Abstract
Background:
Recent studies indicate that glucagon-like peptide (GLP)-1 inhibits appetite in part through regulation of soluble leptin receptors. Thus, during weight loss maintenance, GLP-1 receptor agonist (GLP-1RA) administration may inhibit weight loss-induced increases in soluble leptin receptors thereby preserving free leptin levels and preventing weight regain.
Methods:
In a randomized controlled trial, 52 healthy obese individuals were, after a diet-induced 12% body weight loss, randomized to treatment with or without administration of the GLP-1RA liraglutide (1.2 mg per day). In case of weight gain, low-calorie diet products were allowed to replace up to two meals per day to achieve equal weight maintenance. Glucose tolerance and hormone responses were investigated before and after weight loss and after 52 weeks weight maintenance. Primary end points: increase in soluble leptin receptor plasma levels and decrease in free leptin index after 52 weeks weight loss maintenance.
Results:
Soluble leptin receptor increase was 59% lower; 2.1±0.7 vs 5.1±0.8 ng ml−1 (−3.0 (95% confidence interval (CI)=−0.5 to −5.5)), P<0.001 and free leptin index decrease was 43% smaller; −62±15 vs −109±20 (−47 (95% CI=−11 to −83)), P<0.05 with administration of GLP-1RA compared with control group. The 12% weight loss was successfully maintained in both the groups with no significant change in weight after 52 weeks follow-up. The GLP-1RA group had greater weight loss during the weight maintenance period (−2.3 kg (95% CI=−0.6 to −4.0)), and had fewer meal replacements per day compared with the control group (minus one meal per day (95% CI=−0.6 to −1)), P<0.001. Fasting glucose was decreased by an additional −0.2±0.1 mmol l−1 in the GLP-1RA group in contrast to the control group, where glucose increased 0.3±0.1 mmol l−1 to the level before weight loss (−0.5mmol l−1 (95% CI=−0.1 to −0.9)), P<0.005. Meal response of peptide PYY3–36 was higher at week 52 in the GLP-1RA group compared with the control group, P<0.05.
Conclusions:
The weight maintaining effect of GLP-1RAs may be mediated by smaller decrease in free leptin and higher PYY3–36 response. Low dose GLP-1RA therapy maintained 12% weight loss for 1 year and may prevent pre-diabetes in obesity.
Introduction
Achieving and maintaining a healthy body weight is a primary strategy for the prevention and management of obesity-related comorbidities.1 Many obese people have a history of several successful weight losses, but very few are able to maintain the weight loss over a longer period of time (that is, a year or more).2 After weight loss, several physiological mechanisms favoring weight regain are activated, including declining energy expenditure in combination with increased hunger and reduced satiety during eating. It has been hypothesized that the reduction in free circulating leptin after weight loss is responsible for the weight regain3, 4 and experiments have shown that leptin repletion after weight loss can reduce weight regain by decreasing energy intake.5, 6, 7 Leptin is an appetite-reducing hormone that is secreted by the adipose tissue. Leptin binds to the leptin receptor, which exists in various forms including membrane bound isoforms and a circulating soluble form. The soluble leptin receptor represents the main leptin-binding activity in human blood.8, 9, 10 High levels of soluble leptin receptor appears to be a mechanism, whereby the appetite-inhibiting actions of leptin may be suppressed during energy deficiency11 as high levels of soluble leptin receptor concentrations seem to directly block leptin effects.12 The free leptin index can be calculated as the ratio of leptin to soluble leptin receptor concentrations. A positive correlation between the adipose tissue mass and the level of fasting plasma leptin and free leptin index has been reported, in contrast to a negative correlation to soluble leptin receptor. Fasting plasma leptin levels, as well as free leptin index and soluble leptin receptor are markers of leptin activity.5, 6, 8, 13, 14 Obese people are leptin resistant with a high level of fasting plasma leptin without the expected appetite-reducing effect15 indicating that rather than being an appetite-inhibiting hormone, leptin is an ‘anti-fasting' signal. Hence, when adipose tissue is lost, but independent of the total amount of fat mass, the decrease in fasting plasma leptin levels signals for increased food-seeking behavior.5 This indicates that it is the size of reduction in free leptin from before the weight loss to after weight loss rather than the absolute post-weight loss value that controls food-seeking behavior.
Glucagon-like peptide (GLP)-1 is a hormone secreted from the endocrine L cells in the intestine, which reduces food intake16 and, when administered to obese subjects, induces weight loss.17, 18, 19 It is well established that GLP-1 inhibits food intake by inhibiting appetite,16, 17 but the underlying mechanisms remain unsettled.20, 21 However, the leptin receptor may be involved in the appetite inhibition by GLP-1. Thus, leptin receptor–deficient rats were reported not to respond to GLP-1 at doses that produce significant anorexia in wild-type rats.22 In addition, the anorexic response to GLP-1RA treatment has been shown to involve leptin-dependent mechanisms as demonstrated in experiments where small amounts of leptin enhanced the anorexic effects of GLP-1 and GLP-1RA and where fasting-induced reductions in the anorectic effects could be prevented by leptin replacement.22 Furthermore, recent studies in rodents have shown that GLP-1RAs can restore leptin responsiveness.23, 24 We hypothesized that GLP-1RA administration during weight loss maintenance would inhibit weight loss-induced increases in soluble leptin receptor plasma concentrations resulting in higher level of free leptin (corrected for weight change) thereby preventing weight regain. We therefore investigated changes in free leptin and soluble leptin receptor plasma levels during 52 weeks following a diet-induced 12% total body weight loss in healthy obese individuals with and without administration of the GLP-1RA liraglutide.
Materials and methods
Study participants
In the period from September 2011 to March 2012, 58 obese participants were recruited for the study by advertising in local newspapers according to the following inclusion and exclusion criteria:
Inclusion criteria
BMI>30 and <40 kg m−2; age >18 and <65 years; and healthy.
Exclusion criteria
Any acute or chronic illness other than obesity; any medical treatment with known effects on glucose- and lipid metabolism, appetite or food intake; pregnancy or breast feeding; fasting plasma glucose of ⩾7 mmol l−1.
A diagram of the participant flow is shown in Supplementary Figure 1.
Study design
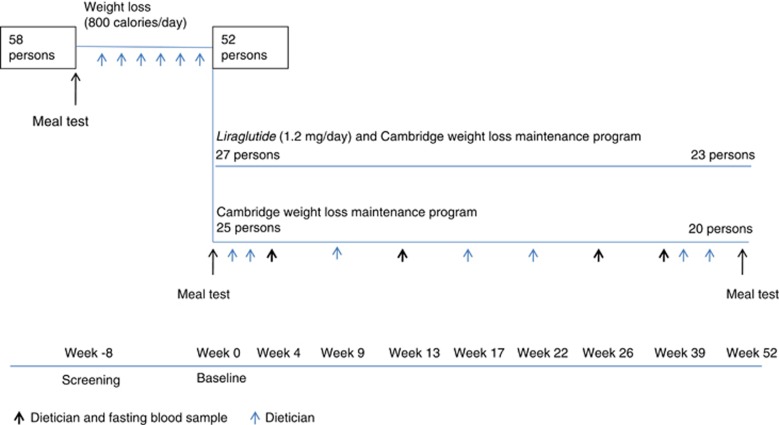
The study design is illustrated in Figure 1.
Figure 1.
Study design.
Weight loss phase
Each study participant met at an individual screening session with the study dietician at the Department of Endocrinology, Hvidovre University Hospital, where the study was conducted. They received thorough instructions on how to follow a very low-calorie powder diet (800 kcal per day; Cambridge Weight Plan, Corby, UK)25 for 8 weeks. During the 8-week weight loss phase, the participants had individual dietician meetings every week with the objective to achieve a weight loss of at least 7.5% of their initial body weight after 8 weeks.
Randomization to GLP-1RA administration or control group
After 8 weeks on very low-calorie diet, the study participants were randomized into two groups: one receiving the GLP-1RA liraglutide 1.2 mg per day and the other a control group. Liraglutide was administered using FlexPen devices (Victoza, Novo Nordisk A/S, Bagsvaerd, Denmark) as subcutaneous injections in the abdomen or thigh. Dosing was initiated at 0.6 mg per day, increasing after 1 week to 1.2 mg per day. Both groups followed Cambridge Weight Loss Maintenance Program with Cambridge Weight Plan products. It was not possible to obtain or buy placebo Victoza pens from the production company (Novo Nordisk). Conduction of study and data analyses were performed with the investigator blinded for randomization group.
The intervention period ran from October 2011 to June 2013.
Weight loss maintenance phase
After the initial 8-week weight loss phase, study participants were instructed to restrict calorie intake according to their estimated daily energy needs subtracted by 600 kcal. The study participants filled in a diet diary and brought it to regular visits with the study dietician where they received education on diet and lifestyle changes (in total 13 visits in 52 weeks). The study participants were encouraged to follow nutritional recommendations (30% calories from fat, 50% from carbohydrates and 20% from protein) and eat four to six meals daily (three main meals). In case of weight gain, up to two meals a day during the weight loss maintenance period were allowed to be replaced by Cambridge Weight Plan products in both groups to ensure a stable maintenance of weight loss. All products used to replace any meals in case of weight gain were recorded by the participants and reported to the dietician.
Meal tests
Each study participant underwent three meal tests: at screening; after 8 weeks on very low calorie powder diet; and after 52 weeks with restrictive weight maintenance diet recommendations. Venous blood samples for measurements of plasma glucose, serum insulin, plasma GLP-1, plasma PYY and plasma ghrelin were taken before the intake of Fresubin Energy drink (600 kcal; 50% carbohydrate, 35% fat and 15% protein; Fresenius Kabi, Bad Homburg, Germany) and every 15 min until 180 min after meal ingestion.
Measurements
Plasma-soluble leptin receptor concentrations were measured by an ELISA technique (R&D Systems, Minneapolis, MN, USA) with a sensitivity of 0.06 ng ml−1. Plasma leptin, plasma PYY3–36 and plasma ghrelin were measured by radioimmunoassay (Millipore, Billerica, MA, USA). All quality controls (prepared by the manufacturer) were within acceptable limits. Radioimmunological determinations of total plasma GLP-1 were performed as described.26, 27, 28 The analytical detection limit was 1 pmol l−1 and intra- and inter-assay coefficients of variation were <6% and <15%, respectively. Plasma glucose was measured with the glucose oxidase technique (YSI model 2300 STAT Plus; Yellow Springs Instruments, Yellow Springs, OH, USA). Serum insulin concentrations were measured using Immulite 2000 solid-phase chemiluminescent immunometric assays (Immulite 2000; Siemens, Erlangen, Germany). Fat percentage was assessed using dual-energy X-ray absorptiometry scanning (Hologic discovery A, Bedford, MA, USA). Blood pressure was measured using semi-automatic upper arm blood pressure monitor (Boso Mercurius E; Bosch+Sohn, Jungingen, Germany).
Ethical issues
The study was approved by the ethical committee in Copenhagen (reference number: H-4-2010-134) and was performed in accordance with the Helsinki Declaration II and with ICH-GCP practice. Participation in the investigation was voluntary and the individuals could at any time retract their consent to participate. ClinicalTrials.gov Identifier: NCT02094183.
Statistical power
We wanted to investigate the primary end point; increase in soluble leptin receptor levels with administration of GLP-1RA compared with a control group. We hypothesized a difference of 3 ng ml−1±0.7 (refs. 9, 11,12) between the groups, thus 20 experimental subjects and 20 control subjects will be able to reject the null hypothesis that the population means of the experimental and control groups are equal with probability (power) 0.8.
Statistics
Delta changes in variables were analyzed using a general linear model contrasting the GLP-1RA treated group vs the control group with weight change, sex and age as covariates. Analysis of changes in variables over time were performed using a repeated measurements analysis of variance (SAS version 9.4; SAS Institute, Cary, NC, USA) contrasting the GLP-1RA group vs the control group. P-values are given for the overall analysis of variance (Pgroup) and the time (Ptime) with asterisks in figures indicating significantly different time points and the interaction is reported (Pgroup × time). Relationships between the maintenance of weight loss and the differences in the leptin-related parameters were analyzed with linear regression (SPSS statistics version 22; IBM Corporation, Armonk, NY, USA). The data are shown as mean±SEM and differences with 95% confidence interval (CI). A P-value <0.05 was considered significant.
Results
Weight loss program
Fifty-eight participants (Danes, White) entered the study and 52 participants completed the 8-week weight loss program, achieving a mean weight loss of −12 kg (−11 to −13 kg) equivalent to 12% of the body weight. The 12% body weight loss led to significant decreases in BMI, total body fat percentage, waist circumference, waist-hip ratio, blood pressure, fasting glucose, triglycerides, insulin, leptin, free leptin index, PYY3–36 and to significant increases in fasting soluble leptin receptor and fasting ghrelin (Table 1).
Table 1. Subject characteristics and fasting variables before and after weight loss run-in with 8 weeks of very low-calorie diet.
|
GLP-1RA (liraglutide 1.2 mg) N=27 (22 women, 5 men; age 46±2) |
Control N=25 (22 women, 3 men; age 45±2) |
|||||
|---|---|---|---|---|---|---|
| At screening | At baseline | Mean of differences | At screening | At baseline | Mean of differences | |
| Bodyweight (kg) | 98.3±1.9 | 86.5±1.5 | −11.9 (−10.6 to −13.1)* | 96.9±1.9 | 84.8±1.8 | 12 (−11.0 to −13.1)* |
| BMI (kg m−2) | 35.0±0.6 | 30.8±0.7 | −4.2 (−3.8 to −4.5)* | 34.0±0.5 | 29.7±0.7 | −4.3 (−3.9 to −4.8)* |
| Total body fat % | 42±1 | 38±1 | −3 (−1 to −4)* | 41±1 | 39±1 | −2 (−2 to −3)* |
| Waist (cm) | 109±2 | 98±2 | −11 (−9 to −13)* | 108±2 | 98±2 | −10 (−8 to −12)* |
| Waist:hip ratio | 0.93±0.02 | 0.89±0.02 | −0.04 (−0.02 to −0.06)* | 0.91±0.02 | 0.88±0.02 | −0.03 (−0.01 to −0.05)* |
| Systolic BP (mmHg) | 124±3 | 114±2 | −10 (−7 to −13)* | 124±4 | 116±3 | −8 (−2 to −13)* |
| Diastolic BP (mmHg) | 80±2 | 73±2 | −7 (−4 to −10)* | 79±2 | 72±2 | −7 (−4 to −10)* |
| Fasting plasma glucose (mM) | 5.4±0.08 | 5.1±0.08 | −0.3 (−0.27 to −0.33)* | 5.3±0.1 | 5.0±0.08 | −0.3 (−0.28 to −0.33)* |
| Plasma triglycerides (mM) | 1.3±0.1 | 0.97±0.06 | −0.4 (−0.2 to −0.5)* | 1.3±0.2 | 0.95±0.06 | −0.4 (−0.2 to −0.5)* |
| Serum insulin (pmol l−1) | 80±8 | 49±3 | −30 (−16 to −46)* | 70±6 | 43±4 | −28 (−20 to −36)* |
| Plasma leptin (ng ml−1) | 32±3 | 13±2 | −19 (−14 to −23)* | 33±3 | 13±1 | −20 (−16 to −24)* |
| Plasma-soluble leptin receptor (ng ml−1) | 16±0.9 | 18±1 | 2 (0.3–4)# | 15±0.7 | 17±0.8 | 2 (0.5–4)# |
| Plasma free leptin index | 198±22 | 74±11 | −124 (−90 to −158)* | 212±23 | 83±16 | −129 (−92 to −166)* |
| Plasma PYY3–36 (pg ml−1) | 78 ±7 | 70 ±6 | −8 (−1 to −15)¤ | 74 ±5 | 66 ±4 | −8 (−1 to −15)¤ |
| Plasma ghrelin (pg ml−1) | 779±54 | 1066±86 | 287 (184–390)* | 855±84 | 1082±90 | 227 (121–334)* |
Abbreviations: BP, blood pressure; BMI, body mass index; GLP, glucagon-like peptide.
Data are shown as mean±s.e.m. and differences with 95% confidence interval. *P<0.0001, #P<0.001, ¤P<0.05. Data were collected before randomization. There were no differences between the groups P>0.05.
Weight loss maintenance program
Soluble leptin receptor, leptin and free leptin index
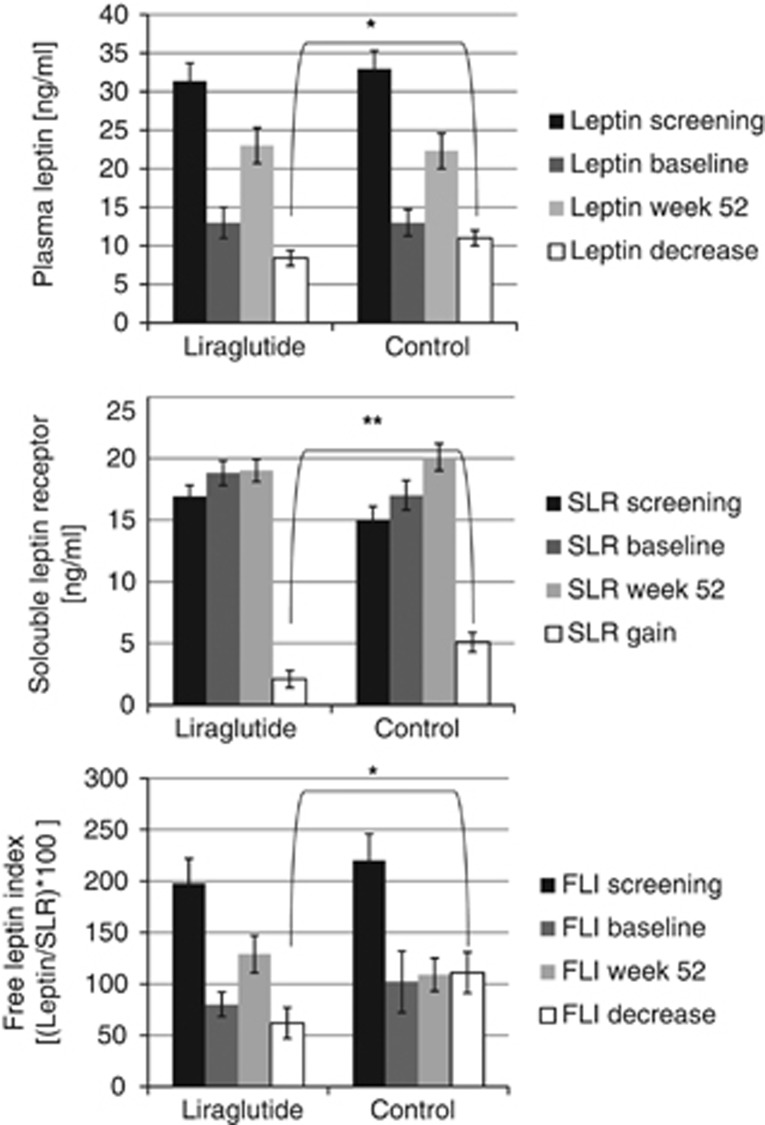
During the 52 weeks follow-up, the soluble leptin receptor increase was 59% lower in the GLP-1RA group compared with the control group, 2.1±0.7 ng ml−1 vs 5.1 ±0.8 ng ml−1, estimated difference −3.0 (95% CI=−0.5 to −5.5), P<0.001 (Figure 2, middle panel). The leptin decrease was significantly smaller in the GLP-1RA group compared with the control group; −9±2 ng ml−1 vs −11±2 ng ml−1, (−2 (95% CI=−0.3 to −4)), P<0.05 (Figure 2, upper panel) and the free leptin index decrease was significantly smaller in the GLP-1RA group compared with the control group; −62±15 vs −109±20, (−47 (95% CI=−11 to −83)), P<0.05, Figure 2, lower panel.
Figure 2.
Change in plasma leptin, soluble leptin receptor (SLR) and free leptin index (FLI). Mean±s.e.m., *P<0.05, **P<0.005.
Weight loss maintenance, meal replacements, fasting plasma glucose, triglycerides and blood pressure
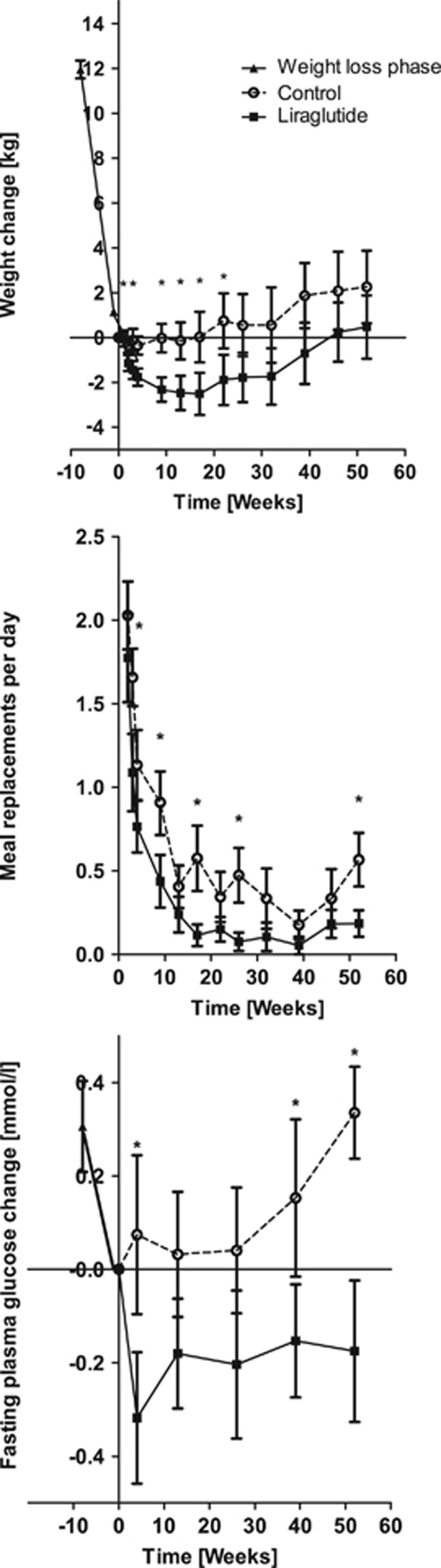
The 12% weight loss was successfully maintained in both the groups with no significant difference in weight between the beginning of the weight loss maintenance program to after 52 weeks; the GLP-1RA group had gained 0.4 kg (95% CI=−2.5 to 3.4 kg) vs 2.3 kg (95% CI=−1.1 to 5.6) in the control group, P>0.05, estimated difference (−1.9 (95% CI=−6.2 to 2.4)), P>0.05 (Figure 3, upper panel). The GLP-1RA group had greater weight loss during the weight maintenance period (−2.3 kg (95% CI=−0.6 to −4.0)), P<0.005 (Figure 3, upper panel), and had fewer meal replacements per day (minus one meal per day (95% CI=−0.6 to −1)), P<0.0001, compared with the control group (Figure 3, middle panel). In the GLP-1RA group, fasting plasma glucose decreased with further −0.2±0.1 mmol l−1 in contrast to the control group where fasting plasma glucose increased by 0.3±0.1 mmol l−1 to the level before weight loss (−0.5 (95% CI=−0.1 to −0.9)), P<0.005 (Figure 3, lower panel). Triglyceride levels increased during weight loss maintenance in the control group as compared with the GLP-1RA group; 0.4±0.08 vs 0.06±0.08 mmol l−1 (−3 (95% CI=−0.5 to −6)), P<0.05. The systolic and diastolic blood pressure increased significantly during the weight loss maintenance period for the control group by 7.2 (95% CI=2.2–12.3) and 6.1 (95% CI=2.3–10.0) mm Hg, respectively, P<0.005. In the GLP-1RA group, the systolic blood pressure increased significantly during the weight loss maintenance period by 9.9 (95% CI=3.9–16.0) mm Hg, P<0.05, in contrast to a nonsignificant diastolic blood pressure increase of 4.7 (−0.1 to 9.5) mm Hg, P>0.05. There was no difference with respect to the blood pressure increases between the two groups (systolic estimated difference 2.7 (95% CI=−5 to 10), diastolic estimated difference of −1.4 (95% CI=−7 to 4), P>0.5).
Figure 3.
Upper panel: Maintenance of weight loss during 52 weeks. Mean weight change (kg)±s.e.m., Pgroup<0.005, Ptime<0.001, Pgroup × time=0.3. Middle panel: Daily meal replacement during the 52 weeks of weight loss maintenance. Mean number of meal replacements per day±s.e.m., Pgroup<0.0001, Ptime<0.0001, Pgroup × time=0.2. Lower panel: Change in fasting plasma blood glucose level during the 52 weeks of weight loss maintenance. Mean blood glucose change (mmol l−1)±s.e.m., Pgroup<0.005, Ptime<0.001, Pgroup × time=0.1. P-values are given for the overall analysis of variance with asterisks indicating significantly different time points. Filled squares indicate GLP-1RA group; open circles indicate control group.
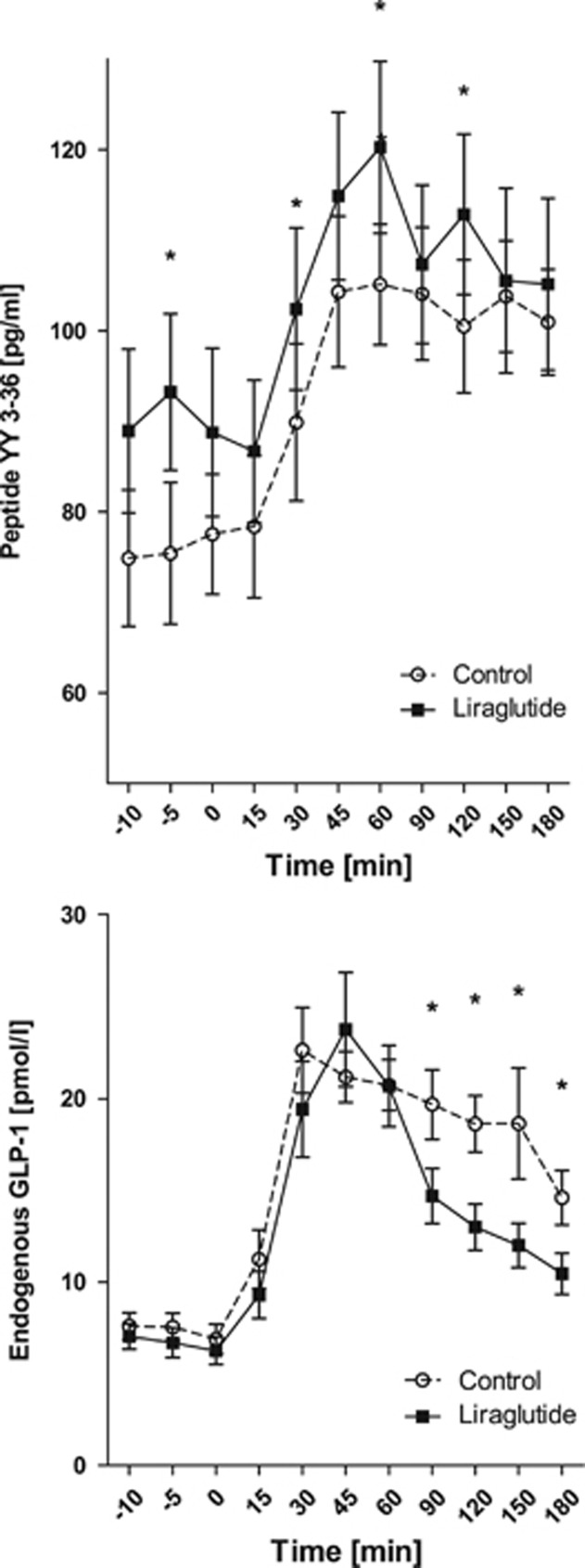
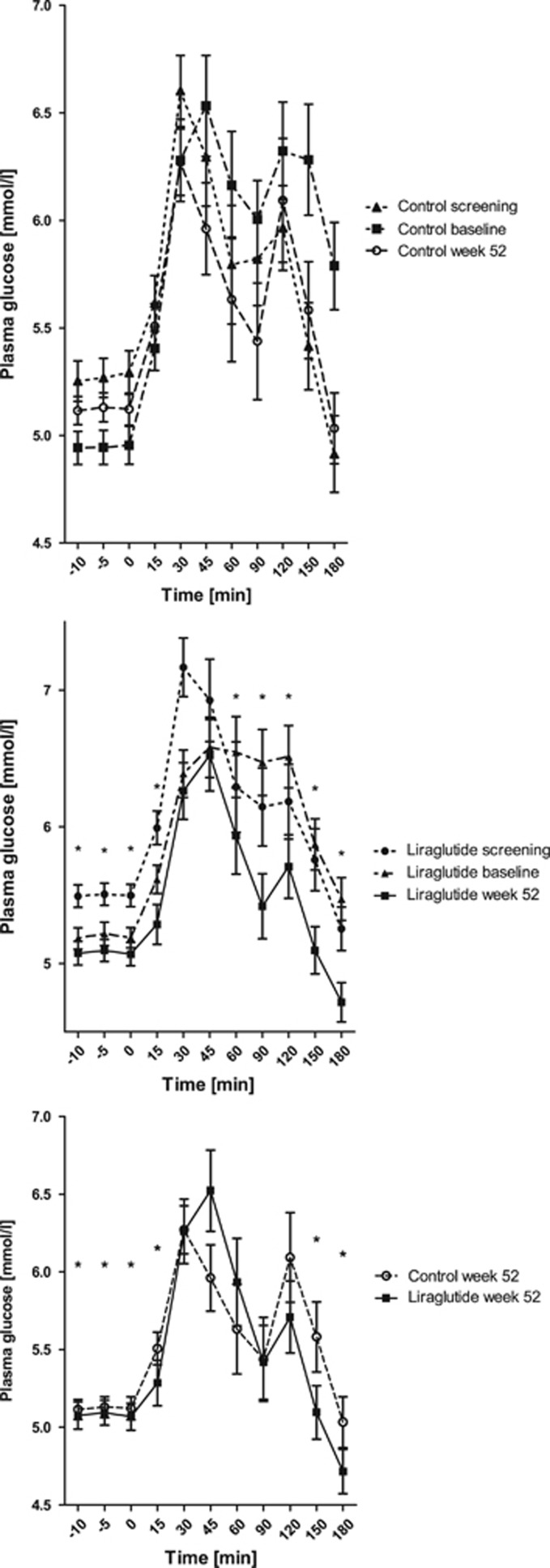
Plasma PYY3–36, endogenous GLP-1, glucose, ghrelin and serum insulin meal responses
The PYY3–36 levels during the meal test were significantly higher at week 52 for the GLP-1RA group compared with the control group, P<0.05; whereas the endogenous GLP-1 levels were significantly lower in the GLP-1RA group compared with the control group, P<0.05 (Figure 4); there were no differences between the groups at screening or baseline, P>0.05. Plasma glucose was significantly lower at 52 weeks compared with screening and baseline for the GLP-1RA group, P<0.001, whereas there was no difference in the control group, P>0.05. Furthermore, plasma glucose was significantly lower in the GLP-1RA group compared with the control group during the meal test after 52 weeks, P<0.05 (Figure 5), whereas there was no difference between the groups at screening or baseline, P>0.05. After 52 weeks, 100% were glucose tolerant in the GLP-1RA group (93% at baseline) vs 90% in the control group (92% at baseline; Supplementary Table 1). Serum insulin was significantly lower at baseline and 52 weeks compared with screening for both groups, P<0.0001. Meal-stimulated serum insulin was significantly higher in the GLP-1RA group at 52 weeks compared with the control group, P<0.05, whereas there was no difference between the groups at screening or baseline, P>0.05 (Supplementary Figure 2). Ghrelin levels did not differ between the groups (Supplementary Figure 3).
Figure 4.
Plasma PYY3–36 and plasma endogenous GLP-1 during a meal test at week 52 during weight loss maintenance. Mean±s.e.m., Pgroup<0.05, Ptime<0.01, Pgroup × time=0.2. P-value is given for the overall analysis of variance with asterisks indicating significantly different time points. Filled squares indicate GLP-1RA group; open circles indicate control group.
Figure 5.
Plasma glucose during the meal tests. Mean±s.e.m., Pgroup<0.005, Ptime<0.05, Pgroup × time=0.4. P-values are given for the overall analysis of variance with asterisks indicating significantly different time points.
Relationship between maintenance of weight loss and differences in leptin-related parameters
Increase in leptin level during weight maintenance correlated positively to an increase in weight; R2=0.46, P>0.0001 (GLP-1-RA group: R2=0.39, P=0.001; control group: R2=0.57, P<0.0001, Supplementary Figure 4). Increase in free leptin index correlated positively to an increase in weight; R2=0.48, P<0.0001 (GLP-1-RA group: R2=0.52, P<0.0001; control group: R2=0.46, P=0.0001, Supplementary Figure 4). Increase in soluble leptin receptor during weight maintenance correlated to a decrease in weight in the control group only, R2=0.09, P>0.05 (GLP-1-RA group: R2=0.06, P=0.7; control group R2=0.18, P<0.05, Supplementary Figure 5).
Adverse effects
Besides nausea which was more frequent in the GLP-1RA group (40 vs 4%), there was no difference in the occurrence of adverse effects. The nausea was mild and transient and disappeared after a few weeks (Supplementary Tables 2–4).
Discussion
We here show that a long-acting GLP-1RA inhibits the normally occurring increase in soluble leptin receptors during weight loss maintenance by 59% compared with a control group. As a result, levels of free leptin were higher in the GLP-1RA group, which may counteract the weight-preserving actions of the physiological leptin decrease during weight loss maintenance as smaller decreases in levels of free leptin would be expected to prevent the leptin-mediated appetite increase and energy expenditure decrease that normally occurs in response to weight loss.5, 6 For optimal hormonal response comparisons, we aimed at equal weight loss maintenance in the two study groups; this was accomplished by closely monitoring weight changes, and, in the case of weight gain, offering up to two low-calorie meal replacements per day. The two groups successfully maintained a 12-kg weight loss during 1 year although the GLP-1RA group was slightly more successful; the GLP-1RA group had gained only 400 g vs 2300 g in the control group 52 weeks after the initial weight loss phase. Our data indicate that the 43% smaller decrease in the free leptin level contributed to maintain weight loss in the GLP-1RA group, whereas in the control group, the weight loss was maintained by replacing one meal per day with a fixed low-calorie meal, thereby keeping the energy intake down.
The soluble leptin receptor represents the main leptin-binding activity in human blood.9 High levels of soluble leptin receptor appears to be a mechanism whereby the appetite-inhibiting action of leptin is suppressed during energy deficiency11 as high levels of soluble leptin receptor concentrations seem to directly inhibit leptin effects.12 Lower leptin levels are associated with a higher risk of weight gain over 2 years in healthy young adults,29 whereas an increase in soluble leptin receptors has been associated with leptin resistance.11 Moreover, leptin receptor expression has previously been coupled to the appetite inhibition of GLP-1,22 and recent studies in rodents have shown that GLP-1RAs can restore leptin responsiveness.23, 24 Our study supports the recently suggested theory that leptin, rather than being an appetite-inhibiting hormone in times of nutrient excess, is an ‘anti-fasting' signal. Thus, when adipose tissue is lost, but independent of the amount of fat mass in the body, the consequent decrease in free leptin levels signals for increased food-seeking behavior.5, 6
This indicates that it is the size of reduction in free leptin, from before the weight loss to 52 weeks after weight loss, rather than the absolute post-weight loss values that control food-seeking behavior.
We analyzed the relationship between maintenance of weight loss and change in leptin-related parameters and found that increases in leptin and free leptin index during the weight loss maintenance period explained 46 and 48%, respectively, of the variation in weight increase in accordance with the previous studies.5, 6, 8, 13, 14 However, only in the control group we found the previously reported relationship wherein increases in soluble leptin receptor levels correlated to decreases in weight.5, 6, 8, 13, 14 This indicates that liraglutide treatment uncouples the increase in soluble leptin receptors that normally occurs during weight loss, thereby preventing the decrease in free leptin and thus preventing the increase in food-seeking behavior during weight loss maintenance.
In humans, the soluble leptin receptor is generated by proteolytic cleavage of membrane-anchored leptin receptors.30 The long isoform of the leptin receptor ObRb, which is highly expressed in the hypothalamus, is considered to be the primary signaling form of the receptor and soluble leptin receptor levels are related to receptor expression of the long isoform ObRb.31 Thus GLP-1RA may, via GLP-1 receptor signaling in the hypothalamus, interfere with mRNA transcription, translation or the cleavage of ObRb to soluble leptin receptors and thereby down-regulate the production of soluble leptin receptors.
The 12% body weight loss during the weight loss phase led to marked decreases in plasma leptin and free leptin index levels and significant increase in soluble leptin receptor levels, confirming previous findings regarding the effect of weight loss on leptin activity.5, 6, 8, 13, 14 In addition, the weight loss led to significant decreases in blood pressure and fasting levels of plasma triglycerides, plasma PYY, plasma glucose, serum insulin, and an increase in fasting plasma ghrelin level.
During the weight loss maintenance period, the fasting plasma glucose level decreased by further 0.2 mmol l−1 in the GLP-1RA group, in contrast to the control group where blood glucose concentrations increased by 0.3 mmol l−1 to the same level as before the weight loss even though the weight loss was maintained. In addition, the triglyceride level increased by 0.4 mmol l−1 during weight loss maintenance in the control group in contrast to the GLP-1RA group where the level remained stable after weight loss, perhaps explained by a GLP-1-induced reduction of the secretion of intestinal triglycerides.32 Furthermore, the GLP-1RA group had significantly lowered plasma glucose levels and increased serum insulin levels during the meal test after 52 weeks, in contrast to the control group, consistent with the glucoregulatory actions of GLP-1.17, 19, 33 In addition, all participants receiving liraglutide became glucose tolerant, in contrast to the control group where 10% remained glucose intolerant. This implies that weight loss maintenance with GLP-1RA may act to prevent pre-diabetes in obesity.
The decrease in blood pressure during weight loss was not maintained during the weight loss period, but increased to the level before weight loss, even though the weight loss was maintained. Similar observations have been made in other studies34, 35 and indicate that the initial large reduction in blood pressure is a transient acute response to hypocaloric states and large acute weight loss. However, in the GLP-1RA group, only the systolic blood pressure reverted significantly, whereas in the control group, both the systolic and diastolic blood pressure returned to pre-intervention level. Of note, a meta-analysis has indicated that in subjects with a systolic blood pressure <130 mm Hg, as the participants in the present study, the effect of liraglutide on blood pressure is minimal.36
PYY3–36 is a known regulator of appetite37, 38 and, interestingly, we found that the PYY3–36 meal response was significantly higher in the GLP-1RA group, although the endogenous GLP-1 production, as expected,39 was blunted in the GLP-1RA group. PYY is predominantly colocalized with GLP-1 and would be expected to be secreted in parallel with GLP-1,40, 41 but previous reports have shown that some conditions may favor release of one hormone over the other42 and that there may be cells that preferentially release PYY or GLP-1 rather than both hormones in parallel.43, 44 Our study suggests that the appetite-inhibiting effect during chronic administration of GLP-1RA also involve an increase in the plasma levels of PYY3–36.
The administration of a relatively low dose of liraglutide (1.2 mg) helped in maintaining a large weight loss of 12% for 52 weeks. The benefit of the combined effect of weight loss with very low-calorie diet and weight maintenance with 1.2 mg liraglutide may be fewer adverse effects and less cost compared with weight maintenance with 3.0 mg liraglutide.18, 45
The strength of the present study is that we were able to successfully maintain a 12% weight loss in both groups thereby being able to make optimal hormonal response comparison in elucidating the potential effect of GLP-1RAs on leptin activity. We were not allowed to buy or obtain placebo Victoza pens from the production company (Novo Nordisk), however, we hold it unlikely that there would be a significant placebo effect on direct leptin activity. We did not assess gastric emptying in this study; however, previous studies have indicated that the weight loss mechanism of liraglutide is mediated by the regulation of appetite signals and not delayed gastric emptying, as the effects on gastric emptying is subject to marked tachyphylaxis in contrast to the body weight-lowering effects.46, 47, 48
In conclusion, administration of a relatively low dose of GLP-1RA successfully maintained a 12% weight loss during 1 year. The appetite-inhibiting effect of GLP-1RA may depend on the 43% smaller decrease in free leptin levels, predominantly mediated by a 59% smaller increase in soluble leptin receptor, and higher PYY3–36 meal responses may also contribute. Furthermore, the GLP-1RA group lowered blood glucose additionally during weight loss maintenance in contrast to the control group where blood glucose levels increased to the same level as before the weight loss even though the weight loss was maintained. Thus, weight loss maintenance with GLP-1RA may act to prevent pre-diabetes in obesity.
Acknowledgments
The project including purchase of Victoza pens was supported by funding from The Danish Research Counsel, Health and Disease (reference number: 11-107683) and the University Investment Capital (UNIK): Food, Fitness and Pharma for Health and Disease from the Danish Ministry of Science, Technology and Innovation. Cambridge Weight Plan products were donated from Cambridge Weight Plan. The funding sponsors were not involved in study design, conduction of the study, data analysis or approval of manuscript. We thank the study participants, the dieticians Jane Hjort and Stine Rasmussen, Hvidovre Hospital and lab technician Lene Albæk, University of Copenhagen.
Author Contributions
SST, JJH, SM, J-EBJ, OP and TH designed the study. EWI, JL and CD conducted the study and collected the data. SST wrote the manuscript and analyzed the data. EWI, JL, CD, J-EBJ, OP, TH, JJH, SM and SST contributed to the discussion, reviewed/edited the manuscript and approved the final version. The corresponding author SST confirms full access to the data and final responsibility for the decision to submit for publication.
SM and JJH have performed consulting services for Novo Nordisk. OP holds stocks in Novo Nordisk. The remaining authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on International Journal of Obesity website (http://www.nature.com/ijo)
Supplementary Material
References
- Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, Franz MJ, et al. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31:S61–S78. doi: 10.2337/dc08-S061. [DOI] [PubMed] [Google Scholar]
- Wadden TA. Treatment of obesity by moderate and severe caloric restriction. Results of clinical research trials. Ann Intern Med. 1993;119:688–693. doi: 10.7326/0003-4819-119-7_part_2-199310011-00012. [DOI] [PubMed] [Google Scholar]
- Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365:1597–1604. doi: 10.1056/NEJMoa1105816. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Leibel RL. Adaptive thermogenesis in humans. Int J Obes (Lond) 2010;34:S47–S55. doi: 10.1038/ijo.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest. 2008;118:2583–2591. doi: 10.1172/JCI35055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115:3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissileff HR, Thornton JC, Torres MI, Pavlovich K, Mayer LS, Kalari V, et al. Leptin reverses declines in satiation in weight-reduced obese humans. Am J Clin Nutr. 2012;95:309–317. doi: 10.3945/ajcn.111.012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owecki M, Nikisch E, Miczke A, Pupek-Musialik D, Sowinski J. Free leptin index as a marker for leptin action in diabetes and obesity: leptin and soluble leptin receptors relationship with HbA1c. Neuro Endocrinol Lett. 2010;31:679–683. [PubMed] [Google Scholar]
- Lammert A, Kiess W, Bottner A, Glasow A, Kratzsch J. Soluble leptin receptor represents the main leptin binding activity in human blood. Biochem Biophys Res Commun. 2001;283:982–988. doi: 10.1006/bbrc.2001.4885. [DOI] [PubMed] [Google Scholar]
- Wu Z, Bidlingmaier M, Liu C, De Souza EB, Tschop M, Morrison KM, et al. Quantification of the soluble leptin receptor in human blood by ligand-mediated immunofunctional assay. J Clin Endocrinol Metab. 2002;87:2931–2939. doi: 10.1210/jcem.87.6.8610. [DOI] [PubMed] [Google Scholar]
- Kratzsch J, Lammert A, Bottner A, Seidel B, Mueller G, Thiery J, et al. Circulating soluble leptin receptor and free leptin index during childhood, puberty, and adolescence. J Clin Endocrinol Metab. 2002;87:4587–4594. doi: 10.1210/jc.2002-020001. [DOI] [PubMed] [Google Scholar]
- Schaab M, Kausch H, Klammt J, Nowicki M, Anderegg U, Gebhardt R, et al. Novel regulatory mechanisms for generation of the soluble leptin receptor: implications for leptin action. PLoS One. 2012;7:e34787. doi: 10.1371/journal.pone.0034787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owecki M, Nikisch E, Miczke A, Pupek-Musialik D, Sowinski J. Leptin, soluble leptin receptors, free leptin index, and their relationship with insulin resistance and BMI: high normal BMI is the threshold for serum leptin increase in humans. Horm Metab Res. 2010;42:585–589. doi: 10.1055/s-0030-1253422. [DOI] [PubMed] [Google Scholar]
- Galgani JE, Greenway FL, Caglayan S, Wong ML, Licinio J, Ravussin E. Leptin replacement prevents weight loss-induced metabolic adaptation in congenital leptin-deficient patients. J Clin Endocrinol Metab. 2010;95:851–855. doi: 10.1210/jc.2009-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101:515–520. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrup A, Rossner S, Van GL, Rissanen A, Niskanen L, Al HM, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374:1606–1616. doi: 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- Wadden TA, Hollander P, Klein S, Niswender K, Woo V, Hale PM, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond) 2013;37:1443–1451. doi: 10.1038/ijo.2013.120. [DOI] [PubMed] [Google Scholar]
- Torekov SS, Holst JJ, Ehlers MR. Dose response of continuous subcutaneous infusion of recombinant glucagon-like peptide-1 in combination with metformin and sulphonylurea over 12 weeks in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2013;16:451–456. doi: 10.1111/dom.12240. [DOI] [PubMed] [Google Scholar]
- Torekov SS, Madsbad S, Holst JJ. Obesity—an indication for GLP-1 treatment? Obesity pathophysiology and GLP-1 treatment potential. Obes Rev. 2011;12:593–601. doi: 10.1111/j.1467-789X.2011.00860.x. [DOI] [PubMed] [Google Scholar]
- Iepsen EW, Torekov SS, Holst JJ.Therapies for inter-relating diabetes and obesity - GLP-1 and obesity Expert Opin Pharmacother 2014. e-pub ahead of print 26 September 2014. [DOI] [PubMed]
- Williams DL, Baskin DG, Schwartz MW. Leptin regulation of the anorexic response to glucagon-like peptide-1 receptor stimulation. Diabetes. 2006;55:3387–3393. doi: 10.2337/db06-0558. [DOI] [PubMed] [Google Scholar]
- Clemmensen C, Chabenne J, Finan B, Sullivan L, Fischer K, Kuchler D, et al. GLP-1/glucagon coagonism restores leptin responsiveness in obese mice chronically maintained on an obesogenic diet. Diabetes. 2014;63:1422–1427. doi: 10.2337/db13-1609. [DOI] [PubMed] [Google Scholar]
- Muller TD, Sullivan LM, Habegger K, Yi CX, Kabra D, Grant E, et al. Restoration of leptin responsiveness in diet-induced obese mice using an optimized leptin analog in combination with exendin-4 or FGF21. J Pept Sci. 2012;18:383–393. doi: 10.1002/psc.2408. [DOI] [PubMed] [Google Scholar]
- Riecke BF, Christensen R, Christensen P, Leeds AR, Boesen M, Lohmander LS, et al. Comparing two low-energy diets for the treatment of knee osteoarthritis symptoms in obese patients: a pragmatic randomized clinical trial. Osteoarthritis Cartilage. 2010;18:746–754. doi: 10.1016/j.joca.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Holst JJ. Molecular heterogeneity of glucagon in normal subjects and in patients with glucagon-producing tumours. Diabetologia. 1983;24:359–365. [PubMed] [Google Scholar]
- Orskov C, Rabenhoj L, Wettergren A, Kofod H, Holst JJ. Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes. 1994;43:535–539. doi: 10.2337/diab.43.4.535. [DOI] [PubMed] [Google Scholar]
- Lindgren O, Carr RD, Deacon CF, Holst JJ, Pacini G, Mari A, et al. Incretin hormone and insulin responses to oral versus intravenous lipid administration in humans. J Clin Endocrinol Metab. 2011;96:2519–2524. doi: 10.1210/jc.2011-0266. [DOI] [PubMed] [Google Scholar]
- Allard C, Doyon M, Brown C, Carpentier AC, Langlois MF, Hivert MF. Lower leptin levels are associated with higher risk of weight gain over 2 years in healthy young adults. Appl Physiol Nutr Metab. 2013;38:280–285. doi: 10.1139/apnm-2012-0225. [DOI] [PubMed] [Google Scholar]
- Schaab M, Kausch H, Klammt J, Nowicki M, Anderegg U, Gebhardt R, et al. Novel regulatory mechanisms for generation of the soluble leptin receptor: implications for leptin action. PLoS One. 2012;7:e34787. doi: 10.1371/journal.pone.0034787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maamra M, Bidlingmaier M, Postel-Vinay MC, Wu Z, Strasburger CJ, Ross RJ. Generation of human soluble leptin receptor by proteolytic cleavage of membrane-anchored receptors. Endocrinology. 2001;142:4389–4393. doi: 10.1210/endo.142.10.8442. [DOI] [PubMed] [Google Scholar]
- Hsieh J, Longuet C, Baker CL, Qin B, Federico LM, Drucker DJ, et al. The glucagon-like peptide 1 receptor is essential for postprandial lipoprotein synthesis and secretion in hamsters and mice. Diabetologia. 2010;53:552–561. doi: 10.1007/s00125-009-1611-5. [DOI] [PubMed] [Google Scholar]
- Torekov SS, Kipnes MS, Harley RE, Holst JJ, Ehlers MR. Dose response of subcutaneous GLP-1 infusion in patients with type 2 diabetes. Diabetes Obes Metab. 2011;13:639–643. doi: 10.1111/j.1463-1326.2011.01388.x. [DOI] [PubMed] [Google Scholar]
- Stevens VJ, Obarzanek E, Cook NR, Lee IM, Appel LJ, Smith West D, et al. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med. 2001;134:1–11. doi: 10.7326/0003-4819-134-1-200101020-00007. [DOI] [PubMed] [Google Scholar]
- Beavers DP, Beavers KM, Lyles MF, Nicklas BJ. Cardiometabolic risk after weight loss and subsequent weight regain in overweight and obese postmenopausal women. J Gerontol A Biol Sci Med Sci. 2013;68:691–698. doi: 10.1093/gerona/gls236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca V, Madsbad S, Falahati, Zychma MJ, Plutzky J. Once-daily human GLP-1 analog liraglutide reduces systolic BP — A meta-analysis of 6 Clinical Trials. Diabetes. 2009;58:A146. [Google Scholar]
- Torekov SS, Larsen LH, Glumer C, Borch-Johnsen K, Jorgensen T, Holst JJ, et al. Evidence of an association between the Arg72 allele of the peptide YY and increased risk of type 2 diabetes. Diabetes. 2005;54:2261–2265. doi: 10.2337/diabetes.54.7.2261. [DOI] [PubMed] [Google Scholar]
- Batterham RL, Cohen MA, Ellis SM, le Roux CW, Withers DJ, Frost GS, et al. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- Berg JK, Shenouda SK, Heilmann CR, Gray AL, Holcombe JH. Effects of exenatide twice daily versus sitagliptin on 24-h glucose, glucoregulatory and hormonal measures: a randomized, double-blind, crossover study. Diabetes Obes Metab. 2011;13:982–989. doi: 10.1111/j.1463-1326.2011.01428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib AM, Richards P, Rogers GJ, Reimann F, Gribble FM. Co-localisation and secretion of glucagon-like peptide 1 and peptide YY from primary cultured human L cells. Diabetologia. 2013;56:1413–1416. doi: 10.1007/s00125-013-2887-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslund E, Bogefors J, Skogar S, Gryback P, Jacobsson H, Holst JJ, et al. GLP-1 slows solid gastric emptying and inhibits insulin, glucagon, and PYY release in humans. Am J Physiol. 1999;277:R910–R916. doi: 10.1152/ajpregu.1999.277.3.R910. [DOI] [PubMed] [Google Scholar]
- Nilsson O, Bilchik AJ, Goldenring JR, Ballantyne GH, Adrian TE, Modlin IM. Distribution and immunocytochemical colocalization of peptide YY and enteroglucagon in endocrine cells of the rabbit colon. Endocrinology. 1991;129:139–148. doi: 10.1210/endo-129-1-139. [DOI] [PubMed] [Google Scholar]
- Mortensen K, Christensen LL, Holst JJ, Orskov C. GLP-1 and GIP are colocalized in a subset of endocrine cells in the small intestine. Regul Pept. 2003;114:189–196. doi: 10.1016/s0167-0115(03)00125-3. [DOI] [PubMed] [Google Scholar]
- Svendsen B, Pedersen J, Holst JJ. Glucagon-like peptide 1 secretion from proximal rat intestine. Diabetologia. 2011;54:S211–S211. [Google Scholar]
- Lean ME, Carraro R, Finer N, Hartvig H, Lindegaard ML, Rossner S, et al. Tolerability of nausea and vomiting and associations with weight loss in a randomized trial of liraglutide in obese, non-diabetic adults. Int J Obes (Lond) 2014;38:689–697. doi: 10.1038/ijo.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelsing J, Vrang N, Hansen G, Raun K, Tang-Christensen M, Knudsen LB. Liraglutide: short-lived effect on gastric emptying — long lasting effects on body weight. Diabetes Obes Metab. 2012;14:531–538. doi: 10.1111/j.1463-1326.2012.01557.x. [DOI] [PubMed] [Google Scholar]
- Nauck MA, Kemmeries G, Holst JJ, Meier JJ. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes. 2011;60:1561–1565. doi: 10.2337/db10-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WHM. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes (Lond) 2014;38:784–793. doi: 10.1038/ijo.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.