Abstract
Introduction:
The irritable bowel syndrome with constipation (IBS-C) and chronic idiopathic constipation (CIC) are associated with substantial symptom and disease burden. Although typically classified as distinct diseases, symptoms frequently overlap.
Aim:
The objective of this study was to characterize symptom and disease burden in IBS-C and CIC sufferers and examine a subset of CIC sufferers with abdominal symptoms.
Methods:
In a US population-based survey, respondents meeting the Rome III criteria for IBS-C or CIC rated symptom frequency and bothersomeness, missed work and disrupted productivity, and degree of obtaining and satisfaction with physician care. CIC respondents were analyzed in two subgroups: those with abdominal symptoms ≥once weekly (CIC-A) and those without (CIC-NA).
Results:
Of the 10,030 respondents, 328 met the criteria for IBS-C and 552 for CIC (363 CIC-A; 189 CIC-NA). All symptoms were significantly more frequent in IBS-C vs. CIC respondents (P<0.0001). Constipation was extremely/very bothersome in 72% of IBS-C respondents, 62% of CIC-A, and 40% of CIC-NA (P<0.01 all pairs). All 11 other measured symptoms were significantly more bothersome in IBS-C and CIC-A vs. CIC-NA respondents. In IBS-C vs. CIC-A, abdominal discomfort, bloating, straining, and pellet-like stools were also significantly more bothersome, with other remaining symptoms similar. Gastrointestinal symptoms disrupted productivity a mean of 4.9 days per month in IBS-C respondents, 3.2 in CIC-A, and 1.2 in CIC-NA (P<0.001 all pairs); missed days were similar in IBS-C and CIC-A respondents.
Conclusion:
CIC respondents with abdominal symptoms experience greater disease burden compared with CIC respondents without frequent abdominal symptoms and have a disease burden profile that is similar to IBS-C respondents.
Introduction
Irritable bowel syndrome (IBS) is a common symptom-based functional gastrointestinal (GI) disorder, estimated to affect between 5 and 20% of the population (1, 2). It is more common in females than males and more common in younger adults (<50 years old) (1). Specifically, the Rome III diagnostic criteria for functional GI disorders define IBS as recurrent abdominal pain or discomfort (at least 3 days per month in the last 3 months) associated with at least two of the following: improvement with defecation, onset associated with a change in stool frequency, and/or onset associated with change in stool form (3). The diagnosis of IBS is subtyped by the predominant stool pattern: constipation (IBS-C), diarrhea (IBS-D), or mixed (IBS-M) (3).
Similar to IBS, chronic idiopathic constipation (CIC), also referred to as functional constipation, is a symptom-based functional GI disorder. Prevalence estimates vary by geographic region and disease definition; a recent meta-analysis based on 41 studies reported a pooled prevalence of 14%, with a confidence interval of 12–17% (4). CIC has been observed to be more common in females and older adults (4). The Rome III diagnostic criteria use the term functional constipation, defining it based on the frequency of specific bowel symptoms (e.g., straining, lumpy/hard stools, incomplete evacuation, sensation of anorectal obstruction, manual maneuvers for defecation, and ≤3 defecations/week) combined with the requirement that the patient does not meet the criteria for IBS (3).
Although the widely accepted Rome III diagnostic criteria maintain a mutually exclusive distinction between IBS-C and CIC (or functional constipation), studies have identified substantial symptom overlap between the two conditions (5, 6, 7, 8). Many CIC patients experience symptoms of abdominal pain, discomfort, and bloating (7, 9). Furthermore, it has been demonstrated that many patients “migrate” over time from one diagnosis to the other, which, coupled with limited knowledge of diagnostic criteria, likely enhances the challenge of making an accurate diagnosis in primary care and specialty practices (10).
Suffering from abdominal symptoms and often exhibiting a range of comorbid conditions, IBS-C and CIC patients have been shown to experience substantial reductions in health-related quality of life (HRQOL) (11, 12, 13, 14). A recent systematic review of the disease burden of IBS and CIC found the attributable direct costs of IBS to range from approximately $1,600 to $7,500 per patient-year, whereas the attributable direct costs of CIC range from approximately $1,900 to $7,500 per patient-year. Specific financial data on the indirect costs and the humanistic burden of CIC are limited, as are data specific to the IBS subtypes (13).
This survey-based study aimed to characterize both symptom experiences of and burden, including impact on work and school, imposed upon individuals who suffer from IBS-C and CIC. We were particularly interested to understand the impact of abdominal symptoms on illness experience and burden of disease in constipation patients who did not fulfill the criteria for IBS-C. We hypothesized that constipation severity would be associated with the presence and severity of abdominal symptoms.
Methods
Cohort selection and survey administration
The Universal Survey Opinionsite consumer panel consists of more than 550,000 members recruited from market research surveys, focus groups, email lists, social networking websites, targeted panel referrals, and other media including banner ads, newspaper ads, and alumni networks. A random group of 37,500 panelists was invited by e-mail to participate in a health-care survey in January of 2010. Although participants are not compensated directly for participating in an individual survey, the consumer panel is a reward-based program in which panelists accumulate redeemable points for survey participation. A stratified sampling procedure was implemented using the 2009 US Census estimate (15); strata were based on age, sex, and ethnicity to be representative of the demographic composition of the estimated 2009 US population. Additional e-mail invitations were sent to male panelists and panelists aged ≥65 years because of the low response rates to the initial invitation in these demographic groups.
E-mail invitations included a link to a 21-item screening questionnaire used to evaluate panelist eligibility to participate in a longer on-line survey. Participants were required to meet the following criteria to be eligible for the longer survey: (i) no conflicts of interest with advertising, marketing research, health care, or pharmaceutical companies; (ii) ≥18 years of age; (iii) never been diagnosed with an organic gut condition (e.g., inflammatory bowel disease, ulcer or significant inflammation in the GI tract, GI cancer); (iv) suffered from any GI symptoms in the past 12 months; and (v) met the diagnostic criteria for IBS-C, CIC, functional dyspepsia, or gastroesophageal reflux disease. The analysis presented here includes participants who, based on the answers to the screening questionnaire, met the Rome III criteria for IBS-C or CIC (3). In keeping with the Rome III criteria, the IBS-C and CIC groups were mutually exclusive. Although CIC respondents may have reported experiencing abdominal pain or discomfort at a frequency that satisfied the Rome III criteria for IBS, they were necessarily excluded from the IBS group if they did not report at least two of the three required conditions (improvement with defecation, onset associated with a change in stool frequency, and onset associated with a change in stool form) associating pain/discomfort with bowel habits. In addition, unlike IBS, which requires the presence of abdominal pain or discomfort, the CIC group could have reported any abdominal symptom (stomach cramping and/or bloating in addition to pain or discomfort), and, as long as they did not satisfy the Rome III criteria for IBS-C, they were classified as suffering from CIC.
Participants who met the screening criteria completed a 31-question survey, which included assessments of overall symptom severity and bothersomeness, frequency and bothersomeness of individual GI symptoms, whether or not they had consulted a physician about any symptom(s), satisfaction with physician care, work/school days missed, and work/school productivity. The survey is included in the Supplementary Materials online. The individual GI symptoms that were assessed in the survey and included in this analysis are “gas pain”, “abdominal pain”, “abdominal discomfort”, “stomach cramping”, “bloating”, “constipation”, “straining when having a bowel movement”, “having hard or lumpy stool”, “having pellet-like stools”, “the inability to have a bowel movement”, “rectal pain when having a bowel movement”, and “not completely emptying your rectum or bowels when having a bowel movement”.
Symptom bothersomeness, symptom severity, and satisfaction with physician care were rated using a 5-point ordinal scale (“Not at all: (1)”, “A little: (2)”, “Somewhat: (3)”, “Very: (4)”, and “Extremely: (5)”). The calculation of mean symptom frequency was based on the choices for the survey question “How frequently did you experience each symptom during the past 12 months? (Select one for each symptom)” as listed below:
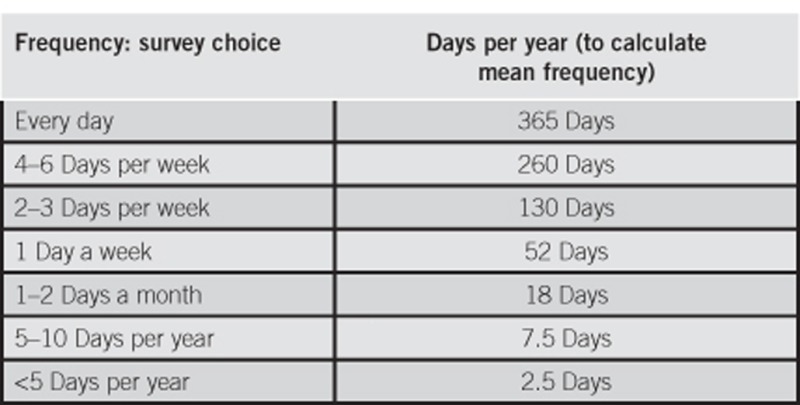
The mean annual frequencies were scaled to weekly frequencies, by dividing by 52.177 (365.242 days per year/7 days per week).
Statistical analysis
Demographic data from the survey did not align completely with the 2009 Current Population Survey (CPS) (15) estimate of the US Census Bureau because of a slight underrepresentation of men and the elderly (≥65 years of age). Therefore, the demographic distribution from this sample of 10,030 respondents was adjusted to match the 2009 CPS for age and sex (15) by applying a weighted adjustment to each respondent and their answers. The adjustment weights for males were 1.17, 0.98, 0.83, and 1.71 for ages 18–39, 40–49, 50–64, and ≥65 years, respectively; for females they were 0.90, 0.84, 0.76, and 1.90 for ages 18–39, 40–49, 50–64, and ≥65 years, respectively. All results reported in this paper are based on the weighted survey data.
Binary data were summarized using the proportion of respondents with the event. Differences between groups were analyzed using the column proportions test. Continuous data were reported using the sample size and mean. Results from IBS-C and CIC sufferers were compared using a two-sided t-test performed at the 95% confidence level. Statistical testing was conducted with the data aggregation program Quantum (version 5.8), and the 95% confidence levels were calculated at the website Raosoft.com. P<0.05 was considered statistically significant.
To assess the burden of abdominal symptoms among respondents who met the criteria for CIC but not IBS-C, the CIC respondents were divided into two subgroups: those experiencing abdominal symptoms (CIC-A) and those without abdominal symptoms (CIC-NA). Experiencing abdominal symptoms was defined as any combination of abdominal pain, abdominal discomfort, stomach cramping, and/or bloating at least once a week during the past 12 months. Analyses of missed work/school days and lost productivity were based on the subsets of respondents who reported being employed or attending school.
Results
Of the 10,030 adults who responded and completed the screening questionnaire, 880 met the inclusion criteria and the diagnostic criteria for this study: 328 met the Rome III criteria for IBS-C and 552 met the Rome III criteria for CIC. Demographic data are summarized in Table 1. Approximately 66% of IBS-C respondents and 62% of CIC were under the age of 50 years. The respondent population was predominantly female (68% of IBS-C; 62% of CIC) and white/Caucasian (79% of IBS-C; 81% of CIC).
Table 1. Demographic characteristics of Rome III IBS-C and CIC cohorts weighted for the US Census population.
| IBS-C, N=328 | CIC (total), N=552 | CIC-A, N=363 | CIC-NA, N=189 | |
|---|---|---|---|---|
| Age in years, % | ||||
| 18–39 | 40.9 | 43.5 | 45.1 | 40.6 |
| 40–49 | 24.9 | 18.2 | 19.8 | 15.1 |
| 50–64 | 25.3 | 20.4 | 19.1 | 23.0 |
| ≥65 | 8.9 | 17.9 | 16.1 | 21.3 |
| Sex, % | ||||
| Male | 31.6 | 37.9 | 36.6 | 40.5 |
| Female | 68.4 | 62.1 | 63.4 | 59.5 |
| Race/ethnicity, % | ||||
| White/Caucasian | 79.4 | 80.7 | 80.1 | 81.9 |
| Black/African-American | 11.7 | 10.4 | 11.5 | 8.3 |
| Asian/Pacific Islander | 3.8 | 3.2 | 2.3 | 5.0 |
| Native American | 0.3 | 0.5 | 0.2 | 0.9 |
| Other | 4.7 | 5.2 | 5.8 | 3.9 |
| Hispanic ethnicity, % | 12.1 | 11.2 | 14.0 | 5.9 |
| Working/school going | ||||
| Employed or attending school, % | 68.3 | 63.0 | 63.4 | 62.4 |
| Household income, mean thousands of dollars | 69.4 | 62.9 | 61.6 | 65.5 |
CIC, chronic idiopathic constipation; CIC-A, CIC with abdominal symptoms ≥weekly; CIC-NA, CIC without abdominal weekly symptoms; IBS-C, constipation-predominant irritable bowel syndrome.
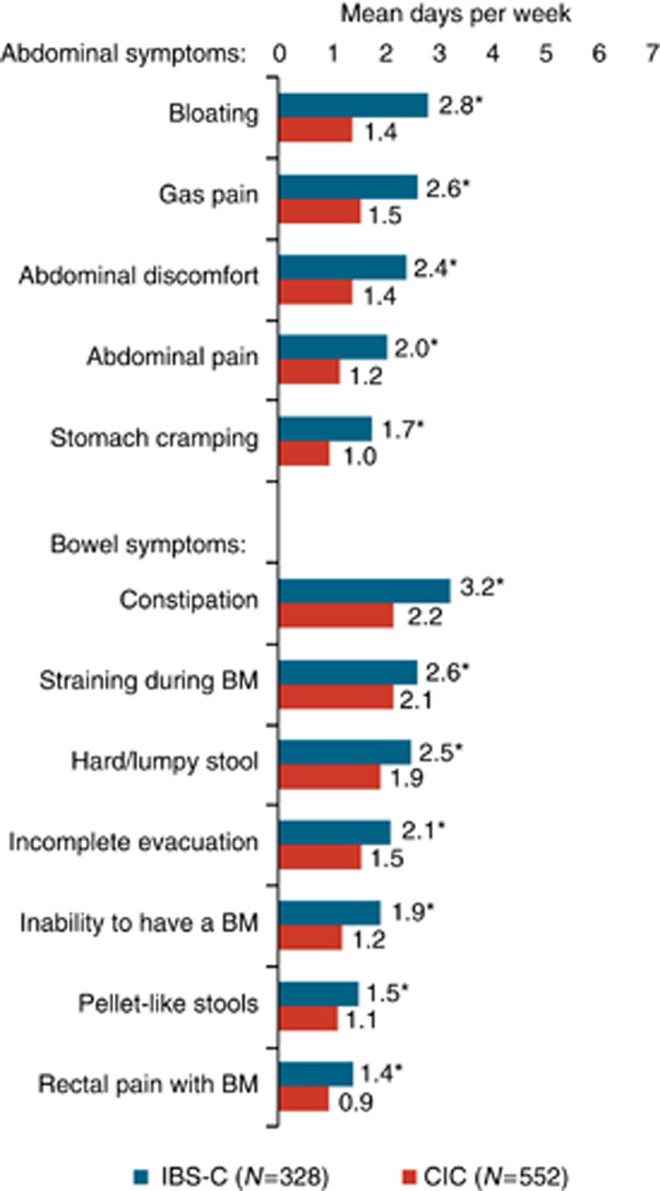
The frequencies of symptoms experienced by IBS-C and CIC respondents are presented in Figure 1. Among IBS-C respondents, the symptoms experienced with the highest frequency were constipation (mean of 3.2 days per week, or 46% of days), bloating (2.8 days (40%)), and straining (2.6 days (37%)). Abdominal symptoms of gas pain, abdominal discomfort, and abdominal pain were experienced by IBS-C respondents at a mean of 2.6 (37%), 2.4 (34%), and 2.0 (29%) days per week (% of days), respectively.
Figure 1.
Frequency of symptoms among constipation-predominant irritable bowel syndrome (IBS-C) and chronic idiopathic constipation (CIC) respondents. BM, bowel movement. *IBS-C vs. CIC, P<0.0001.
Among CIC respondents, the symptoms experienced with the highest frequency were constipation (2.2 days per week or 31% of days), straining (2.1 days (31%)), and hard or lumpy stool (1.9 days (27%)). The abdominal symptoms of bloating, gas pain, abdominal discomfort, and abdominal pain were each experienced with a mean frequency greater than 1 day per week. For each of the abdominal and bowel symptoms evaluated, the frequency was significantly greater in IBS-C respondents compared with CIC respondents (P<0.0001).
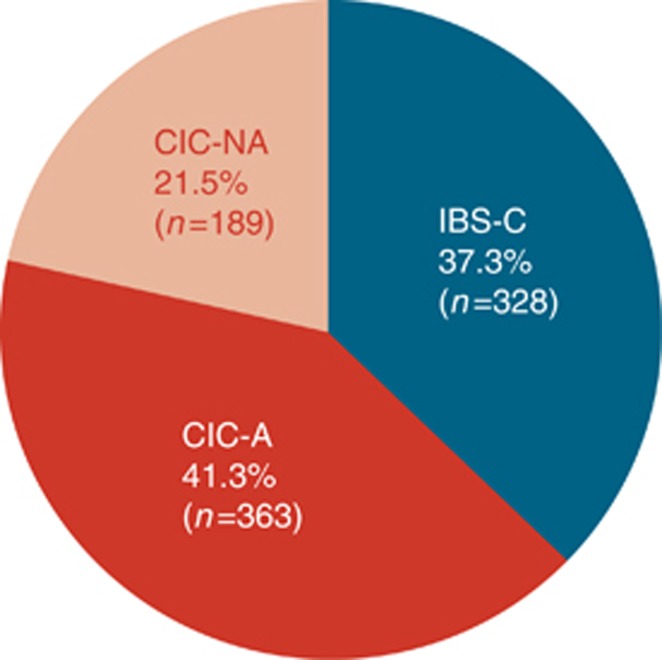
Abdominal symptoms (e.g., abdominal pain, abdominal discomfort, stomach cramping, and/or bloating) were experienced at least once per week by 363 of the CIC respondents. The CIC-A respondents represent 66% of the CIC respondents and 41% of the study population (Figure 2). Demographic information is provided in Table 1.
Figure 2.
Relative proportions of respondents meeting the criteria for chronic idiopathic constipation with abdominal symptoms (CIC-A), chronic idiopathic constipation without abdominal symptoms (CIC-NA), and constipation-predominant irritable bowel syndrome (IBS-C).
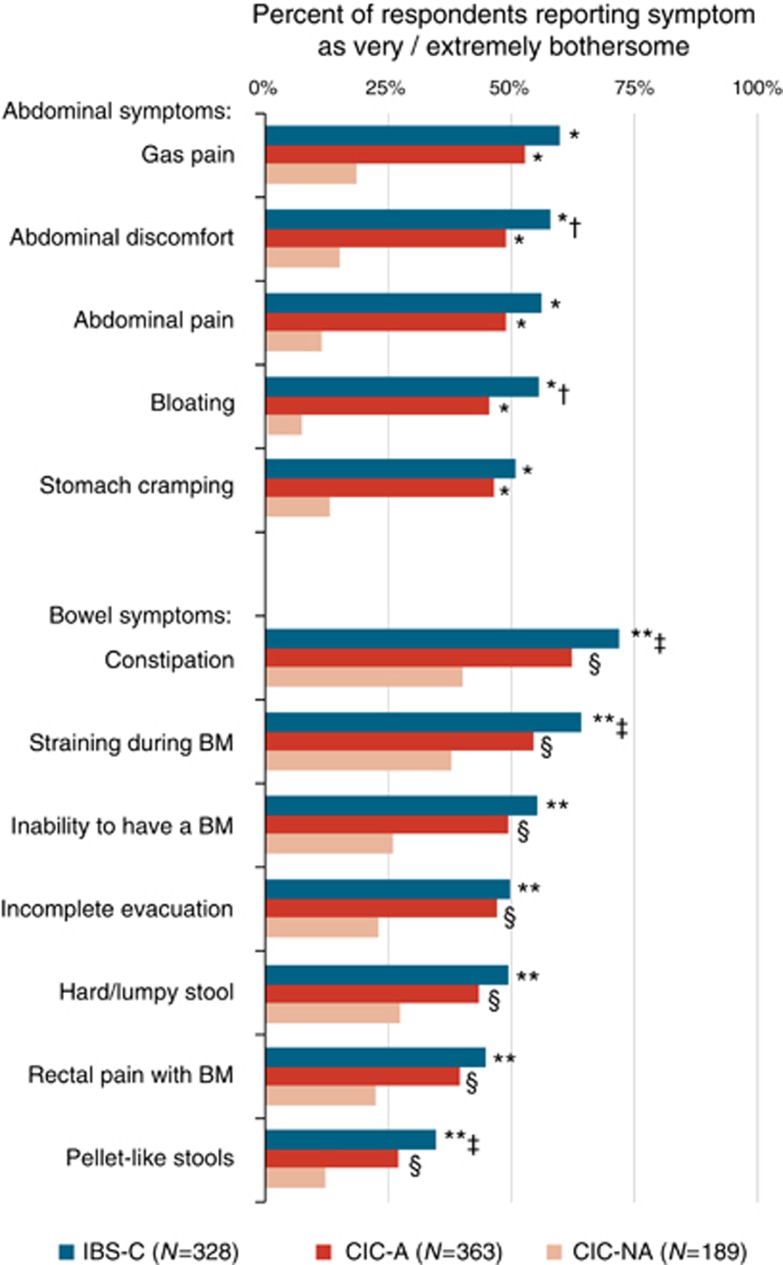
As would be expected, for each of the abdominal symptoms assessed, the percentage of respondents reporting the symptom to be very or extremely bothersome was significantly higher among CIC-A respondents compared with CIC-NA (P<0.0001), as well as among IBS-C respondents compared with CIC-NA (P<0.0001, Figure 3). Among IBS-C respondents, the abdominal symptom most likely to be experienced as very or extremely bothersome was gas pain (60%), followed by abdominal discomfort (58%), abdominal pain (56%), bloating (56%), and stomach cramping (51%). The proportions of CIC-A respondents experiencing very or extremely bothersome abdominal discomfort (49%) and bloating (46%) were significantly lower than in the IBS-C respondents (P<0.03); for the other abdominal symptoms, the differences between IBS-C and CIC-A were not significant.
Figure 3.
Symptoms rated as very or extremely bothersome among respondents with constipation-predominant irritable bowel syndrome (IBS-C), chronic idiopathic constipation with abdominal symptoms (CIC-A), and chronic idiopathic constipation without abdominal symptoms (CIC-NA). Abdominal symptoms: *IBS-C vs. CIC-NA and CIC-A vs. CIC-NA, P<0.0001; †IBS-C vs. CIC-A, P<0.03. Bowel symptoms: **IBS-C vs. CIC-NA, P<0.0001; ‡IBS-C vs. CIC-A, P<0.04; §CIC-A vs. CIC-NA, P<0.001.
For each of the bowel symptoms assessed, the percentage of respondents reporting the symptom to be very or extremely bothersome was significantly greater among CIC-A compared with CIC-NA respondents (P<0.001, Figure 3). For constipation, straining, and pellet-like stools, the percentages of respondents reporting the symptom to be very or extremely bothersome were significantly greater among IBS-C compared with CIC-A respondents (P<0.04); for the other bowel symptoms, the differences between IBS-C and CIC-A were not significant. Constipation, the most bothersome of symptoms, was reported to be very or extremely bothersome in 72% of IBS-C respondents, 62% of CIC-A respondents, and 40% of CIC-NA respondents (P<0.01 all pairs).
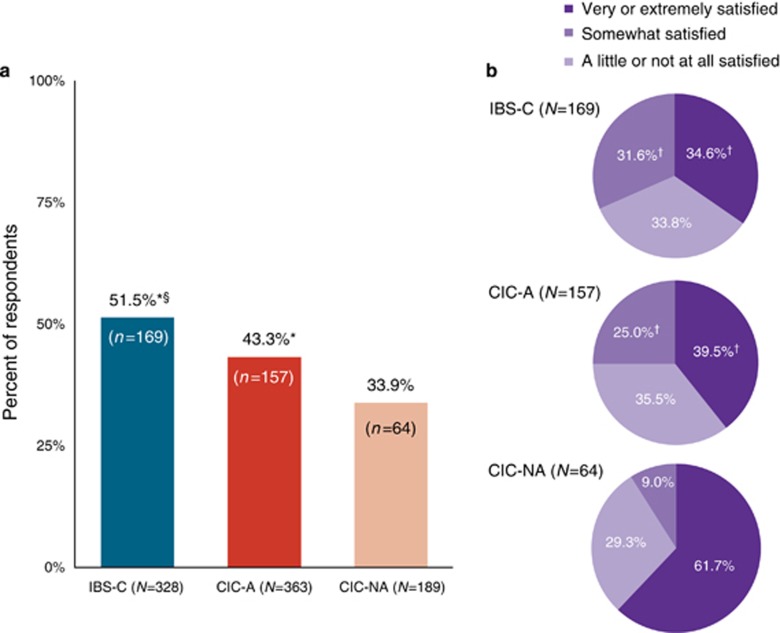
IBS-C respondents were significantly more likely than CIC-A and CIC-NA respondents to seek physician care for GI symptoms in the past 12 months (P<0.04, Figure 4a). Furthermore, CIC-A respondents were significantly more likely than CIC-NA respondents to seek care (P<0.04). Among the respondents who sought physician care, a significantly higher proportion of CIC-NA respondents were very or extremely satisfied with that care as compared with the CIC-A and IBS-C respondents (P<0.01, Figure 4b); the difference between CIC-A and IBS-C was not significant.
Figure 4.
Physician care. (a) Proportion of respondents who sought physician care in the past 12 months for gastrointestinal symptoms. CIC-A, chronic idiopathic constipation with abdominal symptoms; CIC-NA, chronic idiopathic constipation without abdominal symptoms; IBS-C, constipation-predominant irritable bowel syndrome. *IBS-C vs. CIC-NA and IBS-C vs. CIC-A, P<0.04; §CIC-A vs. CIC-NA, P<0.04. (b) Satisfaction with physician care. †IBS-C vs. CIC-NA and CIC-A vs. CIC-NA, P<0.01.
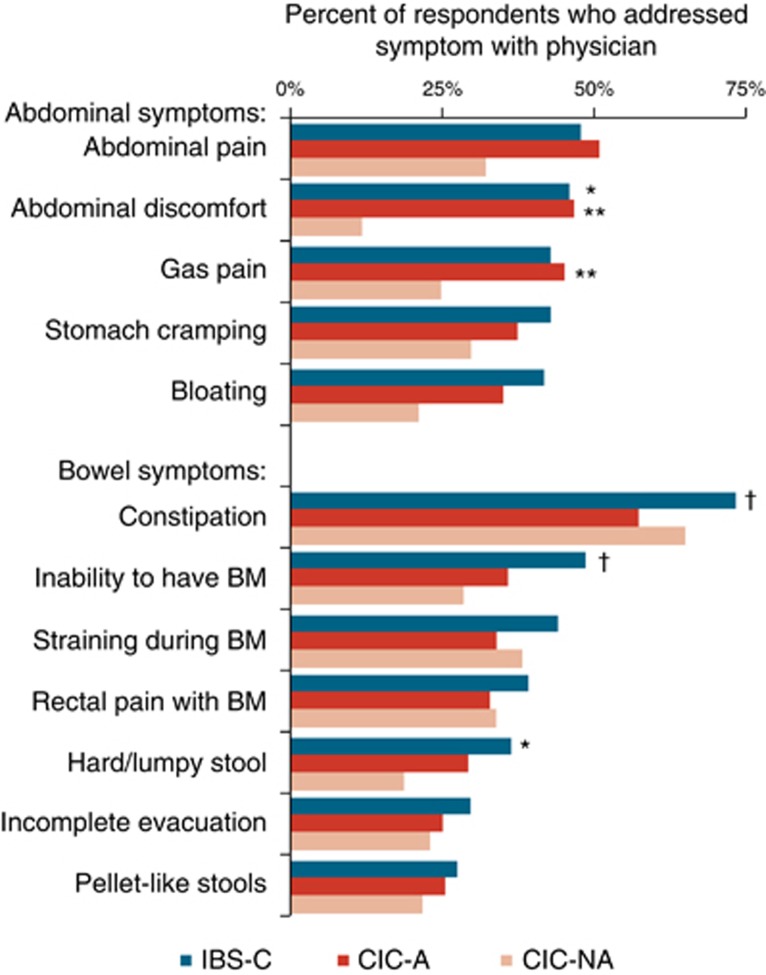
Constipation appeared to be the symptom that, if present, was most likely to be addressed with a physician (Figure 5). Of the 185 IBS-C respondents who both visited a physician within the past 12 months and experienced constipation within the past 12 months, 73% addressed the symptom with a physician. Similarly, of the 158 CIC-A and 62 CIC-NA respondents who both visited a physician and experienced constipation within the past 12 months, 57% and 65%, respectively, addressed the symptom with a physician. For the IBS-C and CIC-A respondents, abdominal pain and abdominal discomfort were the second and third most likely symptoms to be addressed with a physician: 48% of the 163 IBS-C and 51% of the 144 CIC-A respondents who visited a physician and experienced abdominal pain addressed it with a physician; 46% of the 170 IBS-C and 46% of the 159 CIC-A respondents who visited a physician and experienced abdominal discomfort addressed it with a physician.
Figure 5.
Symptoms addressed with a physician. Percentages are based on the number of respondents who visited physician and experienced the symptom within the past year. For constipation-predominant irritable bowel syndrome (IBS-C) respondents, N's ranged from 124 to 185; for chronic idiopathic constipation with abdominal symptoms (CIC-A), N's ranged from 104 to 159; for chronic idiopathic constipation without abdominal symptoms (CIC-NA), N's ranged from 17 to 62. *IBS-C vs. CIC-NA, P<0.03; **CIC-A vs. CIC-NA, P<0.04; †IBS-C vs. CIC-A, P<0.04.
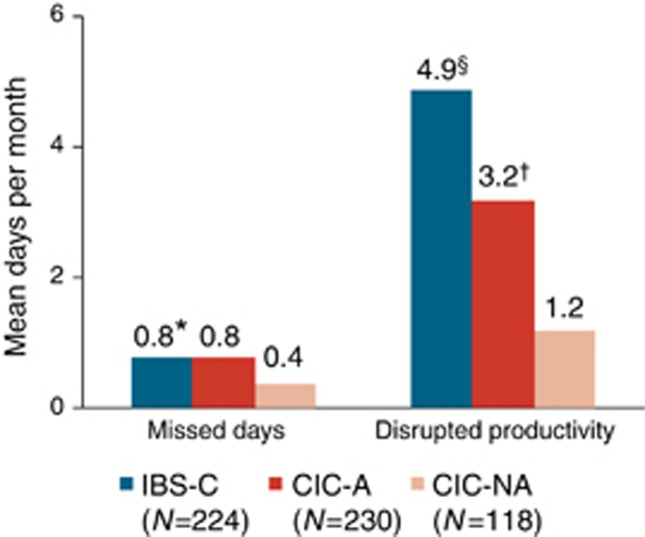
Among the working/school-attending respondents, GI symptoms led to a mean of 0.8 missed days per month in both IBS-C and CIC-A respondents and 0.4 missed days in CIC-NA respondents; the difference was statistically significant between IBS-C and CIC-NA (P<0.01, Figure 6). Disrupted productivity was greatest in IBS-C respondents, with a mean of 4.9 disrupted productivity days per month, compared with 3.2 days in CIC-A and 1.2 days in CIC-NA; the differences in disrupted productivity were significant between each of the groups (P<0.001).
Figure 6.
Missed work and disrupted productivity due to gastrointestinal (GI) symptoms among working/school-going respondents. CIC-A, chronic idiopathic constipation with abdominal symptoms; CIC-NA, chronic idiopathic constipation without abdominal symptoms; IBS-C, constipation-predominant irritable bowel syndrome. *IBS-C vs. CIC-NA, P<0.01; §IBS-C vs. CIC-A and IBS-C vs. CIC-NA, P<0.001 †CIC-A vs. CIC-NA, P<0.001.
Discussion
This study assessed the symptom profile, symptom burden, and disease burden in individuals who met the Rome III criteria for IBS-C or CIC (Rome III criteria use the term functional constipation). For each of the symptoms evaluated in the study (five abdominal symptoms and seven bowel symptoms), the frequency and bothersomeness were significantly greater in the IBS-C respondents than in the CIC respondents.
Among the CIC respondents, the symptoms experienced with the highest frequencies were constipation, straining, and hard/lumpy stool. Also, notable among the CIC respondents, the mean frequencies for the abdominal symptoms of bloating, gas pain, abdominal discomfort, and abdominal pain were each greater than once per week. The reporting of abdominal symptoms in the CIC respondents is consistent with earlier studies that have quantified the presence of abdominal symptoms among CIC patients (16, 17, 18).
In considering reports of abdominal symptoms in CIC patients (16, 17, 18), it is important to note that, according to the Rome III diagnostic criteria, the diagnosis of CIC (or functional constipation) excludes any patient who meets the criteria for IBS. The criterion for IBS, with symptom onset ≥6 months before diagnosis, is recurrent abdominal pain or discomfort at least 3 days per month in the last 3 months that is associated with at least two of the following three conditions: (i) the pain or discomfort is improved with defecation, (ii) the onset of the pain or discomfort is associated with a change in stool frequency, and (iii) the onset of the pain or discomfort is associated with a change in the form of stool (3). Aside from the exclusion of IBS diagnoses, the Rome III criteria for CIC (or functional constipation) do not address abdominal symptoms.
In keeping with the Rome III diagnostic criteria, the IBS and CIC respondents in this study were mutually exclusive groups. Two points are important to emphasize regarding the distinction between the IBS-C and CIC-A groups identified in this study. Although CIC respondents may have reported experiencing abdominal pain or discomfort at a frequency at or above the Rome III IBS frequency threshold, they were necessarily excluded from the IBS group if they did not report at least two of the three required conditions associating pain/discomfort with bowel habits. Also, for the CIC-A group, any combination of abdominal symptoms (cramping and bloating in addition to pain and discomfort) could have been reported at a frequency of more than once per week.
To explore the impact of abdominal symptoms in CIC, we analyzed the CIC respondents in two groups: those who reported experiencing at least one abdominal symptom (abdominal pain, abdominal discomfort, stomach cramping, and/or bloating) at least once per week and those who did not. Of the 552 CIC respondents, 363 experienced abdominal symptoms at least once per week and 189 did not. For the CIC respondents with abdominal symptoms, the bothersomeness of all symptoms (bowel as well as abdominal) was significantly greater compared with the bothersomeness experienced by the CIC respondents without abdominal symptoms. Comparing the IBS-C respondents and the CIC respondents with abdominal symptoms, the bothersomeness of each symptom appeared to be greater in the IBS-C respondents, with 5 of the 12 symptoms—abdominal discomfort, bloating, constipation, straining, and pellet-like stools—statistically significantly more bothersome in IBS-C compared with CIC with abdominal symptoms.
The reports of bothersomeness suggest that the presence of abdominal symptoms in CIC patients may affect patients' overall symptom experience and may be associated with increased intensity of bowel symptoms. The study findings are consistent with a recent analysis of two phase 3 clinical trials in patients meeting the Rome II criteria for CIC that concluded that, in CIC patients with abdominal pain, it is the abdominal symptoms rather than the bowel symptoms alone that may drive patient assessment of constipation severity, HRQOL, and overall response to treatment (16).
Several studies have established the impact of IBS on HRQOL, with IBS patients consistently scoring lower than non-IBS patients (19, 20). Physical HRQOL in IBS is the same or lower when compared with HRQOL in diabetes, depression, and gastroesophageal reflux disease, whereas mental HRQOL is lower than in patients with chronic renal failure (20, 21, 22). Recently published studies have shown that IBS-C and CIC are associated with substantial disease burden, in terms of both overall costs and HRQOL. An economic-burden analysis found that patients with IBS-C incurred significantly higher health-care costs that were nearly double that of matched controls and similar in magnitude to costs of other chronic conditions such as migraine and asthma (23). An analysis of health-care costs in CIC patients found direct health-care costs to be significantly higher compared with matched controls; the analysis also found the costs to be significantly higher for CIC patients with abdominal symptoms compared with costs for those without abdominal symptoms (24).
Our study assessed disease burden in terms of missed days and disrupted productivity. Missed days due to GI symptoms were similar in IBS-C and CIC respondents with abdominal symptoms and somewhat less in CIC respondents without abdominal symptoms; the difference between the two CIC groups, those with abdominal symptoms and those without, did not reach statistical significance. Productivity was disrupted due to GI symptoms significantly more in the IBS-C respondents when compared with each CIC group; productivity disruption was also significantly greater in CIC with abdominal symptoms when compared with CIC respondents without abdominal symptoms.
Among the three groups analyzed in the study, the IBS-C respondents were the most likely to seek physician evaluation for GI symptoms (52%), followed by CIC respondents with abdominal symptoms (43%) and followed in turn by CIC respondents without abdominal symptoms (34%). These results are consistent with those of a population-based survey in Spain that found that approximately 40% of chronic constipation respondents sought medical care for constipation and that seeking medical care was correlated with the symptoms of abdominal pain and prolonged defecation (17). In addition to assessing health-care-seeking behavior, our survey assessed satisfaction with the physician care that respondents received. The IBS-C and CIC patients with abdominal symptoms were significantly less likely to be very or extremely satisfied with their care than CIC patients without abdominal symptoms.
This study is a post-hoc analysis of a cross-sectional population survey, reflecting respondents' retrospective assessment of symptom experience, bothersomeness, and symptom frequency at a single point in time. An inherent limitation is that respondents reported symptoms based on a list of individual symptoms; the responses do not reflect how patients may experience symptoms in conjunction with one another, nor do they reflect how respondents may experience or define individual symptoms (e.g., the term “stomach cramping” may, to some, specifically indicate gastric pain, whereas to others it may be a general cramping; patients may or may not experience gas and bloating as two distinct symptoms).
The study findings highlight the substantial burden of IBS-C and CIC, centered on the impact of the bothersomeness of these diseases, resulting in decreased work productivity and increased physician-seeking care. Furthermore, the severity and impact of disease appears to be greatest in the IBS-C population and greater in the CIC respondents with weekly abdominal symptoms than in the CIC respondents without weekly abdominal symptoms. In total, our data suggest that patient-reported disease severity tracks closely with the presence or absence of abdominal symptoms. Therefore, the presence of abdominal symptoms may provide a proxy for disease severity along a continuum. This notion of a spectrum of disease severity is consistent with observations expressed in recent literature that, despite the research value for treating the mutually exclusive diagnostic categories of IBS-C and CIC established by the Rome III criteria, in clinical practice there is not necessarily a clear separation between the two disorders, with many patients migrating between the diagnoses over time (6, 25, 26). It is this view that prompted the American College of Gastroenterology's Task Force on the management of functional bowel disorders to revise its approach and review treatments for IBS-C and CIC within one integrated clinical framework (26). Viewing these two disorders as part of a spectrum of disease severity conveys the clinical value of considering the full range of symptoms in IBS-C and CIC that patients may experience.
Conclusion
Our survey data demonstrate the substantial symptom overlap and disease burden associated with IBS-C and CIC. IBS-C respondents experience a greater symptom and disease burden compared with that of CIC respondents. CIC respondents experience frequent bowel symptoms, and more than half experience abdominal symptoms at least weekly. Those with CIC and abdominal symptoms experience a greater disease burden, as compared with CIC respondents without frequent abdominal symptoms, and have a disease burden profile that is similar to IBS-C respondents. These results question the current Rome III and regulatory construct of classifying CIC and IBS-C as separate and distinct disorders. Rather, these data suggest that CIC and IBS-C reside along a spectrum of disease where the presence of abdominal symptoms indicates disease severity rather than defines two separate and distinct conditions.
Study Highlights
Acknowledgments
We thank Dr Philip Schoenfeld for insights and participation in an early draft of this study.
Guarantor of the article: Steve A. Miller, BA is the guarantor of this study.
Specific author contributions: J.J. Heidelbaugh contributed to the interpretation of the data, the drafting of the manuscript, and the critical revision of the manuscript. M.A. Stelwagon contributed to the design of the research study, the analysis and interpretation of the data, and the critical revision of the manuscript. S. A. Miller contributed to the design of the research study, participated in data collection, analyzed the data, and contributed to the critical revision of the manuscript. E.P. Shea contributed to the drafting of the manuscript, interpretation of the data, and the critical revision of the manuscript. W.D. Chey contributed to the interpretation of the data and the critical revision of the manuscript. All authors had access to the study data and reviewed and approved the final version of the manuscript.
Financial support: This study was funded by Forest Laboratories, LLC, a subsidiary of Actavis PLC, and Ironwood Pharmaceuticals.
Potential competing interests: J.J. Heidelbaugh has no personal interests to disclose. M.A. Stelwagon and E.P. Shea are full-time employees of and own stock/stock options in Ironwood Pharmaceuticals, Inc. S.A. Miller has served as a market research consultant to Actavis and Ironwood Pharmaceuticals. W.D. Chey has served as an advisor or consultant for Actavis, AstraZeneca, Astellas, Asubio, Ferring, Furiex, Ironwood, Nestle, Proctor & Gamble, Prometheus, Salix, SK, Sucampo, and Takeda, has served as a speaker and received travel expenses from the GI Health Foundation, and has received grant support from Ironwood, Perrigo, and Prometheus.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/ajg
Supplementary Material
References
- Lovell RM, Ford AC. Global prevalence of, and risk factors for, irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–721. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- Quigley EM, Abdel-Hamid H, Barbara G, et al. A global perspective on irritable bowel syndrome: a consensus statement of the world gastroenterology organisation summit task force on irritable bowel syndrome. J Clin Gastroenterol. 2012;46:356–366. doi: 10.1097/MCG.0b013e318247157c. [DOI] [PubMed] [Google Scholar]
- Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- Suares NC, Ford AC. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:1582–1591. doi: 10.1038/ajg.2011.164. [DOI] [PubMed] [Google Scholar]
- Frissora CL, Koch KL. Symptom overlap and comorbidity of irritable bowel syndrome with other conditions. Curr Gastroenterol Rep. 2005;7:264–271. doi: 10.1007/s11894-005-0018-9. [DOI] [PubMed] [Google Scholar]
- Rey E, Balboa A, Mearin F. Chronic constipation, irritable bowel syndrome with constipation and constipation with pain/discomfort: similarities and differences. Am J Gastroenterol. 2014;109:876–884. doi: 10.1038/ajg.2014.18. [DOI] [PubMed] [Google Scholar]
- Wong RK, Palsson OS, Turner MJ, et al. Inability of the Rome III criteria to distinguish functional constipation from constipation-subtype irritable bowel syndrome. Am J Gastroenterol. 2010;105:2228–2234. doi: 10.1038/ajg.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YF, Ma XQ, Wang R, et al. Epidemiology of functional constipation and comparison with constipation-predominant irritable bowel syndrome: the Systematic Investigation of Gastrointestinal Diseases in China (SILC) Aliment Pharmacol Ther. 2011;34:1020–1029. doi: 10.1111/j.1365-2036.2011.04809.x. [DOI] [PubMed] [Google Scholar]
- Faaborg PM, Finnerup NB, Christensen P, et al. Abdominal pain: a comparison between neurogenic bowel dysfunction and chronic idiopathic constipation. Gastroenterol Res Pract. 2013;2013:365037. doi: 10.1155/2013/365037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder SL, Locke GR, III, Schleck CD, et al. Natural history of functional gastrointestinal disorders: a 12-year longitudinal population-based study. Gastroenterology. 2007;133:799–807. doi: 10.1053/j.gastro.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Damon H, Dumas P, Mion F. Impact of anal incontinence and chronic constipation on quality of life. Gastroenterol Clin Biol. 2004;28:16–20. doi: 10.1016/s0399-8320(04)94835-x. [DOI] [PubMed] [Google Scholar]
- Nellesen D, Chawla A, Oh DL, et al. Comorbidities in patients with irritable bowel syndrome with constipation or chronic idiopathic constipation: a review of the literature from the past decade. Postgrad Med. 2013;125:40–50. doi: 10.3810/pgm.2013.03.2640. [DOI] [PubMed] [Google Scholar]
- Nellesen D, Yee K, Chawla A, et al. A systematic review of the economic and humanistic burden of illness in irritable bowel syndrome and chronic constipation. J Manag Care Pharm. 2013;19:755–764. doi: 10.18553/jmcp.2013.19.9.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel BM, Gralnek IM, Bolus R, et al. Clinical determinants of health-related quality of life in patients with irritable bowel syndrome. Arch Intern Med. 2004;164:1773–1780. doi: 10.1001/archinte.164.16.1773. [DOI] [PubMed] [Google Scholar]
- US Census Bureau [internet]. Projected Population by Single Year of Age, Sex, Race, and Hispanic Origin for the United States: July 1, 2000 to July 1, 20502014 http://www.census.gov/population/projections/ . Accessed March 11, 2015.
- Chang L, Lembo AJ, Lavins BJ, et al. The impact of abdominal pain on global measures in patients with chronic idiopathic constipation, before and after treatment with linaclotide: a pooled analysis of two randomised, double-blind, placebo-controlled, phase 3 trials. Aliment Pharmacol Ther. 2014;40:1302–1312. doi: 10.1111/apt.12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez C, Garrigues V, Ortiz V, et al. Healthcare seeking for constipation: a population-based survey in the Mediterranean area of Spain. Aliment Pharmacol Ther. 2006;24:421–428. doi: 10.1111/j.1365-2036.2006.02981.x. [DOI] [PubMed] [Google Scholar]
- Johanson JF, Kralstein J. Chronic constipation: a survey of the patient perspective. Aliment Pharmacol Ther. 2007;25:599–608. doi: 10.1111/j.1365-2036.2006.03238.x. [DOI] [PubMed] [Google Scholar]
- Pare P, Gray J, Lam S, et al. Health-related quality of life, work productivity, and health care resource utilization of subjects with irritable bowel syndrome: baseline results from LOGIC (Longitudinal Outcomes Study of Gastrointestinal Symptoms in Canada), a naturalistic study. Clin Ther. 2006;28:1726–1735. doi: 10.1016/j.clinthera.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Spiegel BM. The burden of IBS: looking at metrics. Curr Gastroenterol Rep. 2009;11:265–269. doi: 10.1007/s11894-009-0039-x. [DOI] [PubMed] [Google Scholar]
- El-Serag HB, Olden K, Bjorkman D. Health-related quality of life among persons with irritable bowel syndrome: a systematic review. Aliment Pharmacol Ther. 2002;16:1171–1185. doi: 10.1046/j.1365-2036.2002.01290.x. [DOI] [PubMed] [Google Scholar]
- Gralnek IM, Hays RD, Kilbourne A, et al. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology. 2000;119:654–660. doi: 10.1053/gast.2000.16484. [DOI] [PubMed] [Google Scholar]
- Doshi JA, Cai Q, Buono JL, et al. Economic burden of irritable bowel syndrome with constipation: a retrospective analysis of health care costs in a commercially insured population. J Manag Care Pharm. 2014;20:382–390. doi: 10.18553/jmcp.2014.20.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Buono JL, Spalding WM, et al. Healthcare costs among patients with chronic constipation: a retrospective claims analysis in a commercially insured population. J Med Econ. 2014;17:148–158. doi: 10.3111/13696998.2013.860375. [DOI] [PubMed] [Google Scholar]
- Cremonini F, Lembo A. IBS with constipation, functional constipation, painful and non-painful constipation: e Pluribus...Plures. Am J Gastroenterol. 2014;109:885–886. doi: 10.1038/ajg.2014.119. [DOI] [PubMed] [Google Scholar]
- Ford AC, Moayyedi P, Lacy BE, et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109:S2–26. doi: 10.1038/ajg.2014.187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.