Background: The mechanisms underlying UCP1-independent thermogenesis are not well understood.
Results: Loss of both SLN and UCP1 results in compromised thermogenic ability and severe sensitivity to acute cold.
Conclusion: Sarcolipin-mediated thermogenesis is required for optimal thermogenesis and is up-regulated in the absence of UCP1.
Significance: Sarcolipin is a crucial contributor to thermogenesis and energy expenditure.
Keywords: adipose tissue, calcium ATPase, muscle physiology, sarcoplasmic reticulum (SR), skeletal muscle, skeletal muscle metabolism, uncoupling protein, sarcolipin, thermogenesis
Abstract
The importance of brown adipose tissue as a site of nonshivering thermogenesis has been well documented. Emerging studies suggest that skeletal muscle is also an important site of thermogenesis especially when brown adipose tissue function is lacking. We recently showed that sarcolipin (SLN), an uncoupler of the sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) pump, could contribute to heat production in skeletal muscle. In this study, we sought to understand how loss of UCP1 or SLN is compensated during cold exposure and whether they are both necessary for thermogenesis. Toward this goal, we generated a UCP1;SLN double knock-out (DKO) mouse model and challenged the single and DKO mice to acute and long-term cold exposures. Results from this study show that there is up-regulation of SLN expression in UCP1-KO mice, and loss of SLN is compensated by increased expression of UCP1 and browning of white adipose tissue. We found that the DKO mice were viable when reared at thermoneutrality. When challenged to acute cold, the DKO were extremely cold-sensitive and became hypothermic. Paradoxically, the DKO mice were able to survive gradual cold challenge, but these mice lost significant weight and depleted their fat stores, despite having higher caloric intake. These studies suggest that UCP1 and SLN are required to maintain optimal thermogenesis and that loss of both systems compromises survival of mice under cold stress.
Introduction
Maintaining constant body temperature is an important homeostatic mechanism essential for normal physiological functions and survival of endotherms. This ability to regulate internal temperature has enabled mammals and birds to colonize vast areas of the world. To maintain body temperature when external temperatures fall below thermoneutrality (1, 2), endotherms rely on facultative thermogenic mechanisms. Among these thermogenic mechanisms, shivering is a first line of defense. However, prolonged shivering can be detrimental to muscle health; therefore, the organism recruits nonshivering thermogenesis (NST)2 to replace and/or supplement shivering thermogenesis. The activation of NST from brown adipose tissue (BAT) is a well studied and well established phenomenon in mammals, in particular rodents (3, 4). The thermogenic capacity of BAT is dependent on the activity of uncoupling protein 1 (UCP1), which generates heat by dissipating the mitochondrial proton gradient (5–7). However, in many adult large mammals, BAT activity is limited to neonatal stages by becoming down-regulated in the adult and is even absent in some endothermic species. Therefore, these animals, including humans, must rely on alternative thermogenic mechanisms to survive when exposed to cold environments (8–13).
Because many studies use rodents, which heavily rely on BAT, as experimental models to understand NST mechanisms, the importance of alternate thermogenic mechanisms has been overlooked. However, there is increasing evidence for the existence of BAT-independent sites of thermogenesis in mammals. This has become especially obvious from studies on large mammals that have limited BAT, as well as from UCP1-knock-out (UCP1-KO) mice. Although UCP1-KO mice are extremely sensitive to acute cold exposure, they can be gradually adapted to 4 °C (14, 15). Additionally, the cold sensitivity of UCP1-KO mice is incompletely penetrant and highly dependent on genetic background; 32.1% of C57Bl/6J mice and 87.5% of 129SV/J mice are cold-sensitive (15). Although BAT is unequivocally a dominant thermogenic mechanism in rodents, several studies have suggested other mechanisms of heat production. Indeed, published studies on cold-adapted UCP1-KO mice have implicated that Ca2+ cycling in skeletal muscle, thermogenesis from white adipose tissue (UCP1-independent), and substrate cycling in skeletal muscle could be recruited to produce heat, but none of these mechanisms were found to be dominant (15–18).
Skeletal muscle has long been viewed as a significant contributor to thermogenesis. Studies on large mammals, including rabbits, dogs, ruminants, marsupials, etc., suggest that muscle is the major site of heat production (8, 9, 13, 19–21). These species have reduced BAT content in adulthood, especially when compared with rodents; thus, they cannot rely entirely on BAT thermogenesis to maintain body temperature. Similarly, it is widely accepted that birds, which completely lack a Ucp1 gene, predominately utilize muscle-based thermogenesis (22–28). Thus, a major research interest of our laboratory has been to investigate the role of skeletal muscle as a site of thermogenesis, independent of shivering. Recently, we showed that skeletal muscle is an important site for thermogenesis even in rodents, especially when BAT function is compromised (29). Our studies highlighted that sarcoplasmic reticulum Ca2+ transport serves as an important mechanism for heat production in muscle. We described that uncoupling of sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) Ca2+ transport from ATP hydrolysis by sarcolipin (SLN), a regulator of sarco(endo)plasmic reticulum Ca2+ ATPase, can increase ATP hydrolysis and heat production via futile cycling of the pump (29). This novel discovery led us to the question: is SLN-mediated heat production the “missing link” underlying UCP1-independent thermogenesis? Thus, we wanted to investigate whether SLN-based thermogenesis could compensate and enable the UCP1-KO mice to adapt to the cold. Furthermore, we hypothesized that a loss of both UCP1 and SLN would render mice unable to survive in the cold. To test this, we generated a UCP1;SLN double knock-out (DKO) mouse model and challenged the single and DKO mice to acute and long-term cold exposures. Our studies highlight the compensatory and unique roles of these two thermogenic systems in a mouse model.
EXPERIMENTAL PROCEDURES
Animals
The generation of UCP1−/− and SLN−/− mice have been described previously (6, 30). UCP1−/−·SLN−/− mice on a C57Bl/6J background were generated by crossing UCP1−/−·SLN+/+ and UCP1+/+·SLN−/− to obtain the first generation of UCP1+/−·SLN+/−. The double heterozygotes were intercrossed to obtain the DKO and littermate controls. The mice were maintained in a temperature-controlled room at either 22 °C or 28 °C and fed a chow diet (Harlan Laboratories, rodent diet 17% kcal/fat). Male and female mice were used for the acute cold exposure studies. Only male mice were used for the gradual cold adaptation experiments.
Acute Cold Exposures
All cold exposures were carried out in a temperature-controlled unit. Mice maintained at either 22 °C or 28 °C were transferred to the pre-cooled 4 °C unit and housed in individual cages. Body temperature was monitored every 20 min during the first hour and then every 30–60 min thereafter using implanted thermal transponders (29). Body weight was measured immediately before the cold exposure. Numbers of mice maintained at 22 °C were as follows: WT (n = 3), SLN-KO (n = 4), UCP1-KO (n = 10), DKO (n = 12). Numbers of mice maintained at 28 °C were as follows: WT (n = 11), SLN-KO (n = 9), UCP1-KO (n = 7), DKO (n = 7).
Gradual Cold Adaptation
Mice previously maintained at 28 °C were individually housed in the temperature-controlled unit where the temperature was decreased to 22 °C and maintained for 2 days, decreased to 18 °C for 2 days, and then decreased by 2 °C/day until the temperature reached 4 °C. The mice were housed at 4 °C for a further 9–10 days until the termination of the experiment. Using implanted thermal transponders, body temperature was monitored at the same time everyday. Body weight was measured twice weekly. Numbers of mice used were as follows: WT (n = 6), SLN-KO (n = 6), UCP1-KO (n = 5), DKO (n = 6).
Metabolic Monitoring
During the cold exposures, oxygen consumption and carbon dioxide production were continuously measured by the Oxymax Comprehensive Lab Animal Monitoring System (CLAMS, Columbus Instruments, Columbus, OH). Food intake was measured by physical weighing of the food provided.
Histology of Adipose Tissues
Brown adipose tissue, epididymal, and inguinal fat pads were fixed in 10% formalin. Paraffin-embedded sections were stained with hematoxylin and eosin. Images were collected with a Richter Optica U2D digital microscope with a digital camera (5mp ToupCam) attached.
Urinary Catecholamine Output
Urine was obtained by a one-time collection at the same time of day at four temperatures during the gradual cold adaptation: 28, 18, 10, and 4 °C. Hydrochloric acid was added to a final concentration of 0.1 m to maintain the integrity of the catecholamine levels until analysis. The collected urine was stored at −80 °C until analysis. Norepinephrine and epinephrine levels were determined by ELISA (Rocky Mountain Diagnostics). Catecholamine levels were normalized to creatinine content, determined by a creatinine (urinary) colorimetric assay (Cayman Chemical).
Western Blotting
Protein expression was analyzed as described previously (29). Briefly, muscle homogenates were resolved by SDS-PAGE (16% Tris-Tricine for SLN, 10% Tris-glycine for UCP1). Proteins were transferred to a nitrocellulose membrane (0.2 μm for SLN, 0.45 μm for UCP1). The membranes were blocked in 5% BSA or milk and probed with primary antibodies to SLN (Millipore, rabbit polyclonal antibody), UCP1 (rabbit polyclonal antibody, a kind gift from Dr. Barbara Cannon), myoglobin (Santa Cruz Biotechnology, rabbit polyclonal antibody), and GAPDH (Thermo Scientific, mouse monoclonal antibody). Secondary horseradish peroxidase antibodies were applied for 1 h at a 1:25,000–1:50,000 dilution. Expression was detected with chemiluminescent substrate.
Statistical Analysis
Data are expressed as means ± S.E. Differences among groups were determined by one-way analysis of variance. Statistically significant differences were accepted at p < 0.05.
RESULTS
Gradual Cold Adaptation of UCP1-KO and SLN-KO Mice
It has previously been shown that UCP1-KO mice can readily adapt to cold (14, 15, 31, 32), but the mechanism(s) underlying their adaptation has remained incompletely understood. In this study, we reinvestigated the mechanism underlying cold adaptation in UCP1-KO mice. We tested the hypothesis that increased SLN expression could potentially compensate for loss of BAT function. We adapted WT, SLN-KO, and UCP1-KO mice to 4 °C by gradually reducing the temperature from 28 °C and then maintaining the mice at 4 °C for ∼10 days. After cold adaptation, we observed an increased redness of the muscles of UCP1-KO mice, when compared with WT (Fig. 1A), accompanied by an increased myoglobin content (Fig. 1B). Interestingly, we observed a strong induction of SLN in the red quadriceps (Fig. 1C), and to a lesser extent in the whole gastrocnemius, infraspinatus, and soleus muscles (Fig. 1C and data not shown). However, SLN expression was not altered in the diaphragm, which already expresses high levels in WT and UCP1-KO mice (Fig. 1C).
FIGURE 1.
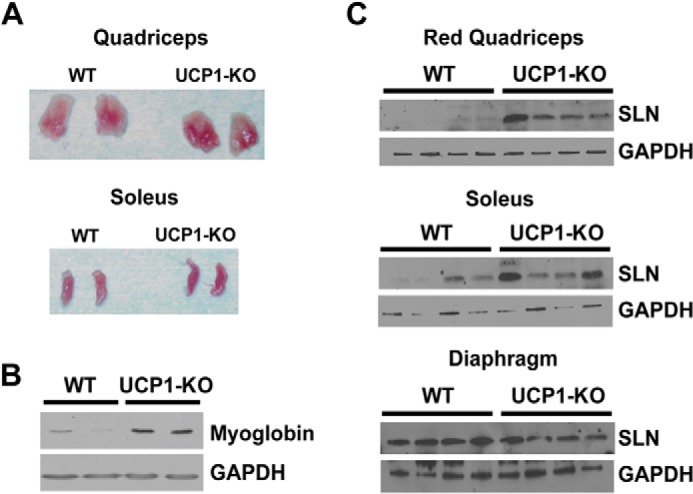
Muscle of cold-adapted UCP1-KO mice. A, representative images of cold-adapted quadriceps and soleus muscles are presented to show the greater reddening of the muscle in UCP1-KO. B, myoglobin protein expression in quadriceps muscles of cold-adapted WT and UCP1-KO mice. C, SLN protein expression in the red quadriceps, soleus, and diaphragm muscles of WT and UCP1-KO mice after cold adaptation.
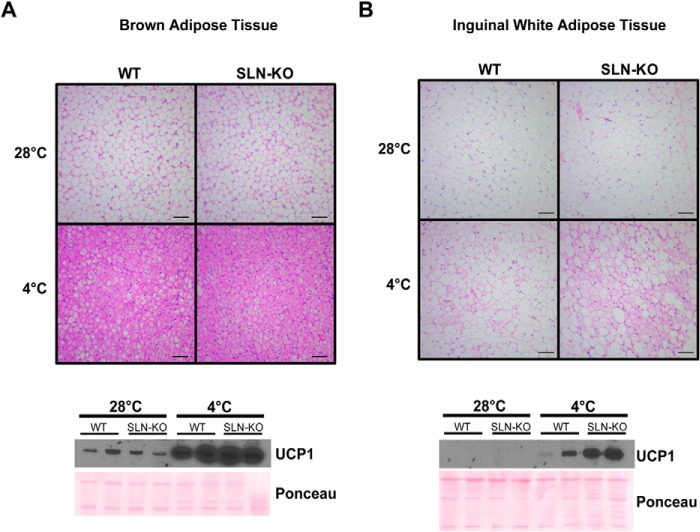
As expected, the SLN-KO mice were able to adapt to gradual cold exposure. H&E staining of brown adipose tissue depots of cold-adapted WT and SLN-KO mice showed extreme lipid depletion, which was slightly greater in the SLN-KO (Fig. 2A). UCP1 protein was ∼2-fold greater in the cold-adapted SLN-KO when compared with WT (Fig. 2A). We next examined whether there is additional recruitment of subcutaneous white adipose tissue (WAT) to increase thermogenesis by what is known as “browning.” Interestingly, H&E staining of subcutaneous WAT in SLN-KO mice showed morphological characteristics consistent with browning: large areas of fat depletion and multilocular adipocytes (Fig. 2B). Moreover, UCP1 expression was increased ∼3-fold in the SLN-KO when compared with WT, and mitochondrial protein content was higher in the SLN-KO (Fig. 2B and data not shown). Therefore, to overcome the loss of SLN-based thermogenesis, SLN-KO mice must increase UCP1-based thermogenesis by increasing BAT activity and browning of WAT.
FIGURE 2.
A and B, hematoxylin and eosin staining of BAT (A) and inguinal (subcutaneous) WAT (B) from thermoneutral (28 °C) and cold-adapted (4 °C) WT and SLN-KO mice. UCP1 protein expression in thermoneutral (28 °C) and cold-adapted (4 °C) WT and SLN-KO mice from BAT (A) and inguinal WAT (B) is presented below. Scale bars are equal to 100 μm.
Increased Energy Expenditure in SLN-KO Mice
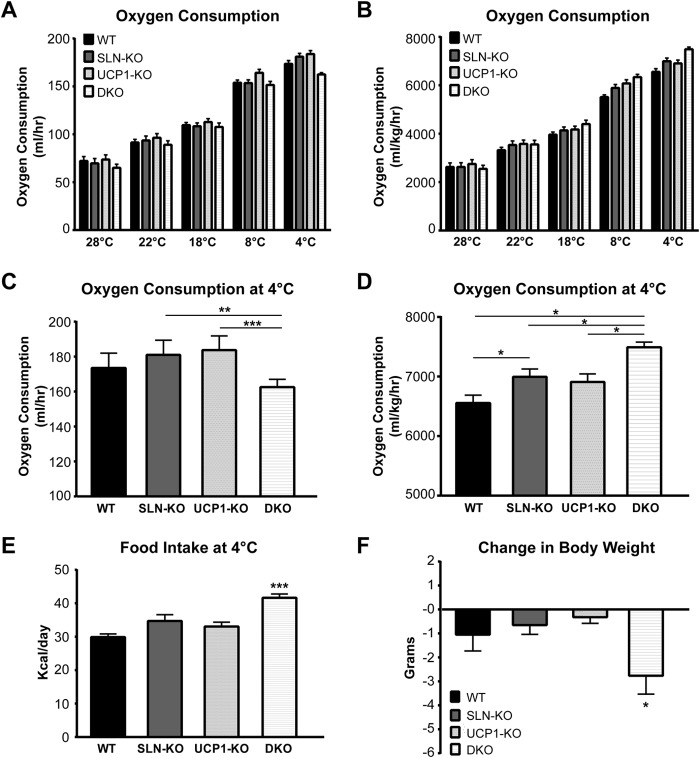
We also investigated the energetic cost of cold adaptation in these mice. Throughout the cold adaptation period, oxygen consumption, food intake, and body weight were monitored. We found that SLN-KO mice exhibited higher oxygen consumption at 4 °C than WT mice (Fig. 3, A–D) and also trended toward greater food intake (Fig. 3E). Interestingly, measurement of fat pad weights from thermoneutral controls and cold-adapted mice trended toward a greater depletion of fat stores from SLN-KO than WT mice (Fig. 4A). Similarly, UCP1-KO mice, as reported previously (16), exhibited higher oxygen consumption (Fig. 3, A–D) and significantly greater fat loss than WT (Fig. 4A), although food intake was not significantly different (Fig. 3E). Together, these data suggest that a loss of either SLN or UCP1 places a greater energetic cost to maintain thermogenesis and would be detrimental to the survival of mice during long-term cold exposure.
FIGURE 3.
Energetics of cold adaptation. A and B, average oxygen consumption over 24-h periods at each temperature indicated during the cold adaptation. A, not normalized to body weight; B, normalized to body weight. C and D, oxygen consumption over a 24-h period at 4 °C. C, not normalized to body weight; D, normalized to body weight. * = p < 0.05, ** = p < 0.01, *** = p < 0.001. E, daily caloric intake per mouse at 4 °C. *** = WT versus DKO, p < 0.001; SLN-KO versus DKO, p < 0.05; UCP1-KO versus DKO, p < 0.01. F, change in body weight (in grams) from the start of the cold adaptation period to the end. * = WT versus DKO, SLN-KO versus DKO, UCP1-KO versus DKO, p < 0.05. Data are expressed as means ± S.E.
FIGURE 4.
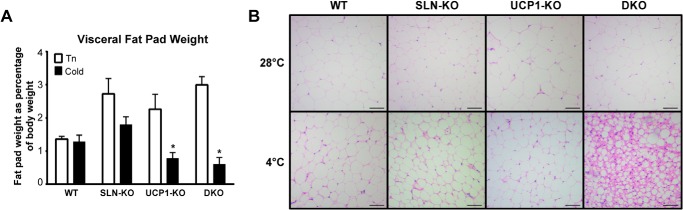
A, epididymal (visceral white fat) fat pad weights from thermoneutral (Tn) control mice and cold-acclimated (Cold) mice at the end of the cold adaptation protocol. Fat pad weights are expressed as a percentage of body weight. * = UCP1-KO Tn versus UCP1-KO Cold, DKO Tn versus DKO Cold, p < 0.05. Data are expressed as means ± S.E. B, hematoxylin and eosin staining of epididymal white adipose tissue from thermoneutral (28 °C) and cold-adapted mice (4 °C). Adipocyte size is mildly reduced in WT, SLN-KO, and UCP1-KO mice after cold adaptation; however, cold adaptation of DKO mice results in a severe reduction in adipocyte size. Scale bars are equal to 100 μm.
UCP1/SLN DKO Mice Show Poor Survival Rate at 22 °C
We hypothesized that a loss of both UCP1 and SLN would render mice unable to survive in the cold. To test this, we generated a UCP1;SLN DKO mouse model by breeding double heterozygotes (UCP1+/−·SLN+/−). When bred at standard vivarium temperature (22 °C), very few DKO mice were obtained, and they were found to be at less than the expected Mendelian frequencies, whereas single knock-out mice were not affected (Table 1). Although 22 °C is a mild cold stress, it is sufficient to cause death in newborns because neonates are much more vulnerable to cold than adult mice (33, 34). Therefore, we bred the double heterozygotes at thermoneutrality (28 °C) and found that the DKO mice were born at normal Mendelian frequencies. These findings suggest that a loss of both SLN and UCP1 reduces the thermogenic capacity significantly and compromises the survival of newborn mice.
TABLE 1.
Observed and predicted frequencies of offspring from UCP1+/−·SLN+/− intercrosses when reared at 22 and 28 °C
| Genotype | 22 °C |
28 °C |
||
|---|---|---|---|---|
| Observed (n = 136) | Predicted | Observed (n = 113) | Predicted | |
| UCP1+/+·SLN+/+ | 15 | 8.5 | 9 | 7.0625 |
| UCP1+/+·SLN−/− | 11 | 8.5 | 11 | 7.0625 |
| UCP1−/−·SLN+/+ | 11 | 8.5 | 9 | 7.0625 |
| UCP1−/−·SLN−/− | 4 | 8.5 | 9 | 7.0625 |
Acute Cold Challenge of Mice Housed at 22 °C versus 28 °C
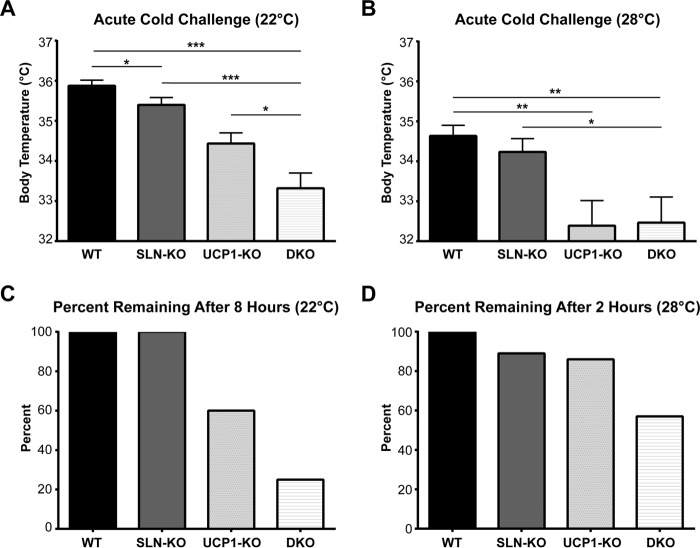
It is known that UCP1-KO mice are sensitive to an acute cold challenge; however, their sensitivity is highly variable and is influenced by factors including housing temperature (15, 32). Therefore, we maintained WT, SLN-KO, UCP1-KO, and DKO mice at two different temperatures, 22 °C and 28 °C, and then challenged them to a 4 °C exposure. When reared at 22 °C, the DKO mice were severely cold-sensitive, as indicated by reduced body temperature and a greater number (75%) reaching early removal criteria (ERC, defined by a body temperature reaching 30 °C) within 8 h of cold challenge (Fig. 5, A and C). In agreement with published data, UCP1-KO mice were also cold-sensitive, although to a lesser degree than the DKO. As expected, SLN-KO mice were found to be cold-sensitive, as indicated by a lower body temperature than WT mice, but did not develop hypothermia.
FIGURE 5.
Acute cold exposure of DKO mice. A and B, average core body temperature during an acute cold exposure to 4 °C in WT, SLN-KO, UCP1-KO, and DKO mice reared at 22 °C (A) and 28 °C (B). * = p < 0.05, ** = p < 0.01, *** = p < 0.001. C and D, the percentage of mice reaching ERC, defined by a body temperature ≤30 °C, during the acute cold challenge in mice reared at 22 °C (C) and 28 °C (D). Data are expressed as means ± S.E.
On the other hand, when maintained at thermoneutrality (28 °C), NST mechanisms (BAT-dependent and -independent) are down-regulated because there is no requirement for facultative thermogenesis to maintain body temperature (1). Therefore, when these mice were challenged acutely to 4 °C, all mice showed increased cold sensitivity as indicated by a greater drop in body temperature (Fig. 5B), due to an inability to fully recruit NST mechanisms. Interestingly, the DKO mice reared at 28 °C were highly cold-sensitive; 43% had reached ERC within 2 h of cold challenge, whereas only 14% of UCP1-KO mice reached ERC (Fig. 5D). When the cold challenge was continued beyond 2 h, 100% of the DKO and UCP1-KO mice reached ERC (data not shown). Another interesting observation was that when mice were reared at 28 °C, the effect of loss of SLN on thermogenic capacity became much more apparent. Here, we observed that ∼33% of SLN-KO mice are cold-sensitive (reaching ERC within 4 h of cold challenge, data not shown), when compared with ∼11% of WT. In contrast, none of the SLN-KO mice reached ERC when reared at 22 °C (Fig. 5C).
DKO Mice Can Survive Gradual Cold Exposure
Paradoxically, the majority of DKO mice were able to adapt to the cold by a gradual reduction in temperature, although one out of six developed hypothermia and lost ∼26% of body weight within 20 days of cold adaptation. Overall, the DKO mice had similar oxygen consumption rates as WT when expressed per mouse (Fig. 3, A–D) throughout the adaptation period. However, the DKO mice lose the most weight (11% of starting weight, when compared with 3.8% for WT) (Fig. 3F). Considering this, the DKOs had the highest oxygen consumption per body weight (Fig. 3, B and D). Interestingly, they also consumed 39% more food than WT mice at 4 °C (Fig. 3E). Despite higher caloric intake, the white fat stores of the DKO mice were almost completely depleted by the end of the exposure (Fig. 4A). Histological analyses of visceral fat depots further substantiate these data by showing much smaller adipocyte size in the DKO after cold adaptation, when compared with other genotypes (Fig. 4B). These histological analyses along with fat mass quantification suggest that DKO mice have to completely mobilize their fat stores, despite increased food intake, to maintain thermogenesis at all costs. These data point out that mice lacking both SLN-based and UCP1-based adaptive thermogenesis can survive in the cold but at an extremely high energetic cost.
DKO and UCP1-KO Mice Have Elevated Catecholamine Levels during Their Exposure to Cold
The sympathoadrenal system is an important regulator of whole body temperature homeostasis, and this is achieved by activating heat-generating as well as heat conservation mechanisms (35, 36). We hypothesized that the ability of DKO mice to adapt to cold was due, in part, to higher sympathoadrenal tone/levels. Therefore, we measured sympathoadrenal output by quantifying epinephrine and norepinephrine content in the urine from four time points during the cold adaptation (28, 18, 10, and 4 °C). We found that when mice were housed at 28 °C, there was no difference in norepinephrine or epinephrine among the genotypes, whereas their levels were elevated upon cold stress (Fig. 6). During cold adaptation, beginning at 18 °C, both UCP1-KO and DKO had elevated epinephrine and norepinephrine levels over WT and SLN-KO. Both UCP1-KO and DKO reached maximal epinephrine and norepinephrine levels by 10 °C; however, there was no difference between the two at any time point. These findings suggest that in the absence of UCP1, elevated adrenergic signaling is required to recruit BAT-independent thermogenic mechanisms.
FIGURE 6.
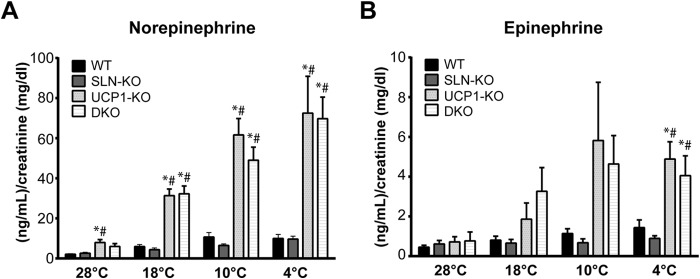
Urine was collected at each temperature shown during the cold adaptation. A and B, norepinephrine (A) and epinephrine (B) levels were determined by ELISA and normalized to creatinine levels. * = significant difference when compared with WT at the same temperature, # = significant difference when compared with SLN-KO at the same temperature. Data are expressed as means ± S.E.
DISCUSSION
Temperature homeostasis is a major homeostatic mechanism that is required for proper physiological functions. Despite its central importance to human physiology, the mechanisms that contribute to temperature homeostasis, including thermogenesis, are still poorly understood. However, during the past decade, there has been renewed interested in defining the mechanisms that contribute to thermogenesis. This is largely because thermogenic processes are unique in that they expend vast amounts of energy (releasing heat as a byproduct) when activated and can affect whole body metabolism and fat stores (37, 38). Thus, they are also ideal targets for increasing energy expenditure to combat weight gain and obesity.
BAT is a well studied component of mammalian thermogenesis. Multiple lines of evidence demonstrate the existence of BAT-independent thermogenesis, although the mechanisms have remained less well understood. Studies have shown that UCP1-KO mice, generated by Kozak and colleagues (6), can be gradually adapted to survive and maintain body temperature during prolonged exposure to 4 °C. It has been argued that constant shivering could be the major source of heat that replaces UCP1-mediated thermogenesis (31, 39). However, prolonged shivering can compromise muscle function and is detrimental to muscle health (39). Furthermore, UCP1-KO mice are severely sensitive to acute cold exposure, despite shivering. This suggests that shivering alone is not sufficient and that additional thermogenic mechanisms must be recruited to fully compensate for the absence of UCP1 thermogenesis.
Previous studies from our laboratory and others indicated that SLN is an important component of muscle thermogenesis and metabolism (29, 40–44). We found that SLN-KO mice were sensitive to acute cold exposure and developed hypothermia when interscapular BAT was ablated, suggesting that both SLN and BAT are major contributors to rodent thermogenesis (29). However, it has been shown that neither SLN nor BAT is absolutely necessary for survival during cold exposure. In this study, we therefore studied whether the presence of one can compensate for a lack of the other. Indeed, our studies suggest that SLN and UCP1 could compensate for each other's deficiency, indicating a functional interplay between the two systems. We found that SLN protein was up-regulated in the skeletal muscles of UCP1-KO mice after cold adaptation, accompanied by a deep reddening of the muscle and increased myoglobin content, which is indicative of a greater oxidative capacity. These data are consistent with previous studies that have shown increased mitochondrial content and fatty acid oxidative capacity in muscles of cold-adapted UCP1-KO mice (31). In addition, we found compensatory browning occurring in the subcutaneous and visceral (data not shown) white adipose tissue of SLN-KO mice after cold adaptation, which suggests that classical BAT alone is insufficient. Compensatory browning has been shown to occur in mouse models of defective thermogenesis (45, 46), which further substantiates the imperative contribution of SLN to thermogenesis.
Our oxygen consumption data provided interesting insight into the process of gradual cold adaptation. SLN-KO mice can readily adapt to the cold and maintain body temperature close to that of WT mice, like UCP1-KO mice. Both UCP1-KO and SLN-KO mice showed higher oxygen consumption than the WT mice, whereas both knock-out groups had similar oxygen consumption rates. Higher oxygen consumption is suggestive of an increased reliance on less efficient heat-producing mechanisms. Therefore, the above data indicate that when either of these mechanisms is not present, heat production becomes energetically costly, despite the compensatory recruitment of the other mechanism. Based on this observation, we propose that although BAT is the major heat producer in rodents, SLN is also a considerable contributor to thermogenesis, especially when considering that BAT constitutes less than 1% of mouse weight when compared with muscle, which constitutes about 40% of mouse weight. This argument is further supported by the fact that both the knockouts lost similar amounts of their fat stores. Further, with muscle being dispersed throughout the body, it can provide heat locally, rather than needing to be circulated by the blood from a localized BAT. Hence, during the evolution of endothermy in individual species, a combination of BAT and muscle-based thermogenesis has been selected that provides the best survival outcome (13).
An important question that has not been addressed is whether animals can survive cold in the absence of both SLN and UCP1. Thus, we compared cold tolerance in single and DKO mice using littermates in the same genetic background. Our data showed that the DKO mice were extremely sensitive to an acute 4 °C cold challenge and developed hypothermia rapidly. Paradoxically, the DKO mice were able to maintain body temperature and survive during gradual cold exposure. Despite consuming the most food, the DKOs lost the most weight and had the highest oxygen consumption per body weight. In fact, at the end of the cold exposure, the DKO mice had almost completely depleted their adipose tissue stores, suggesting that these mice have to mobilize their white fat, in addition to increasing food intake, to fuel thermogenesis. The finding that the DKO mice had significantly elevated catecholamine levels suggests that the mice are highly cold-stressed and recruiting every other mechanism to maintain body temperature. Thus, the ability to maintain body temperature without UCP1 and SLN came at a huge energetic cost. These data indicate that in the absence of UCP1 and SLN, the DKO mice must rely on extremely inefficient thermogenic mechanisms (possibly due to sustained elevation of metabolic heat) to maintain body temperature. It appears that the DKO mice would not be able to survive for longer periods of time in the cold, once fat stores are depleted. Hence, metabolic heat production will not be sufficient to meet the thermogenic demand during prolonged cold exposure, which is why mammals have evolved more energetically efficient thermogenic mechanisms.
Taken together, the present study shows that the presence of both UCP1 and SLN provides an effective combination to meet the thermogenic demand and that loss of either one increases the energetic cost of cold adaptation. Loss of both mechanisms makes the system almost unsustainable, and after ∼3 weeks of survival in the cold, most of the WAT stores are depleted in the DKO mice. The unexpected survival of the DKO mice in this study highlights the resiliency of mice and how species have evolved with a multitude of mechanisms to accomplish vital physiological functions. A species will not rely on only one or two mechanisms but will have the ability to recruit additional methods to ensure survival at all costs. Finally, these studies further substantiate the critical role of SLN and muscle in thermogenesis and provide exciting insight into mechanisms of energy expenditure and thermogenesis in animals with minimal BAT, such as humans.
This work was supported, in whole or in part, by National Institutes of Health Grants R01-HL088555 and R01-DK098240-01.
- NST
- nonshivering thermogenesis
- BAT
- brown adipose tissue
- WAT
- white adipose tissue
- UCP1
- uncoupling protein 1
- SLN
- sarcolipin
- DKO
- double knock-out
- ERC
- early removal criteria
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine.
REFERENCES
- 1. Golozoubova V., Gullberg H., Matthias A., Cannon B., Vennström B., Nedergaard J. (2004) Depressed thermogenesis but competent brown adipose tissue recruitment in mice devoid of all hormone-binding thyroid hormone receptors. Mol. Endocrinol. 18, 384–401 [DOI] [PubMed] [Google Scholar]
- 2. Chaffee R. R., Roberts J. C. (1971) Temperature acclimation in birds and mammals. Annu. Rev. Physiol. 33, 155–202 [DOI] [PubMed] [Google Scholar]
- 3. Cannon B., Nedergaard J. (2004) Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 [DOI] [PubMed] [Google Scholar]
- 4. Rothwell N. J., Stock M. J. (1985) Biological distribution and significance of brown adipose tissue. Comp. Biochem. Physiol. A Comp. Physiol. 82, 745–751 [DOI] [PubMed] [Google Scholar]
- 5. Golozoubova V., Cannon B., Nedergaard J. (2006) UCP1 is essential for adaptive adrenergic nonshivering thermogenesis. Am. J. Physiol. Endocrinol. Metab. 291, E350–E357 [DOI] [PubMed] [Google Scholar]
- 6. Enerbäck S., Jacobsson A., Simpson E. M., Guerra C., Yamashita H., Harper M. E., Kozak L. P. (1997) Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 387, 90–94, 10.1038/387090a0 [DOI] [PubMed] [Google Scholar]
- 7. Fedorenko A., Lishko P. V., Kirichok Y. (2012) Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 151, 400–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arruda A. P., Ketzer L. A., Nigro M., Galina A., Carvalho D. P., de Meis L. (2008) Cold tolerance in hypothyroid rabbits: role of skeletal muscle mitochondria and sarcoplasmic reticulum Ca2+ ATPase isoform 1 heat production. Endocrinology 149, 6262–6271 [DOI] [PubMed] [Google Scholar]
- 9. Rose R. W., West A. K., Ye J. M., McCormick G. H., Colquhoun E. Q. (1999) Nonshivering thermogenesis in a marsupial (the Tasmanian Bettong Bettongia gaimardi) is not attributable to brown adipose tissue. Physiol. Biochem. Zool. 72, 699–704 [DOI] [PubMed] [Google Scholar]
- 10. Cambon B., Reyne Y., Nouguès J. (1998) In vitro induction of UCP1 mRNA in preadipocytes from rabbit considered as a model of large mammals brown adipose tissue development: importance of PPARγ agonists for cells isolated in the postnatal period. Mol. Cell Endocrinol. 146, 49–58 [DOI] [PubMed] [Google Scholar]
- 11. Casteilla L., Champigny O., Bouillaud F., Robelin J., Ricquier D. (1989) Sequential changes in the expression of mitochondrial protein mRNA during the development of brown adipose tissue in bovine and ovine species: sudden occurrence of uncoupling protein mRNA during embryogenesis and its disappearance after birth. Biochem. J. 257, 665–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lean M. E., James W. P., Jennings G., Trayhurn P. (1986) Brown adipose tissue uncoupling protein content in human infants, children and adults. Clin. Sci. (Lond.) 71, 291–297 [DOI] [PubMed] [Google Scholar]
- 13. Rowland L. A., Bal N. C., Periasamy M. (2014) The role of skeletal-muscle-based thermogenic mechanisms in vertebrate endothermy. Biol. Rev. Camb. Philos. Soc. 10.1111/brv.12157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ukropec J., Anunciado R. V., Ravussin Y., Kozak L. P. (2006) Leptin is required for uncoupling protein-1-independent thermogenesis during cold stress. Endocrinology 147, 2468–2480 [DOI] [PubMed] [Google Scholar]
- 15. Ukropec J., Anunciado R. P., Ravussin Y., Hulver M. W., Kozak L. P. (2006) UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1−/− mice. J. Biol. Chem. 281, 31894–31908 [DOI] [PubMed] [Google Scholar]
- 16. Anunciado-Koza R. P., Zhang J., Ukropec J., Bajpeyi S., Koza R. A., Rogers R. C., Cefalu W. T., Mynatt R. L., Kozak L. P. (2011) Inactivation of the mitochondrial carrier SLC25A25 (ATP-Mg2+/Pi transporter) reduces physical endurance and metabolic efficiency in mice. J. Biol. Chem. 286, 11659–11671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bruton J. D., Aydin J., Yamada T., Shabalina I. G., Ivarsson N., Zhang S. J., Wada M., Tavi P., Nedergaard J., Katz A., Westerblad H. (2010) Increased fatigue resistance linked to Ca2+-stimulated mitochondrial biogenesis in muscle fibres of cold-acclimated mice. J. Physiol. 588, 4275–4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Solinas G., Summermatter S., Mainieri D., Gubler M., Pirola L., Wymann M. P., Rusconi S., Montani J. P., Seydoux J., Dulloo A. G. (2004) The direct effect of leptin on skeletal muscle thermogenesis is mediated by substrate cycling between de novo lipogenesis and lipid oxidation. FEBS Lett. 577, 539–544 [DOI] [PubMed] [Google Scholar]
- 19. Davis T. R. (1967) Contribution of skeletal muscle to nonshivering thermogenesis in the dog. Am. J. Physiol. 213, 1423–1426 [DOI] [PubMed] [Google Scholar]
- 20. Clarke S. D., Lee K., Andrews Z. B., Bischof R., Fahri F., Evans R. G., Clarke I. J., Henry B. A. (2012) Postprandial heat production in skeletal muscle is associated with altered mitochondrial function and altered futile calcium cycling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 303, R1071–1079 [DOI] [PubMed] [Google Scholar]
- 21. Block B. A. (1994) Thermogenesis in muscle. Annu. Rev. Physiol. 56, 535–577 [DOI] [PubMed] [Google Scholar]
- 22. Eldershaw T. P. D., Duchamp C., Ye J., Clark M. G., Colquhoun E. Q. (1997) Potential for non-shivering thermogenesis in perfused chicken (Gallus domesticus) muscle. Comp. Biochem. Physiol. A Physiol. 117, 545–554 [DOI] [PubMed] [Google Scholar]
- 23. Duchamp C., Barré H., Rouanet J. L., Lanni A., Cohen-Adad F., Berne G., Brebion P. (1991) Nonshivering thermogenesis in king penguin chicks. I. Role of skeletal muscle. Am. J. Physiol. 261, R1438–1445 [DOI] [PubMed] [Google Scholar]
- 24. Duchamp C., Barré H. (1993) Skeletal muscle as the major site of nonshivering thermogenesis in cold-acclimated ducklings. Am. J. Physiol. 265, R1076–1083 [DOI] [PubMed] [Google Scholar]
- 25. Dumonteil E., Barré H., Meissner G. (1993) Sarcoplasmic reticulum Ca2+-ATPase and ryanodine receptor in cold-acclimated ducklings and thermogenesis. Am. J. Physiol. 265, C507–513 [DOI] [PubMed] [Google Scholar]
- 26. Dumonteil E., Barré H., Meissner G. (1994) Effects of palmitoyl carnitine and related metabolites on the avian Ca2+-ATPase and Ca2+ release channel. J. Physiol. 479, 29–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dumonteil E., Barré H., Meissner G. (1995) Expression of sarcoplasmic reticulum Ca2+ transport proteins in cold-acclimating ducklings. Am. J. Physiol. 269, C955–C960 [DOI] [PubMed] [Google Scholar]
- 28. Bicudo J. E., Vianna C. R., Chaui-Berlinck J. G. (2001) Thermogenesis in birds. Biosci. Rep. 21, 181–188 [DOI] [PubMed] [Google Scholar]
- 29. Bal N. C., Maurya S. K., Sopariwala D. H., Sahoo S. K., Gupta S. C., Shaikh S. A., Pant M., Rowland L. A., Bombardier E., Goonasekera S. A., Tupling A. R., Molkentin J. D., Periasamy M. (2012) Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat. Med. 18, 1575–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Babu G. J., Bhupathy P., Timofeyev V., Petrashevskaya N. N., Reiser P. J., Chiamvimonvat N., Periasamy M. (2007) Ablation of sarcolipin enhances sarcoplasmic reticulum calcium transport and atrial contractility. Proc. Natl. Acad. Sci. U.S.A. 104, 17867–17872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shabalina I. G., Hoeks J., Kramarova T. V., Schrauwen P., Cannon B., Nedergaard J. (2010) Cold tolerance of UCP1-ablated mice: a skeletal muscle mitochondria switch toward lipid oxidation with marked UCP3 up-regulation not associated with increased basal, fatty acid- or ROS-induced uncoupling or enhanced GDP effects. Biochim. Biophys. Acta 1797, 968–980 [DOI] [PubMed] [Google Scholar]
- 32. Meyer C. W., Willershäuser M., Jastroch M., Rourke B. C., Fromme T., Oelkrug R., Heldmaier G., Klingenspor M. (2010) Adaptive thermogenesis and thermal conductance in wild-type and UCP1-KO mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 299, R1396–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arjamaa O., Lagerspetz K. Y. H. (1979) Postnatal-development of shivering in the mouse. J. Therm. Biol. 4, 35–39 [Google Scholar]
- 34. Bryant D. M., Hails C. J. (1975) Mechanisms of heat conservation in litters of mice (Mus musculus L). Comp. Biochem. Physiol. A. Comp. Physiol. 50, 99–104 [DOI] [PubMed] [Google Scholar]
- 35. Thomas S. A., Palmiter R. D. (1997) Thermoregulatory and metabolic phenotypes of mice lacking noradrenaline and adrenaline. Nature 387, 94–97 [DOI] [PubMed] [Google Scholar]
- 36. Landsberg L., Saville M. E., Young J. B. (1984) Sympathoadrenal system and regulation of thermogenesis. Am. J. Physiol. 247, E181–189 [DOI] [PubMed] [Google Scholar]
- 37. Tseng Y. H., Cypess A. M., Kahn C. R. (2010) Cellular bioenergetics as a target for obesity therapy. Nat. Rev. Drug. Discov. 9, 465–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lowell B. B., Spiegelman B. M. (2000) Towards a molecular understanding of adaptive thermogenesis. Nature 404, 652–660 [DOI] [PubMed] [Google Scholar]
- 39. Aydin J., Shabalina I. G., Place N., Reiken S., Zhang S. J., Bellinger A. M., Nedergaard J., Cannon B., Marks A. R., Bruton J. D., Westerblad H. (2008) Nonshivering thermogenesis protects against defective calcium handling in muscle. FASEB J. 22, 3919–3924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bombardier E., Smith I. C., Gamu D., Fajardo V. A., Vigna C., Sayer R. A., Gupta S. C., Bal N. C., Periasamy M., Tupling A. R. (2013) Sarcolipin trumps β-adrenergic receptor signaling as the favored mechanism for muscle-based diet-induced thermogenesis. FASEB J. 27, 3871–3878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mall S., Broadbridge R., Harrison S. L., Gore M. G., Lee A. G., East J. M. (2006) The presence of sarcolipin results in increased heat production by Ca2+-ATPase. J. Biol. Chem. 281, 36597–36602 [DOI] [PubMed] [Google Scholar]
- 42. Smith W. S., Broadbridge R., East J. M., Lee A. G. (2002) Sarcolipin uncouples hydrolysis of ATP from accumulation of Ca2+ by the Ca2+-ATPase of skeletal-muscle sarcoplasmic reticulum. Biochem. J. 361, 277–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sopariwala D. H., Pant M., Shaikh S. A., Goonasekera S. A., Molkentin J. D., Weisleder N., Ma J., Pan Z., Periasamy M. (2015) Sarcolipin overexpression improves muscle energetics and reduces fatigue. J. Appl. Physiol. 10.1152/japplphysiol.01066.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Maurya S. K., Bal N. C., Sopariwala D. H., Pant M., Rowland L. A., Shaikh S. A., Periasamy M. (2015) Sarcolipin is a key determinant of basal metabolic rate and its overexpression enhances energy expenditure and resistance against diet induced obesity. J. Biol. Chem. 290, 10840–10849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schulz T. J., Huang P., Huang T. L., Xue R., McDougall L. E., Townsend K. L., Cypess A. M., Mishina Y., Gussoni E., Tseng Y. H. (2013) Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature 495, 379–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xue B., Rim J. S., Hogan J. C., Coulter A. A., Koza R. A., Kozak L. P. (2007) Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. J. Lipid Res. 48, 41–51 [DOI] [PubMed] [Google Scholar]