.2 | 12 |
| -R668E/R672E | 5.3 ± 0.5 | 27 |
| -R668E | 0.50 ± 0.05 | 2.5 |
| -R672E | 0.24 ± 0.02 | 1.2 |
| -G662P/G665P | 0.039 ± 0.005 | 0.20 |
| -L656K/E657K | 0.24 ± 0.02 | 1.2 |
| hcLeuRS | 0.18 ± 0.02 | 1.0 |
| -R766A | 2.74 ± 0.25 | 15 |
As the refined structure of the EcLeuRS-EctRNAUAALeu complex (PDB codes 4ARC and 4AQ7) displayed disorder in the anticodon loop, there was no clear evidence to show whether residues Arg668 and Arg672 from helix α3 of the SC-fold participated in interactions with the D-stem and anticodon stem of EctRNAUAALeu (11). In an attempt to verify and identify the determinants of the editing reaction on the tRNA, we constructed a set of mutants of tRNALeu. Because EcLeuRS recognizes the synthetic transcript of MmtRNAUAALeu produced in vitro as well as the fully modified EctRNAGAGLeu purified from an E. coli overproducing strain (kcat/Km = 2.6 and 2.2 μm−1 s−1, respectively), we chose to mutate the MmtRNAUAALeu transcript, which is remarkably similar in sequence to EctRNAGAGLeu (Fig. 4). We constructed seven mutants with specific nucleotide substitutions in the stems of MmtRNAUAALeu: -G24U and -C25U in the D-stem and -U39G, -C40U, -C41U, -C42U, and -C41U/C42U in the anticodon stem (Fig. 4). The catalytic efficiency (kcat/Km) in the aminoacylation reaction and the kobs in the editing reaction were measured, and the values for WT MmtRNAUAALeu and the -G24U, -C25U, and -U39G mutants were indistinguishable, indicating that these nucleotides do not critically interact with LeuRS (data not shown). However, the catalytic efficiencies (kcat/Km) of EcLeuRS for the -C40U, -C41U, and -C42U variants were 73, 51, and 26% that of EcLeuRS for WT MmtRNAUAALeu (Table 5). Consistently, the kcat/Km value of the EcLeuRS for double mutant -C41U/C42U was even lower (12% that of WT MmtRNAUAALeu), with a value of 0.31 μm−1 s−1 (Table 5).
FIGURE 4.
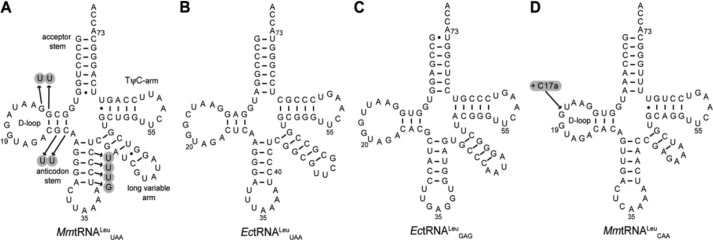
Cloverleaf structures of MmtRNAUAALeu (A); EctRNAUAALeu (B); EctRNAGAGLeu (C); and MmtRNACAALeu (D). Mutations constructed during this study are indicated with arrows.
TABLE 5.
Various parameters of EcLeuRS for MmtRNAUAALeu and its derived variants
The Km and kcat values were determined using the tRNA charging assay described under “Experimental Procedures.” The kobs values are the observed rate constants for AMP formation with Nva and MmtRNAUAALeu. The kd values are the dissociation constants of the EcLeuRS and MmtRNAUAALeu complex determined by filter binding assays in the presence of 0.01 to 10 μm EcLeuRS. Parameters are the average of three repeats with the standard deviations indicated.
| MmtRNAGAGLeu | Km | kcat | kcat/Km | Relative value | kobs of AMP formation | kd |
|---|---|---|---|---|---|---|
| μm | s−1 | s−1μm−1 | s−1 | μm | ||
| WT | 1.6 ± 0.1 | 4.2 ± 0.5 | 2.6 | 1.0 | 2.6 ± 0.3 | 0.27 ± 0.03 |
| -G12U | 1.7 ± 0.2 | 3.9 ± 0.3 | 2.3 | 0.88 | 2.7 ± 0.2 | 0.27 ± 0.02 |
| -G13U | 1.7 ± 0.2 | 4.0 ± 0.5 | 2.4 | 0.92 | 2.6 ± 0.2 | 0.29 ± 0.03 |
| -C40U | 1.5 ± 0.1 | 3.5 ± 0.3 | 1.9 | 0.73 | 2.4 ± 0.3 | 0.38 ± 0.02 |
| -C41U | 1.4 ± 0.1 | 1.9 ± 0.1 | 1.3 | 0.51 | 2.5 ± 0.3 | 1.7 ± 0.2 |
| -C42U | 2.7 ± 0.2 | 1.8 ± 0.1 | 0.67 | 0.26 | 1.9 ± 0.2 | 1.6 ± 0.2 |
| -C41U/C42U | 4.2 ± 0.4 | 1.3 ± 0.1 | 0.31 | 0.12 | 1.4 ± 0.1 | 1.6 ± 0.2 |
Further analysis revealed that the decreased catalytic efficiency may result from an altered affinity for the tRNA substrate. In the filter binding assay, the kd values of EcLeuRS for MmtRNAUAALeu-C41U (1.7 μm) and -C42U (1.6 μm) mutants were ∼5-fold greater than that of WT MmtRNAUAALeu (0.27 μm; Table 4). In addition, we showed that the double mutant MmtRNAUAALeu-C41U/C42U was even less capable of editing. In the presence of 15 mm Nva, the kobs value of MmtRNAUAALeu-C41U/C42U was 1.4 s−1, compared with 2.6 s−1 for WT MmtRNAUAALeu (Table 4). Together, these results suggest that LeuRS employs these basic residues of the SC-fold domain to modulate both aminoacylation and editing activities through interaction with the anticodon stem of tRNALeu.
Two Gly Residues in Helix α3 Participate in the Aminoacylation and Editing Functions of LeuRS
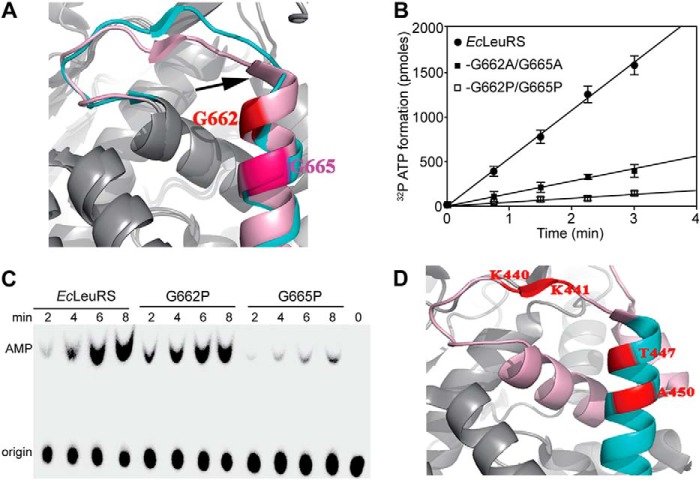
Analysis of the SC-fold of EcLeuRS showed that both the β-strand and helix α3 undergo substantial conformational changes during aminoacylation and editing (PDB code 4AQ7 and 4ARC; Fig. 5A). Mutating the first two Gly residues of α3 to Ala (EcLeuRS-G662A/G665A) was accompanied by a substantial drop in aminoacylation activity (Table 6), whereas mutating to Pro did not impact the aminoacylation activity of EcLeuRS-G662P or EcLeuRS-G665P (Table 6). However, the catalytic efficiency of the double mutant EcLeuRS-G662P/G665P was decreased to 9.5% that of WT EcLeuRS with EctRNAGAGLeu, but exhibited similar secondary structure with WT EcLeuRS (Table 6 and Fig. 2A).
FIGURE 5.
Effects of mutating the helix α3 Gly residues on synthetic and editing activities. A, structure of the EcLeuRS-tRNALeu complex in aminoacylation (pink) and editing (cyan) conformations (PDB codes 4AQ7 and 4ARC, respectively) (11). Helix α3 residues Gly662 and Gly665 are numbered and highlighted in red and pink. Arrows indicate conformational changes of β-strand of the SC-fold facilitated by the Gly residues. B, Leu activation properties measured in the ATP/PPi exchange reaction with 10 nm EcLeuRS, EcLeuRS-G662A/G665A, and EcLeuRS-G662P/G665P. Leu was used at a final concentration of 5 mm. C, AMP formation assays used to measure total editing activity of 1 μm EcLeuRS, EcLeuRS-G662P, and EcLeuRS-G665P in the presence of 5 μm EctRNAGAGLeu and 15 mm Nva. kobs values of AMP formation are reported in Table 7. D, structure of MmLeuRS (gray; PDB code 3ZIU (39). Residues shown on helix α3 (cyan) are equivalent to Gly662 and Gly665 of EcLeuRS. Other helices of the SC-fold are represented in pink, and the β-strand is in red.
TABLE 6.
Kinetic constants of LeuRSs from various species and their mutants at Gly residues for their cognate tRNAs in aminoacylation reaction
Kinetic constants were determined using the tRNA charging assay described under “Experimental Procedures.” All parameters are the average of three repeats with the standard deviations indicated.
| Enzymes | Km | kcat | kcat/Km | Relative value |
|---|---|---|---|---|
| μm | s−1 | s−1 μm−1 | ||
| EcLeuRSa | 2.2 ± 0.1 | 4.9 ± 0.3 | 2.2 | 1.0 |
| -G662P | 1.1 ± 0.3 | 2.2 ± 0.3 | 2.0 | 0.91 |
| -G665P | 1.3 ± 0.1 | 2.1 ± 0.1 | 1.6 | 0.73 |
| -G662A/G665A | 1.2 ± 0.1 | 0.70 ± 0.07 | 0.58 | 0.26 |
| -G662P/G665P | 1.4 ± 0.2 | 0.30 ± 0.03 | 0.21 | 0.095 |
| -G662R/G665R | 1.3 ± 0.1 | 0.80 ± 0.08 | 0.61 | 0.28 |
| PhLeuRS | 0.20 ± 0.01 | 0.020 ± 0.003 | 0.11 | 1.0 |
| -G695P | 0.19 ± 0.05 | 0.010 ± 0.001 | 0.053 | 0.48 |
| hcLeuRS | 0.74 ± 0.05 | 2.6 ± 0.2 | 3.5 | 1.0 |
| -G763P | 0.61 ± 0.06 | 1.1 ± 0.2 | 1.8 | 0.51 |
a Data are from Ref. 10.
The mutations also diminished the Leu activation activity, as measured using the ATP-PPi exchange reaction (Fig. 4B). Compared with the kobs of WT EcLeuRS (57 s−1), the activity of the double mutant G662P/G665P (3.6 s−1) was impacted to a much greater extent than the double mutant G662A/G665A (16.5 s−1). These results suggest that the decreased aminoacylation activity may be the consequence of a reduction in the formation of adenylate. Additionally, these double mutants were also shown to be defective in their editing capacity. The kobs value of the double mutant G662P/G665P in the presence of Nva and EctRNAGAGLeu was only 9.4% that of the WT enzyme. Mutation G665P alone reduced the kobs to 12% that of the WT enzyme, whereas the G662P mutation had only a minor impact (Table 7 and Fig. 5C). Additionally, the post-transfer editing activity of mutation G665P was also decreased (Fig. 2B). These observations suggest that the editing-defective mutant EcLeuRS-G665P, which exhibited a standard synthetic efficiency, could be used to produce mischarged aa-tRNALeu molecules.
TABLE 7.
Observed rate constants of EcLeuRS and its mutants at G of helix α3, crucial residues in β-sheet for AMP synthesis in the presence of Nva
All rates are the average of three repeats with the standard deviations indicated.
| LeuRS | Rate of AMP formation kobs(s−1) |
|||
|---|---|---|---|---|
| −EctRNAGAGLeu | Relative value | + EctRNAGAGLeu | Relative value | |
| EcLeuRSa | 0.33 ± 0.04 | 1.0 | 3.4 ± 0.5 | 1.0 |
| -G662P | 0.29 ± 0.03 | 0.88 | 2.4 ± 0.3 | 0.70 |
| -G665P | 0.059 ± 0.007 | 0.18 | 0.42 ± 0.05 | 0.12 |
| -G662P/G665P | 0.027 ± 0.003 | 0.082 | 0.32 ± 0.03 | 0.094 |
| -D653K | 0.33 ± 0.03 | 1.0 | 1.5 ± 0.1 | 0.45 |
| -L656K/E657K | 0.30 ± 0.03 | 0.91 | 0.31 ± 0.03 | 0.091 |
a Data are from Ref. 10.
To further explore the hypothesis that Gly residues might facilitate conformational changes of the SC-fold module during the transition between the aminoacylation and editing stages, we examined the sequences of PhLeuRS and hcLeuRS. Both enzymes contain a single Gly in helix α3, albeit at different positions (PDB code 1WZ2; Fig. 1C). Mutations to Pro in PhLeuRS-G695P decreased the catalytic efficiency of aminoacylation to 5.3% that of WT PhLeuRS. Similarly, a mutant replaced Gly763 of hcLeuRS with Pro, and hcLeuRS-G763P had only half-catalytic efficiency of hcLeuRS (Table 6). Although not conserved, these data suggest that the Gly residues play a substantial role in retaining aminoacylation activity.
Sequence alignment revealed that MmLeuRS lacks any Gly residues in helix α3; the two Gly residues found in EcLeuRS are replaced by Thr and Ala (PDB code 3ZIU; Figs. 1C and 5D). Additionally, the β-strand of the SC-fold of MmLeuRS contains two Lys residues, whereas other LeuRS enzymes usually contain two or three acidic residues in this vicinity (Fig. 1C). This suggests that MmLeuRS employs a different strategy for interaction with its cognate tRNA, which may be linked to the absence of the CP1 domain and its associated editing function. Indeed, without the need to transfer the tRNA from the synthetic to the editing site, the role of the SC-fold may have diverged in these enzymes, and this may explain the altered charge distribution and the absence of flexibility-inducing Gly residues.
β-Strand of the SC-fold Participates in the Editing Function of LeuRS
To study the role of the key residues of the SC-fold β-strand, we replaced residues Asp653, Glu657, and Leu656 of EcLeuRS with Lys. In the aminoacylation reaction, the catalytic efficiency of mutant EctRNAGAGLeu-D653K and double mutant EctRNAGAGLeu-L656K/E657K was not significantly different from that of the native enzyme (Table 8). However, in the editing step, in the presence of Nva and tRNA, the kobs value of EctRNAGAGLeu-D653K dropped to 45% that of WT EcLeuRS for AMP formation, which was only half that of the native enzyme (Table 7). For the double mutant L656K/E657K, the kobs value was decreased to 9.1% that of WT EcLeuRS in the presence of tRNA, which suggested that the mutant had lost its tRNA-dependent editing activity (Fig. 6A and Table 7). Therefore, we performed an Ile-tRNALeu deacylation assay in the presence of mutant D653K or L656K/E657K. The deacylation curves showed that both mutants were severely affected in their capacity to deacylate mischarged EctRNAGAGLeu, suggesting that the SC-fold β-strand is involved in tRNA-dependent editing of LeuRS following the transfer step (Fig. 6B).
TABLE 8.
Kinetic constants of EcLeuRS, chimeric LeuRS and their mutants for tRNALeu in aminoacylation reaction
Kinetic constants were determined using the tRNA charging assay described under “Experimental Procedures.” All parameters are the average of three repeats with the standard deviations indicated. NM means not measurable (too low to be measured). WT indicates the chimeric enzyme MmLeuRS-CP1/LSD obtained by inserting the LSD and CP1 domain of EcLeuRS into MmLeuRS.
| Enzymes | LeuRS | Km | kcat | kcat/Km | Relative value |
|---|---|---|---|---|---|
| μm | s−1 | s−1 μm−1 | |||
| EcLeuRSa | WT | 2.2 ± 0.1 | 4.9 ± 0.3 | 2.2 | 1.0 |
| -L656K/E657K | 0.66 ± 0.07 | 1.1 ± 0.01 | 1.7 | 0.77 | |
| -D653K | 2.2 ± 0.2 | 3.1 ± 0.3 | 1.4 | 0.64 | |
| MmLeuRS-CP1/LSD | WT | 2.8 ± 0.3 | 2.1 ± 0.2 | 0.78 | 1.0 |
| -K440E/K441E | NM | NM | NM | NM | |
| -K440L/K441E | 5.2 ± 0.5 | 0.69 ± 0.02 | 0.13 | 0.33 |
a Data are from Ref. 10.
FIGURE 6.
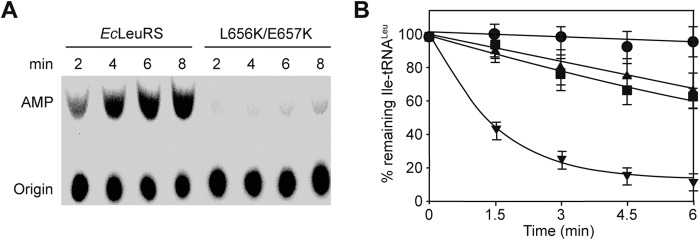
Residues of the SC-fold β-strand are involved in tRNA-dependent editing. A, TLC showing AMP formation catalyzed by 0.2 μm EcLeuRS and EcLeuRS-L656K/E657K in the presence of 5 μm EctRNAGAGLeu and 15 mm Nva. kobs values are reported in Table 7. B, deacylation curves of [3H]Ile-EctRNALeu (1 μm) induced by EcLeuRS enzymes (5 nm). No enzyme (●), EcLeuRS (▾), EcLeuRS-D653K (■), and EcLeuRS-L656K/E657K (▴).
In the crystal structure of the complex containing EcLeuRS and EctRNAUAALeu (11), nucleotides G12 and G13 are located in the vicinity of the β-strand of the SC-fold. Although these residues are not within bonding distance of the acidic residues 653 and 657, we mutated them to U in MmtRNAUAALeu to probe their role during the complete aminoacylation/editing cycle. However, both aminoacylation and editing activity properties were indistinguishable from the WT tRNA (Table 4).
We further explored the possibility that, due to its appropriate placement in the structure of the complex, the SC-fold may sense the size of the D-loop during the editing step. We chose to use a different MM tRNALeu with a CAA anticodon that contains only 10 nucleotides in the D-loop instead of the usual 11 nucleotides in EctRNAUAALeu and MmtRNAUAALeu (Fig. 4). Remarkably, during the editing reaction, MmtRNACAALeu could not stimulate AMP formation as well as MmtRNAUAALeu; in the presence of Nva and EcLeuRS, the kobs was only 0.91 s−1 compared with 2.6 s−1 for both MmtRNACAALeu and EctRNAUAALeu. This result strongly suggested that the size of the D-loop is a key determinant of editing activity. To confirm this hypothesis, we inserted in the D-loop after cytosine 17 of MmtRNACAALeu (17a), just before the crucial nucleotides G18 and G19. In the resulting mutant MmtRNACAALeu (+C17a), the loop was identical to that of MmtRNAUAALeu and EctRNAUAALeu (Fig. 4). Consistently, in the presence of Nva, the kobs value of EcLeuRS for mutant (+C17a) was increased to 2.1 s−1, reaching a level comparable with MmtRNAUAALeu and EctRNAUAALeu (Tables 7 and 9). This result further indicates that the β-strand of the SC-fold may participate in editing by sensing the size of the tRNALeu D-loop. The editing activity of EcLeuRS was magnified when the native size of the D-loop of tRNALeu was introduced into MmtRNACAALeu. These results show that the β-strand adjacent to the SC-fold influences the editing activities of EcLeuRS, probably via the conserved acidic residues.
TABLE 9.
Observed rate constants of EcLeuRS and chimeric LeuRS and its mutant for AMP formation in the presence of Nva and MmtRNACAALeu
Rates are the average of three repeats with the standard deviations indicated.
| LeuRS | MmtRNACAALeu | kobs of AMP formation | Relative value |
|---|---|---|---|
| s−1 | |||
| EcLeuRS | 0.33 ± 0.04a | 1.0 | |
| +WT | 0.91 ± 0.10 | 2.8 | |
| + (+C17a) | 2.1 ± 0.2 | 6.4 | |
| MmLeuRS-CP1/LSD | (3.7 ± 0.8) × 10−2 | 1.0 | |
| +WT | (4.1 ± 0.8) × 10−2 | 1.1 | |
| + (+ C17a) | 0.10 ± 0.04 | 2.7 | |
| MmLeuRS-CP1/LSD-K440L/K441E | (3.7 ± 0.8) × 10−2 | 1.0 | |
| +WT | 0.11 ± 0.02 | 3.0 | |
| + (+ C17a) | 0.31 ± 0.04 | 8.4 |
a Data are from Ref. 10.
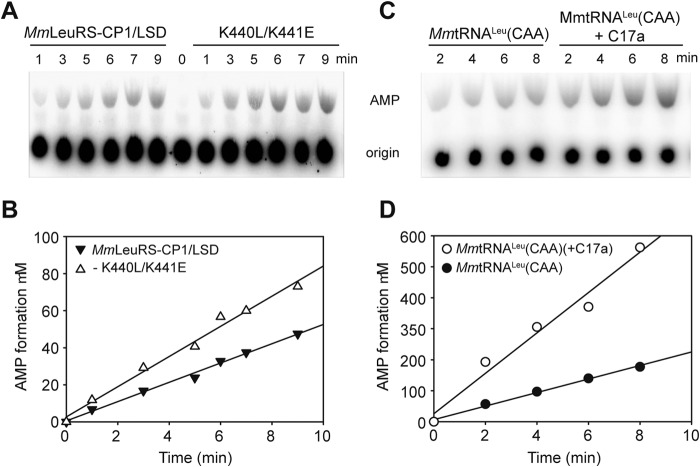
Engineering of the Chimeric MmLeuRS-CP1/LSD and MmtRNACAALeu to Improve Fidelity
We previously engineered a variant of MmLeuRS containing an insertion of CP1 and LSD from EcLeuRS, and this chimera exhibited improved tRNA-dependent editing activity for isoacceptor MmtRNAUAALeu (21). In this study, the kobs value of AMP formation during the editing reaction of the chimera was 0.037 and 0.041 s−1 in the absence or presence of the isoacceptor MmtRNACAALeu, respectively (Table 9 and Fig. 7A). This result suggests the tRNA-dependent editing pathway was inefficient in the presence of the MmtRNACAALeu isoacceptor. Therefore, MmLeuRS-CP1/LSD was able to discriminate between the AUG and CAA isoacceptors during the editing reaction. Considering the contribution of the SC-fold to the catalytic function and fidelity of LeuRS, we first introduced into MmLeuRS two Glu residues in place of the two unusual basic residues (Lys440 and Lys441) found in the β-strand. However, the double mutant MmLeuRS-CP1/LSD-K440E/K441E did not possess any aminoacylation activity (Table 7), suggesting that both Lys residues contribute to the catalytic activity in MmLeuRS. We further substituted Lys440 with Leu and Lys441 with Glu to mimic the residues in EcLeuRS. Remarkably, the kobs value of MmLeuRS-CP1/LSD-K440L/K441E for MmtRNACAALeu was twice that of the starting MmLeuRS-CP1/LSD chimera in the editing reaction (0.11 s−1 versus 0.041 s−1 in Table 9 and Fig. 7, A and B). In contrast, the kcat/Km values of the double mutant -K440L/K441E exhibited only 33% compared with the starting chimera MmLeuRS-CP1/LSD (Table 7). We next measured the editing capacity of the double mutant K440L/K441E in the presence of MmtRNACAALeu with the (+C17a) insertion in the D-loop, and the kobs value (0.31 s−1) was almost 3-fold higher than that of MmtRNACAALeu (0.11 s−1), which confirmed the importance of the size of the D-loop (Table 9 and Fig. 7, C and D).
FIGURE 7.
Editing of the engineered MmLeuRS-CP1/LSD for MmtRNACAALeu and mutated derivatives. A, AMP formation assay used to measure total editing activity of 1 μm MmLeuRS-CP1/LSD and mutated derivative K440L/K441E in the presence of 5 μm MmtRNACAALeu and 15 mm Nva. B, graphical representation of AMP formation as a function of time for MmLeuRS-CP1/LSD and derivative K440L/K441E. C, AMP formation assays used to measure total editing activity with 1 μm MmLeuRS-CP1/LSD-K440L/K441E in the presence of 5 μm MmtRNACAALeu and mutant (+C17a) with 15 mm Nva. D, graphical representation of AMP formation as a function of time for MmtRNACAALeu and mutant (+C17a). kobs values for AMP formation were calculated from the slopes (Table 9).
Taken together, these results show that the SC-fold β-strand influences both aminoacylation and editing activities of EcLeuRS via conserved acidic residues. Revealingly, the editing activity of MmLeuRS that ordinarily lacks these residues was enhanced when acidic residues were introduced into this enzyme.
DISCUSSION
Conserved SC-fold Is Crucial for Both Aminoacylation and Editing of LeuRSs
Systematic comparison of class I synthetases identified a region connecting the Rossmann fold and the anticodon binding domain (7, 15–18) named the SC-fold. This module is poorly conserved in sequence and is widely considered to function in a structure-dependent manner rather than in a sequence-dependent manner. However, this study showed that several crucial SC-fold residues contribute to both aminoacylation and editing functions of LeuRS and interact specifically with the tRNA. Although archaeal and eukaryotic cytoplasmic LeuRSs are architecturally distinct from bacterial LeuRSs (38), all share crucial elements, including one or two crucial basic residues on helix α3 of the SC-fold. Interestingly, many bacterial and archaeal LeuRSs contain additional basic residues, such as EcLeuRS that harbors an extra Lys residue at position 671 between the two crucial basic residues. However, mutation of this additional basic residue had no effect on the aminoacylation activity of the enzyme (Table 1). Moreover, of the five basic residues on helix α3 of PhLeuRS, only Arg703 and Lys696 were essential for catalytic activity. In contrast, hcLeuRS contains only one basic residue (Arg766) that is involved in both synthetic and editing catalytic steps. These results suggest that despite the evident separation in the three kingdoms of life, evolutionary pressure has caused the SC-fold to retain these functional elements.
Specific Gly residues form the second critical element that modulates the aminoacylation and editing properties of LeuRS, and mutation of these residues affected the Leu activation activity in EcLeuRS, suggesting the synthetic active site pocket may undergo conformational changes during catalysis. In this study we identified a mutant (EcLeuRS-G665P) with diminished editing properties reminiscent of a previously characterized CP1-editing site mutant (EcLeuRS-Y330D) (34). Compared with the basic residues, mutants with impaired aminoacylation activity, both EcLeuRS-G665P and EcLeuRS-Y330D, retained tRNA aminoacylation activity, and these variants could therefore be used in the future to mischarged aa-tRNALeu for deacylation studies.
The crystal structure of EcLeuRS in complex with tRNALeu (PDB codes 4ARC and 4AQ7) revealed Gly residues that may contribute to conformational changes of the SC-fold β-strand containing several acidic residues (Glu657 and Asp653) involved in the fidelity of EcLeuRS (Fig. 4A). This structure also highlighted the proximity of residue Met654 and tRNALeu, which is an even shorter distance than between Glu657, Asp653, and the tRNA (PDB code 4AQ7) (11). However, mutation of Met654 to Ala or Lys did not affect either the synthetic or editing properties of LeuRS, suggesting Met654 may not be functionally important or may act in a different way (data not shown).
Enzymes Lacking the SC-fold Crucial Residues Exhibit Impaired Editing Capacity
To date, only two LeuRSs lacking the editing activity have been described (9, 10, 38, 39) as follows: MmLeuRS lacks the entire CP1 editing domain, and human mitochondrial LeuRS (hmLeuRS) has lost only the catalytic residues of the CP1 domain. As a consequence, both enzymes are deprived of post-transfer editing activity. Sequence alignment revealed that neither MmLeuRS nor hmLeuRS contain the Gly or acidic residues found in helix α3 of the SC-fold (Fig. 1C). Additionally, hmLeuRS utilizes a Thr instead of the first Gly of helix α3, and it has a Leu in place of the crucial Glu in the β-strand. The lack of editing activity in these enzymes further suggested that they may be functionally important and may be involved in mediating communication between the SC-fold and the CP1 editing domain.
Improving the Aminoacylation Fidelity by Modifying the Editing Determinants of Both Synthetase and tRNA
The discovery of residues of the SC-fold that influence the editing capacity of LeuRS and the interaction with the anticodon stem and D-loop of tRNALeu provide valuable new information that may be of use in the engineering of both synthetase enzymes and tRNAs. Incorporation of two key EcLeuRS β-strand residues, including a conserved acidic residue, into the chimeric MmLeuRS-CP1-LSD enzyme significantly enhanced the editing activity, which opens up exciting possibilities for improving the fidelity of this and other artificial synthetases.
Similarly, we engineered the tRNA to improve the fidelity of LeuRS by enhancing the tRNA-dependent editing capacity. We observed that the isoacceptor MmtRNACAALeu was only weakly able to stimulate the editing activity of the chimeric LeuRS, compared with other isoacceptors. By inserting a single nucleotide in the D-loop of MmtRNACAALeu that is naturally smaller than the D-loop of other tRNALeu molecules, the editing activity of the synthetic MmLeuRS enzyme was markedly enhanced. In concert with enzyme engineering, such tRNA modification approaches are paving the way for improving the fidelity of artificial synthetase enzymes. Although error-prone systems are presumably advantageous for proteome diversity in some living organisms (9, 19), constructing artificial enzymes with high fidelity from naturally occurring enzymes lacking proofreading activity remains challenging. Remarkably, the work described here suggests that at least two nonexclusive pathways may exist to achieve this goal.
In summary, this study reveals the functional importance of the conserved SC-fold module in both the aminoacylation and editing activities of LeuRS enzymes. The SC-fold is therefore not only a connecting linker between the tRNA-binding module and the catalytic site. Furthermore, the SC-fold interacts with the inner side of the L-shaped tRNA, thereby positioning the anticodon stem. Given that the KMSKS motif is presumed to contribute to the catalytic site, the SC-fold carrying this module also forms a functional link between the catalytic activity and tRNA binding. This feature senses the size of the D-loop of tRNALeu and controls the editing reaction. In addition, engineering of the chimeric MmLeuRS-CP1/LSD and tRNA provides evidence that the SC-fold module has co-evolved with the CP1 editing domain in LeuRS enzymes.
Acknowledgments
We thank Drs. Michael T. Bethune and Shuai Jiang at Caltech and Drs. Xiaolong Zhou and Rujuan Liu in our laboratory for their good suggestions.
This work was supported by National Key Basic Research Foundation of China Grant 2012CB911000, Natural Science Foundation of China Grant 31130064, and Committee of Science and Technology in Shanghai Grant 12JC1409700.
- aaRS
- aminoacyl-tRNA synthetase
- CP1
- connective peptide 1
- LeuRS
- leucyl-tRNA synthetase
- LSD
- leucine-specific domain
- Nva
- norvaline
- SC-fold
- stem contact-fold
- PhLeuRS
- P. horikoshii LeuRS
- hcLeuRS
- human cytoplasm LeuRS
- MmLeuRS
- M. mobile LeuRS
- EcLeuR
- E. coli LeuRS
- hmLeuRS
- human mitochondrial LeuRS
- PDB
- Protein Data Bank.
REFERENCES
- 1. Ling J., Reynolds N., Ibba M. (2009) Aminoacyl-tRNA synthesis and translational quality control. Annu. Rev. Microbiol. 63, 61–78 [DOI] [PubMed] [Google Scholar]
- 2. Ibba M., Soll D. (2000) Aminoacyl-tRNA synthesis. Annu. Rev. Biochem. 69, 617–650 [DOI] [PubMed] [Google Scholar]
- 3. Woese C. R., Olsen G. J., Ibba M., Söll D. (2000) Aminoacyl-tRNA synthetases, the genetic code, and the evolutionary process. Microbiol. Mol. Biol. Rev. 64, 202–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eriani G., Delarue M., Poch O., Gangloff J., Moras D. (1990) Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence modules. Nature 347, 203–206 [DOI] [PubMed] [Google Scholar]
- 5. Cusack S., Berthet-Colominas C., Härtlein M., Nassar N., Leberman R. (1990) A second class of synthetase structure revealed by x-ray analysis of Escherichia coli seryl-tRNA synthetase at 2.5 Å. Nature 347, 249–255 [DOI] [PubMed] [Google Scholar]
- 6. Burbaum J. J., Schimmel P. (1991) Structural relationships and the classification of aminoacyl-tRNA synthetases. J. Biol. Chem. 266, 16965–16968 [PubMed] [Google Scholar]
- 7. Cusack S., Yaremchuk A., Tukalo M. (2000) The 2 Å crystal structure of leucyl-tRNA synthetase and its complex with a leucyl-adenylate analogue. EMBO J. 19, 2351–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lincecum T. L., Jr., Tukalo M., Yaremchuk A., Mursinna R. S., Williams A. M., Sproat B. S., Van Den Eynde W., Link A., Van Calenbergh S., Grøtli M., Martinis S. A., Cusack S. (2003) Structural and mechanistic basis of pre- and posttransfer editing by leucyl-tRNA synthetase. Mol. Cell 11, 951–963 [DOI] [PubMed] [Google Scholar]
- 9. Li L., Boniecki M. T., Jaffe J. D., Imai B. S., Yau P. M., Luthey-Schulten Z. A., Martinis S. A. (2011) Naturally occurring aminoacyl-tRNA synthetases editing-domain mutations that cause mistranslation in Mycoplasma parasites. Proc. Natl. Acad. Sci. U.S.A. 108, 9378–9383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tan M., Yan W., Liu R. J., Wang M., Chen X., Zhou X. L., Wang E. D. (2012) A naturally occurring nonapeptide functionally compensates for the CP1 domain of leucyl-tRNA synthetase to modulate aminoacylation capability. Biochem. J. 443, 477–484 [DOI] [PubMed] [Google Scholar]
- 11. Palencia A., Crépin T., Vu M. T., Lincecum T. L., Jr., Martinis S. A., Cusack S. (2012) Structural dynamics of the aminoacylation and proofreading functional cycle of bacterial leucyl-tRNA synthetase. Nat. Struct. Mol. Biol. 19, 677–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tukalo M., Yaremchuk A., Fukunaga R., Yokoyama S., Cusack S. (2005) The crystal structure of leucyl-tRNA synthetase complexed with tRNALeu in the post-transfer-editing conformation. Nat. Struct. Mol. Biol. 12, 923–930 [DOI] [PubMed] [Google Scholar]
- 13. Sugiura I., Nureki O., Ugaji-Yoshikawa Y., Kuwabara S., Shimada A., Tateno M., Lorber B., Giegé R., Moras D., Yokoyama S., Konno M. (2000) The 2.0 Å crystal structure of Thermus thermophilus methionyl-tRNA synthetase reveals two RNA-binding modules. Structure 8, 197–208 [DOI] [PubMed] [Google Scholar]
- 14. Rould M. A., Perona J. J., Söll D., Steitz T. A. (1989) Structure of E. coli glutaminyl-tRNA synthetase complexed with tRNA(Gln) and ATP at 2.8 Å resolution. Science 246, 1135–1142 [DOI] [PubMed] [Google Scholar]
- 15. Fukunaga R., Yokoyama S. (2005) Crystal structure of leucyl-tRNA synthetase from the archaeon Pyrococcus horikoshii reveals a novel editing domain orientation. J. Mol. Biol. 346, 57–71 [DOI] [PubMed] [Google Scholar]
- 16. Fukunaga R., Yokoyama S. (2005) Aminoacylation complex structures of leucyl-tRNA synthetase and tRNALeu reveal two modes of discriminator-base recognition. Nat. Struct. Mol. Biol. 12, 915–922 [DOI] [PubMed] [Google Scholar]
- 17. Casina V. C., Lobashevsky A. A., McKinney W. E., Brown C. L., Alexander R. W. (2011) Role for a conserved structural module in assembly of a class I aminoacyl-tRNA synthetase active site. Biochemistry 50, 763–769 [DOI] [PubMed] [Google Scholar]
- 18. Cavarelli J., Delagoutte B., Eriani G., Gangloff J., Moras D. (1998) l-Arginine recognition by yeast arginyl-tRNA synthetase. EMBO J. 17, 5438–5448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gomes A. C., Miranda I., Silva R. M., Moura G. R., Thomas B., Akoulitchev A., Santos M. A. (2007) A genetic code alteration generates a proteome of high diversity in the human pathogen Candida albicans. Genome Biol. 8, R206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hu Q. H., Huang Q., Wang E. D. (2013) Crucial role of the C-terminal domain of Mycobacterium tuberculosis leucyl-tRNA synthetase in aminoacylation and editing. Nucleic Acids Res. 41, 1859–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yan W., Tan M., Eriani G., Wang E. D. (2013) Leucine-specific domain modulates the aminoacylation and proofreading functional cycle of bacterial leucyl-tRNA synthetase. Nucleic Acids Res. 41, 4988–4998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rogers H. H., Griffiths-Jones S. (2014) tRNA anticodon shifts in eukaryotic genomes. RNA 20, 269–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhou X. L., Du D. H., Tan M., Lei H. Y., Ruan L. L., Eriani G., Wang E. D. (2011) Role of tRNA amino acid-accepting end in aminoacylation and its quality control. Nucleic Acids Res. 39, 8857–8868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Crothers D. M., Seno T., Söll G. (1972) Is there a discriminator site in transfer RNA? Proc. Natl. Acad. Sci. U.S.A. 69, 3063–3067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McClain W. H. (1993) Rules that govern tRNA identity in protein synthesis. J. Mol. Biol. 234, 257–280 [DOI] [PubMed] [Google Scholar]
- 26. Du X., Wang E. D. (2003) Tertiary structure base pairs between D- and T C-loops of Escherichia coli tRNA(Leu) play important roles in both aminoacylation and editing. Nucleic Acids Res. 31, 2865–2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yao P., Zhou X. L., He R., Xue M. Q., Zheng Y. G., Wang Y. F., Wang E. D. (2008) Unique residues crucial for optimal editing in yeast cytoplasmic leucyl-tRNA synthetase are revealed by using a novel knockout yeast strain. J. Biol. Chem. 283, 22591–22600 [DOI] [PubMed] [Google Scholar]
- 28. Huang Q., Yao P., Eriani G., Wang E. D. (2012) In vivo identification of essential nucleotides in tRNA(Leu) to its functions by using a constructed yeast tRNALeu knockout strain. Nucleic Acids Res. 40, 10463–10477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nordin B. E., Schimmel P. (1999) RNA determinants for translational editing. Mischarging a minihelix substrate by a tRNA synthetase. J. Biol. Chem. 274, 6835–6838 [DOI] [PubMed] [Google Scholar]
- 30. Chen X., Ma J. J., Tan M., Yao P., Hu Q. H., Eriani G., Wang E. D. (2011) Modular pathways for editing non-cognate amino acids by human cytoplasmic leucyl-tRNA synthetase. Nucleic Acids Res. 39, 235–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhou X. L., Wang M., Tan M., Huang Q., Eriani G., Wang E. D. (2010) Functional characterization of leucine-specific domain 1 from eukaryal and archaeal leucyl-tRNA synthetases. Biochem. J. 429, 505–513 [DOI] [PubMed] [Google Scholar]
- 32. Zhu B., Yao P., Tan M., Eriani G., Wang E. D. (2009) tRNA-independent pretransfer editing by class I leucyl-tRNA synthetase. J. Biol. Chem. 284, 3418–3424 [DOI] [PubMed] [Google Scholar]
- 33. Li Y., Wang E. D., Wang Y. L. (1998) Overproduction and purification of Escherichia coli tRNA(Leu). Sci. China Ser. C-Life Sci. 41, 225–231 [DOI] [PubMed] [Google Scholar]
- 34. Tan M., Zhu B., Zhou X. L., He R., Chen X., Eriani G., Wang E. D. (2010) tRNA-dependent pre-transfer editing by prokaryotic leucyl-tRNA synthetase. J. Biol. Chem. 285, 3235–3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yarus M., Berg P. (1970) On the properties and utility of a membrane filter assay in the study of isoleucyl-tRNA synthetase. Anal. Biochem. 35, 450–465 [DOI] [PubMed] [Google Scholar]
- 36. Michel S. L., Guerrerio A. L., Berg J. M. (2003) Selective RNA binding by a single CCCH zinc-binding domain from Nup475 (Tristetraprolin). Biochemistry 42, 4626–4630 [DOI] [PubMed] [Google Scholar]
- 37. Guijarro J. I., Pintar A., Prochnicka-Chalufour A., Guez V., Gilquin B., Bedouelle H., Delepierre M. (2002) Structure and dynamics of the anticodon arm binding domain of Bacillus stearothermophilus tyrosyl-tRNA synthetase. Structure 10, 311–317 [DOI] [PubMed] [Google Scholar]
- 38. Lue S. W., Kelley S. O. (2005) An aminoacyl-tRNA synthetase with a defunct editing site. Biochemistry 44, 3010–3016 [DOI] [PubMed] [Google Scholar]
- 39. Li L., Palencia A., Lukk T., Li Z., Luthey-Schulten Z. A., Cusack S., Martinis S. A., Boniecki M. T. (2013) Leucyl-tRNA synthetase editing domain functions as a molecular rheostat to control codon ambiguity in Mycoplasma pathogens. Proc. Natl. Acad. Sci. U.S.A. 110, 3817–3822 [DOI] [PMC free article] [PubMed] [Google Scholar]