Abstract
Lyme disease is the most important vector-borne disease in the Northern hemisphere and represents a major public health challenge with insufficient means of reliable diagnosis. Skin is rarely investigated in proteomics but constitutes in the case of Lyme disease the key interface where the pathogens can enter, persist, and multiply. Therefore, we investigated proteomics on skin samples to detect Borrelia proteins directly in cutaneous biopsies in a robust and specific way. We first set up a discovery gel prefractionation-LC-MS/MS approach on a murine model infected by Borrelia burgdorferi sensu stricto that allowed the identification of 25 Borrelia proteins among more than 1300 mouse proteins. Then we developed a targeted gel prefractionation-LC-selected reaction monitoring (SRM) assay to detect 9/33 Borrelia proteins/peptides in mouse skin tissue samples using heavy labeled synthetic peptides. We successfully transferred this assay from the mouse model to human skin biopsies (naturally infected by Borrelia), and we were able to detect two Borrelia proteins: OspC and flagellin. Considering the extreme variability of OspC, we developed an extended SRM assay to target a large set of variants. This assay afforded the detection of nine peptides belonging to either OspC or flagellin in human skin biopsies. We further shortened the sample preparation and showed that Borrelia is detectable in mouse and human skin biopsies by directly using a liquid digestion followed by LC-SRM analysis without any prefractionation. This study thus shows that a targeted SRM approach is a promising tool for the early direct diagnosis of Lyme disease with high sensitivity (<10 fmol of OspC/mg of human skin biopsy).
Lyme borreliosis is an arthropod-borne disease transmitted by hard ticks (Ixodes spp.). The causative agents are bacteria belonging to the Borrelia burgdorferi sensu lato group. In the United States, more than 30,000 cases have been reported to the Centers for Disease Control and Prevention in 2012. There, the unique pathogenic species of Borrelia is B. burgdorferi sensu stricto (s.s.). In Europe, between 65,000 and 85,000 cases are reported depending on the epidemiological study (1, 2), and the three most prevalent pathogenic species of Borrelia are Borrelia afzelii, Borrelia garinii, and B. burgdorferi s.s. The disease in both Europe and the United States is first characterized in most patients by an inflammatory skin lesion, erythema migrans (EM),1 which is the most frequent manifestation of the disease. Dissemination to other sites occurs secondarily and can involve among others articulation, nervous system, heart, and skin at other sites (3, 4). The diagnosis can be a real challenge because of the proteiform clinical manifestations. When an EM is present, which is the case for 80% of patients (3), early diagnosis is facilitated. However, EM presentation can be clinically atypical, making the recognition of this manifestation of Lyme borreliosis difficult (5). Later on, when Borrelia has disseminated to the target organs, biological diagnosis is based either on the direct detection of the pathogen in different patient body fluids and biopsies by means of culture and/or PCR or on the indirect demonstration of presence of Borrelia by detection of anti-pathogen-directed IgM and IgG antibodies (enzyme-linked immunosorbent assay (ELISA) and Western blot) (6).
Concerning the direct detection of Borrelia, culture of the bacteria has allowed the spirochete isolation since the 80s in different specific Barbour-Stoenner-Kelly-based media by using skin biopsies or biological fluids such as blood or cerebrospinal fluid (7, 8). However, Borrelia culture is not routinely used as a diagnostic test because the bacterial growth takes several weeks and does not yield timely results. Indeed, it requires the use of the specific and expensive Barbour-Stoenner-Kelly medium and a dark field microscope to detect, frequently after at least 2 weeks of incubation, the presence of Borrelia in tissues or biological fluids. When performed from patients with EM, only 40–80% of the cultures are positive (6). In addition, the success of culture varies greatly according to the Borrelia species. PCR is quicker and generally more sensitive than culture with a range of 36–88%, although the success of bacterial detection varies with the gene selected for the assay (6). PCR is efficient for Borrelia detection in synovial liquid (60–85% of the cases) in the case of arthritis (9, 10) but less sensitive in cases of neuroborreliosis in cerebrospinal fluid (<20–40% of the cases) (9, 11). Moreover, PCR detects DNA and not proteins and therefore prevents the detection of active infection. As far as the skin biopsies are concerned, the sensitivity of detection is variable in cases of EM or acrodermatitis chronica atrophicans (12). Conversely, indirect detection using serological tests is not adapted to the early diagnosis as it relies on antibodies only detectable after at least 4–6 weeks after the infectious tick bite. These tests also suffer from lack of specificity (13). New diagnostic approaches are therefore required. Selected reaction monitoring (SRM) has been recognized as an efficient mass spectrometry-based technique for the biomarker verification and validation in several biological fluids (blood, plasma, and urine) (14 –18). The demonstrated specificity, selectivity, and high sensitivity (low attomole range) of the technique (19) makes it promising for the development of an SRM-based method for early diagnosis of Lyme disease. To our knowledge, this strategy has only rarely been used on skin tissue (20). It would allow the direct and rapid detection of Borrelia proteins in the skin, demonstrating the presence of an active infection very early after the tick transmission.
In the present study, we set up a workflow to develop a robust and sensitive SRM assay to detect Borrelia in human skin samples (Fig. 1). First, we looked for Borrelia proteins in infected mouse skin samples by using a classical shotgun/discovery strategy. This experiment afforded a list of bacterial proteins that are expressed in vivo in the skin of an infected mammalian host. Then, we selected protein targets and optimized a Ge-LC-SRM assay to specifically detect and quantify these proteins in mouse skin samples. We demonstrated the transferability of the SRM assay for the detection of the targeted proteins in human skin samples naturally infected with Borrelia. Finally, we improved the experimental protocol to avoid gel prefractionation.
Fig. 1.
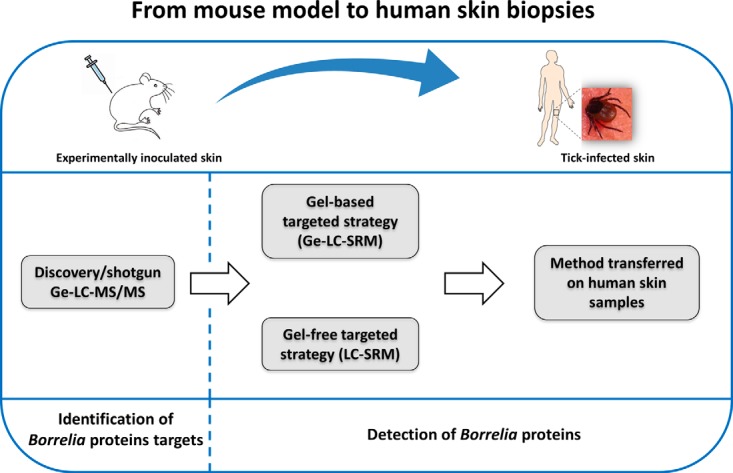
Summary of the experimental workflow. Experimentally infected mouse skin biopsies were analyzed by a shotgun Ge-LC-MS/MS strategy to identify Borrelia target proteins. Then we developed targeted LC-SRM assays with or without gel prefractionation. Finally, these targeted methods were transferred on tick-infected human skin samples.
EXPERIMENTAL PROCEDURES
Chemicals
Modified porcine trypsin was obtained from Promega (Madison, WI). High quality and crude isotopically labeled standard peptides with C-terminal 15N- and 13C-labeled arginine and lysine residues (HeavyPeptide AQUATM Ultimate at 5 pmol/μl ± 5% and PEPotecsTM) were synthesized by Thermo Fisher Scientific (Bremen, Germany). C18 Sep-Pak cartridges (Sep-Pak Vac, 1 ml, 50 mg, tC18) were obtained from Waters (Milford, MA), and all other reagents and chemicals were purchased from Sigma-Aldrich. All buffers were prepared with ultrapure water.
Inoculation and Incubation of Bacteria
Mice were infected intradermally with 103 spirochetes of B. burgdorferi sensu stricto (strain 297) in 0.1 ml of Barbour-Stoenner-Kelly culture medium in the dorsal thoracic area. At two time points after the inoculation (days 5 and 7) mice were killed by isoflurane. An approximately 1-cm area of mouse skin was collected at the inoculation site. For the reproducibility study, mouse skin samples were cut in three equal parts around the inoculation point. Samples were stored at −80 °C until analysis.
For human samples, 4-mm skin biopsies were taken after informed consent and local anesthesia from six patients with EM lesions (H1–H6) after a tick bite in France (ClinicalTrials.gov Identifier NCT00576082). Half of each biopsy was used for both culture and PCR, and half of the biopsy was immediately frozen and kept at −80 °C until use. These six biopsies were all positive by both culture and PCR, and the causative species were identified by a species-specific real time PCR assay (21). Biopsies H1, H2, H3, and H5 were taken from solitary EM lesions and were infected with B. afzelii, and biopsy H6 was infected with B. garinii. The causative species in biopsy H4, taken from a patient with multiple EM, was B. afzelii.
Protein Fractionation and In-gel Digestion
For mouse and human skin biopsies, 4-mg samples were manually extracted by 200 μl of Laemmli sample buffer in a 0.1-ml Potter tissue grinder, Wheaton, Millville, NJ. After 5 min of sonication and 10 min of centrifugation (14,000 × g at 4 °C), the protein content of the supernatant was determined by using a detergent-compatible assay (Bio-Rad). Proteins (50 μg) were subjected to 12% SDS-PAGE and stained overnight with colloidal Coomassie Brilliant Blue (22). 25 gel bands of 2 mm were excised manually. In-gel digestion was carried out as described previously (23), and the tryptic peptides were extracted (60% ACN, 0.1% HCO2H) prior to mass spectrometry analyses.
Liquid Digestion Protocols
Six different extraction protocols compatible with liquid digestion were tested: (i) 1% RapiGestTM SF (Waters) in 50 mm NH4HCO3 buffer, (ii) 1% deoxycholate (DOC) in 25 mm NH4HCO3 buffer, (iii) 8 m urea in 100 mm NH4HCO3 buffer, (iv) 1% octyl β-d-glucopyranoside (octyl glucoside) in 10 mm NaCl, 10 mm NH4HCO3 buffer, (v) 2% SDS in 25 mm NH4CO3 buffer, and (vi) filter-aided sample preparation as described by Wiśniewski et al. (24).
The mouse skin biopsies were extracted in 200 μl of buffer in a 0.1-ml Potter tissue grinder. After 5 min of sonication and 10 min of centrifugation (14,000 × g at 4 °C), the protein content of the supernatant was determined by using a detergent-compatible assay. The filter-aided sample preparation protocol (vi) was then performed as described (24) starting from 100 μg of proteins. For the other protocols (i–v), 100 μg of each extract was reduced for 1 h at 60 °C (except 37 °C for the urea protocol (iii)) by adding dithiothreitol to a final concentration of 10 mm. Alkylation was performed by adding iodoacetamide to a final concentration of 40 mm at room temperature. To carry out the digestion in an optimal way, the sample was diluted to 1 m urea (i) and 0.07% SDS (vi). For the octyl glucoside protocol (iv), proteins were precipitated by addition of ice-cold acetone overnight and solubilized in 0.1 m NH4HCO3 before digestion. An overnight digestion was performed by adding trypsin in a 1:50 enzyme to protein ratio. After digestion, trifluoroacetic acid was added to a final concentration of 0.5% (v/v) for protocols i, ii, iii, and iv. For protocol vi, 4 m KCl was added just after digestion to precipitate the SDS. A centrifugation step was then necessary to eliminate the by-products of RapiGest SF (i), the precipitated DOC (ii), and SDS (vi). All samples were desalted on Sep-Pak C18 cartridges and recovered in 100 μl of 0.1% HCO2H. The peptide content was determined by a detergent-compatible assay. One microliter of a mixture of heavy labeled peptides was finally added to a volume of sample solution containing 1 μg of peptides prior to SRM analyses. The efficiency of the different protocols was evaluated by calculating the extraction yield and the sample recovery. The extraction yield corresponds to the protein content obtained after the extraction step divided by the weight of the biopsy. The sample recovery is the peptide content after Sep-Pak desalting divided by the initial protein content and multiplied by 100.
The RapiGest protocol was applied to one human skin biopsy (H1). The extraction, reduction, alkylation, and digestion steps were performed as for mouse skin biopsies.
Nano-LC-MS/MS Analyses
Peptides were analyzed on a nano-LC-Chip/Cube (Agilent Technologies, Palo Alto, CA) hyphenated to an amaZon ion trap (Bruker Daltonics, Bremen, Germany). The chip contained a Zorbax 300SB-C18 column (150 mm × 75 μm, 5 μm) and a Zorbax 300SB-C18 enrichment column (40 nl, 5 μm). The solvent system consisted of 2% ACN, 0.1% HCO2H in water (solvent A) and 2% water, 0.1% HCO2H in ACN (solvent B). Trapping was done at a flow rate set to 3.75 μl/min with solvent A. Elution was performed at a flow rate of 300 nl/min with a 8–40% linear gradient (solvent B) in 30 min followed by a 4-min stage at 70% solvent B before reconditioning the column at 8% solvent B. The MS spectra were acquired in the positive ion mode on the mass range 250–1500 m/z using the standard enhanced resolution mode at a scan rate of 8100 m/z/s. The eight most abundant peptides were selected on each MS spectrum for further isolation and fragmentation with a preference for doubly charged ions (absolute threshold of 100,000). Fragmentation was performed using argon as the collision gas. Ions were excluded after the acquisition of one MS/MS spectrum, and the exclusion was released after 0.60 min. MS/MS spectra were acquired on the mass range 100–2000 m/z. The complete system was fully controlled by HyStar 3.2 (Bruker Daltonics) software.
MS/MS Data Interpretation
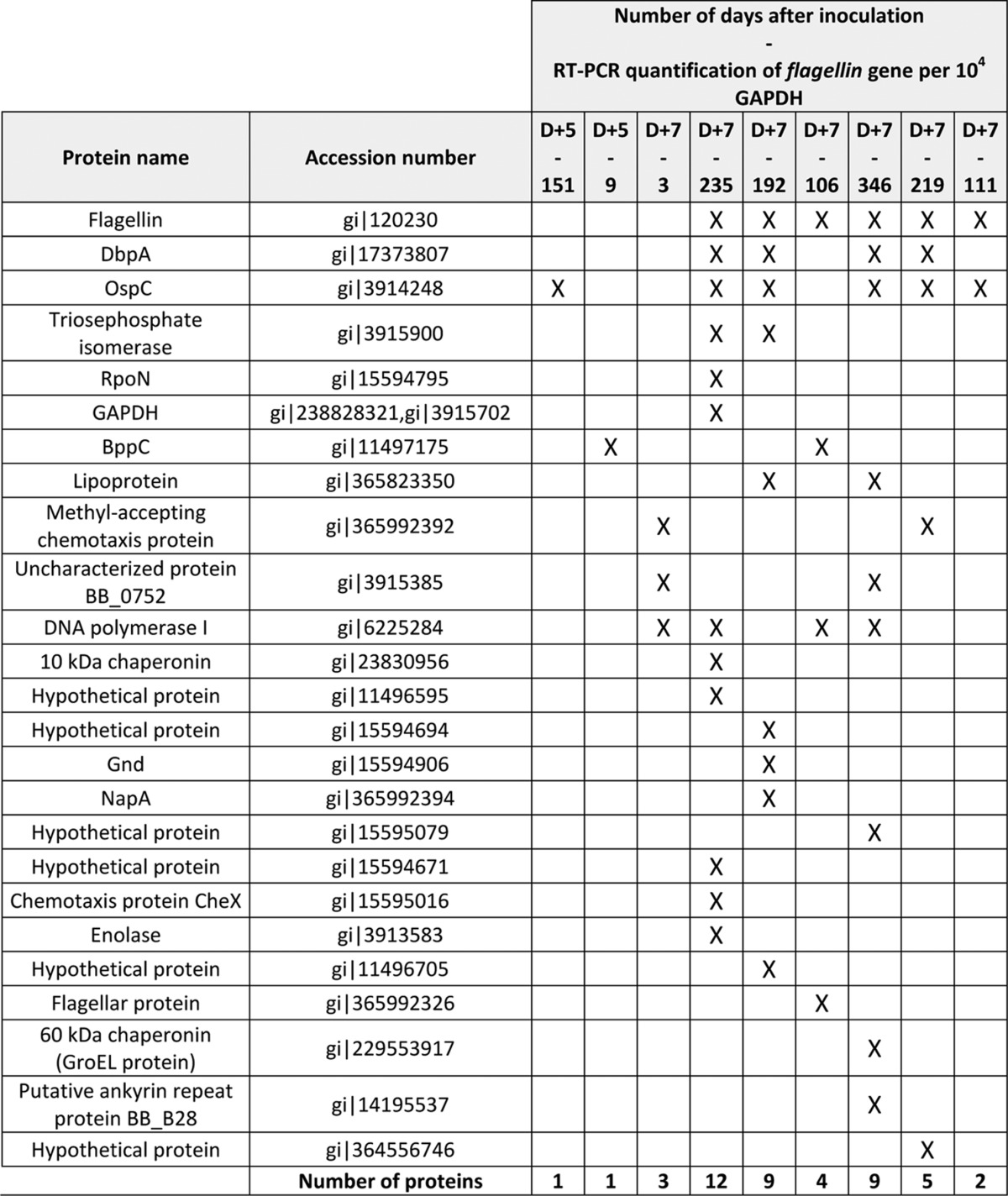
Mass data collected during nano-LC-MS/MS were processed, converted into “.mgf” files with DataAnalysis 4.0 (Bruker Daltonics), and interpreted using Mascot 2.4.3 (Matrix Science, London, UK) and Open Mass Spectrometry Search Algorithm (OMSSA) 2.1.7 (25) algorithm run on the Mass Spectrometry Data Analysis (MSDA) software suite (26). Searches were performed without any molecular weight or isoelectric point restrictions against an in house-generated protein database composed of all protein sequences of B. burgdorferi B31 and mouse (extracted from NCBInr and UniProtKB-Swiss-Prot, respectively). Known contaminant proteins such as human keratins and trypsin were added to the database and concatenated with reversed copies of all sequences (B. burgdorferi B31, August 16, 2012, 1758 entries; mouse, April 19, 2013, 16,722 entries). The database for B. burgdorferi B31 was used because the B. burgdorferi 297 strain has not been sequenced yet. Trypsin was selected as enzyme, and for MS/MS data, a parent and fragment mass tolerance of 0.5 Da was used. A maximum of two missed cleavages was allowed, and some modifications were taken into account: carbamidomethyl (Cys), acetyl N terminus of protein, and oxidation (Met). The Mascot and OMSSA results were independently loaded into Scaffold software (Proteome Software, Portland, OR) to validate peptide identifications. The target-decoy database search allowed us to control the false positive identification rate, which was set to 1% with a minimum of one peptide per protein. Borrelia protein identifications in mouse skin biopsies analyzed are listed in Table I (peptide identification scores and sequence coverage are given in supplemental Table S1). The shotgun data have been deposited to the ProteomeXchange Consortium (proteomecentral.proteomexchange.org) via the PRIDE partner repository (27) with the data set identifier PXD000879 and DOI 10.6019/PXD000879.
Table 1. Shotgun identifications of 9 mouse skin biopsies using a Ge-LC-MS/MS strategy (25 bands). Biopsies were collected 5 days (D+5) or 7 days (D+7) after the inoculation of Borrelia burgdorferi ss 297. Each sample was analyzed once and the identifications were performed according to two search engines (Mascot and OMSSA).
Micro-LC-SRM Assay Development
Peptide/Transition Selection and Concentration-balanced Mixture of Heavy Labeled Synthetic Peptides
We developed two LC-SRM methods, M1 and M2. The first method (M1) monitored 33 peptides corresponding to nine Borrelia proteins. The second method (M2) was focused on four Borrelia proteins only but was extended for OspC variants and flagellin (76 monitored peptides).
For M1, 33 proteotypic peptides were selected for nine targeted proteins (OspC, flagellin, DbpA, GAPDH, RpoN, BB0081, BBP42, HSP 90, and sensory transduction histidine kinase), and isotopically labeled equivalent peptides were purchased (32 crude PEPotecs peptides and one high purity AQUA peptide (GPNLTEISK, OspC). For M2, 76 proteotypic peptides were selected for only four (OspC, flagellin, DbpA, and GAPDH) among the nine initially targeted proteins in M1, and isotopically labeled equivalent peptides were purchased (74 crude PEPotecs peptides and two high purity AQUA peptides (GPNLTEISK, OspC; ANLGAFQNR, flagellin).
For the lower limits of quantification and detection determination (LLOQ and LLOD, respectively), a dilution series of the peptides was realized by spiking crude peptides at different dilution values (1:10,000 to 1:10) and the high purity peptides at different concentrations (500 amol/μl to 50 fmol/μl) in a blank mouse skin matrix and injected in triplicate on a TSQ Vantage triple quadrupole mass spectrometer (Thermo Fisher Scientific). The area under curve of all transitions for each peptide were summed and drawn versus the peptide concentration.
According to the LLOQ of each peptide for both methods, we separated all the crude peptides into several groups of different dilutions to prepare a concentration-balanced mixture of heavy labeled peptides. Four picomoles of the GPNLTEISK (OspC) and 4 pmol of the ANLGAFQNR (flagellin) AQUA peptides were added to this mixture with a final concentration of 25 fmol/μl.
For both methods, nano-LC-MS/MS analysis of the mixture of isotopically labeled peptides afforded a representative MS/MS spectrum for each peptide. Four transitions corresponding to the most abundant y monocharged ions with an m/z value above the doubly charged precursor m/z and four most abundant transitions (if different) were selected from the fragmentation spectrum. Four to eight transitions were monitored for both endogenous and heavy labeled peptides. Thus, a total of 314 transitions corresponding to 66 precursors and nine proteins were measured in M1. A total of 758 transitions corresponding to 152 precursors and four proteins were measured in M2. For the SRM analyses, 1 μl of a mixture of heavy labeled peptides was added to a volume of sample solution containing 2 μg on average (50 μg of protein/25 bands gel) and 6.6 μg of peptides for the gel-based and the gel-free strategies, respectively.
Micro-LC-SRM
All separations were carried out on an Ultimate 3000 RSLCnano system (Thermo Fisher Scientific). For each analysis, the sample was loaded into a trapping column (ZORBAX SB MicroBore Guard (5 μm, 1.0 × 17 mm), Agilent) at 50 μl/min with aqueous solution containing 0.1% (v/v) HCO2H and 2% ACN. After 3 min of trapping, the column was put on line with an Acclaim PepMap RSLC column (15 cm × 300 μm inner diameter, C18, 3 μm, 100 Å; Thermo Fisher Scientific). Peptide elution was performed at 5 μl/min by applying a mixture of solvents A/B. Solvent A was water with 2% ACN and 0.1% (v/v) HCO2H, and solvent B was ACN with 2% water and 0.1% (v/v) HCO2H. Separations were performed by applying three gradients either (i) a linear gradient from 8 to 30% solvent B over 25 min followed by a washing step (3 min at 80% solvent B) and an equilibration step (7 min at 8% solvent B) for the gel bands of both mouse and human skin biopsies H1, H2, H3, H4 and for the liquid digested mouse skin samples; (ii) a linear gradient from 2% to 35% solvent B over 90 min followed by a washing step (5 min at 80% solvent B) and an equilibration step (10 min at 2% solvent B) for the liquid digested human skin sample H1; or (iii) a linear gradient from 5 to 30% solvent B over 57 min followed by a washing step (1 min at 80% solvent B) and an equilibration step (20 min at 5% solvent B) for the gel bands of human skin samples H5 and H6. SRM analyses were performed using the TSQ Vantage triple quadrupole mass spectrometer. The isolation width for both Q1 and Q3 was set to 0.7 m/z unit. The collision gas pressure in Q2 was set at 1.5 millitorr argon. For M1, the collision energy was calculated using the optimized formula CE = 0.03 × m/z + 2.905 for doubly charged precursor ions provided by the supplier. For M2, the collision energy was experimentally optimized by testing nine values centered on the calculated value from the previous formula. Both time-scheduled SRM methods targeted the pairs of isotopically labeled peptides/endogenous peptides in ±2.5-min (except ±1.5-min for human skin sample H4) retention time windows by monitoring a minimum of three transitions for each peptide within a cycle time of 3 s (the target peptides and transitions are given in supplemental Table S2). Mass data collected during LC-SRM were processed with the Skyline open source software package 2.0.9 (28). Area intensity ratios of the heavy and light forms of each peptide were determined automatically by the software. Manual integration was performed when necessary. The endogenous peptide amount calculation was performed by multiplying light/heavy ratios by the known amount of injected heavy standard peptide. The SRM data have been deposited in the PeptideAtlas (PASSEL component) with the data set identifiers PASS00471 (for M1) and PASS00590 (for M2).
RESULTS
Discovery/Shotgun Identification of Borrelia Proteins in Mouse Skin Biopsies
Previous analyses have shown a peak of multiplication of Borrelia 5–7 days after inoculation in the skin (29). To identify the bacterial proteins that are present in the skin, we sampled at the inoculation site and analyzed nine mouse skin biopsies infected by B. burgdorferi s.s. (strain 297) by using a Ge-LC-MS/MS strategy (25 bands). As expected, we identified mostly mouse proteins with a mean number of 1316 ± 84 proteins. However, we also detected a total of 25 Borrelia proteins (Table I). Several Borrelia proteins were reproducibly detected in different biopsies, but 16 proteins were only detected in one biopsy and generally with a low number of peptides. All the Borrelia assigned spectra were manually validated to ensure confidence in the identifications. Overall, eight proteins were validated by both Mascot and OMSSA algorithms, double identifications that increase the confidence in identification. The lowest Mascot score and OMSSA e-value for a Borrelia peptide identified in the mouse skin biopsies were 40.9 and 4.5, respectively (supplemental Table S1).
Among the 25 detected proteins, we selected five proteins (OspC, flagellin, DbpA, GAPDH, and RpoN) that were detected either in several biopsies or simultaneously by both search algorithms. Despite its detection in four biopsies, DNA polymerase I was not selected because only one peptide was detected and solely with the OMSSA algorithm. We added to our selection four proteins (BB0081, BBP42, HSP 90, and sensory transduction histidine kinase) that were detected in a preliminary Ge-LC-MS/MS (75-band) analysis (data not shown). Thus, a total of nine proteins were selected to be further targeted using SRM assays.
LLOD and LLOQ Determination for the LC-SRM Assays
The SRM assays were optimized as described under “Experimental Procedures.” Briefly, we dnine9 Borrelia proteins, and method M2 was focused on four Borrelia proteins only but was extended for OspC variants and flagellin. 314 and 758 transitions were selected for method M1 and method M2, respectively. A linearity study was performed for both assays by mixing all heavy labeled peptides in a background extract of mouse skin biopsies. Fig. 2 shows the results obtained for GPNLTEISK, a high purity OspC AQUA peptide. As shown, the dilution curve was linear with an excellent correlation coefficient (R2 = 0.999). We evaluated the LLOQ and LLOD by applying recognized definitions (30). The LLOQ was indeed the point at which the accuracy of the recalculated concentration (according to the dilution curve equation) is between 80 and 120% and the coefficient of variation among the triplicates is below 20%. The LLOD was the last point at which we can observe a co-elution of the transitions corresponding to the peptide of interest. According to these definitions, we evaluated the LLOD for both GPNLTEISK (OspC) and ANLGAFQNR (flagellin) high purity peptides at 1 fmol. The LLOQ for both AQUA peptides was evaluated at 5 fmol. The evaluation of the LLOD and LLOQ for the other peptides was limited because of the low purity of the labeled peptides, but the large majority showed an excellent linearity (R2 > 0.995) over more than 3 orders of magnitude (not shown).
Fig. 2.
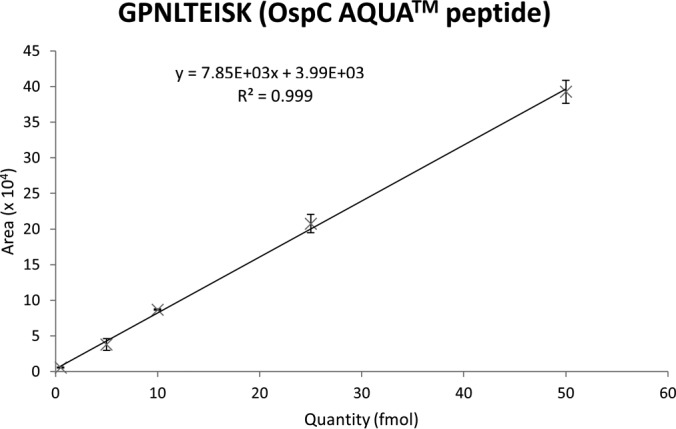
Linearity study for the high purity GPNLTEISK AQUA peptide. Equations for the curve and correlation coefficient (R2) are indicated on the graph. A dilution series of the peptides was realized by spiking crude peptides at different dilution values (1:10,000 to 1:10) and the two high purity peptides at different concentrations (500 amol/μl to 50 fmol/μl) in a blank mouse skin matrix. Analyses were performed in triplicate on a TSQ Vantage triple quadrupole mass spectrometer. Error bars represent S.D. for triplicates.
Targeted Analyses of Infected Mouse Skin Biopsies by Using a Ge-LC-SRM Strategy
We assessed the detection of Borrelia proteins in one experimentally infected mouse skin biopsy by using the first SRM assay (M1). As indicated, this method monitored 33 peptides corresponding to nine Borrelia proteins. After prefractionation by SDS-PAGE (25 bands) and LC-SRM analysis (33 peptides monitored), we detected 10 peptides corresponding to four proteins (OspC, flagellin, GAPDH, and DbpA). These peptides were validated as we observed (i) a co-elution for all the transitions followed, (ii) a co-elution between the heavy labeled and the endogenous peptides, and (iii) a consistent ratio between the different transition area for the endogenous peptide in comparison with the heavy labeled peptide. An example of validation is shown in Fig. 3 for the TAEELGMQPAK flagellin peptide. The amounts of GAPDH and DbpA peptides were near the LLOD, whereas the amounts of flagellin and OspC peptides were higher than the LLOQ. Using the GPNLTEISK AQUA peptide and the heavy/light ratio, we estimated an amount of 300 fmol of OspC in 1 mg of experimentally infected mouse skin biopsy (Fig. 4).
Fig. 3.
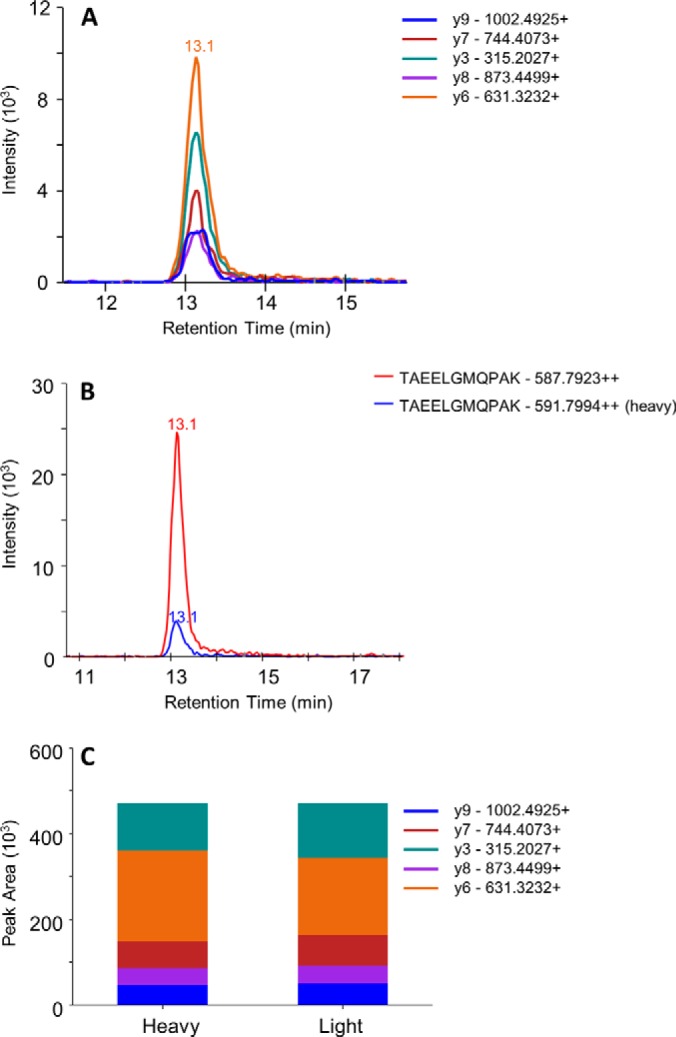
Conditions required to validate the detection of a targeted peptide (TAEELGMQPAK; flagellin peptide). A, co-elution of the transitions followed. B, co-elution of the heavy and endogenous peptides. C, similar transition ratios between the heavy and endogenous peptides.
Fig. 4.
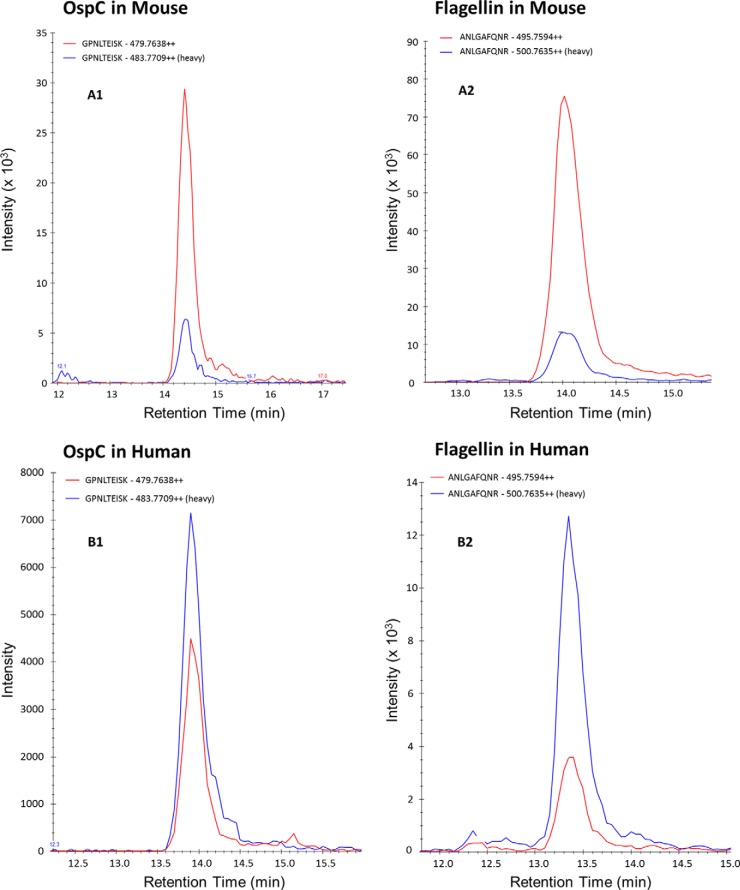
SRM traces showing the co-elution of heavy and light peptides for both flagellin (ANLGAFQNR) and OspC (GPNLTEISK) in mouse (A1, OspC; A2, flagellin) and human (B1, OspC; B2, flagellin) infected samples. The analyses were performed using the first SRM method M1 (33 peptides, 314 transitions). Area intensity ratios of the heavy and the light forms allowed calculation of the amount of targeted peptides in the biopsies.
The technical reproducibility of the gel-based approach was evaluated by loading the same infected mouse skin extract on three different gels and performing a relative quantification of one OspC peptide and one flagellin peptide. We determined a coefficient of variation of around 10%. We also evaluated the intramouse reproducibility by analyzing in triplicate three aliquots of the same biopsy and found a coefficient of variation of around 50%, which highlights the heterogeneous dissemination of the bacteria in the skin. Finally, we evaluated the intermouse reproducibility by analyzing three different infected biopsies and observed a coefficient of variation of around 50%.
Targeted Analyses of Infected Human Skin Biopsies by Using a Ge-LC-SRM Strategy
The results obtained on experimentally inoculated mouse biopsies prompted us to investigate tick-infected human samples. Thus, we applied the same 25-band Ge-LC-SRM strategy to three human skin biopsies (H1, H2, and H3) collected from patients presenting an EM and infected by B. afzelii. The yield of extraction obtained for the human skin was lower (33.5 ± 7.0 μg of protein/mg of biopsy, n = 3) than for the mouse skin (155.1 ± 45.4 μg of protein/mg of biopsy, n = 28). After in-gel digestion and LC-SRM analysis using M1 (33 peptides monitored), we detected the GPNLTEISK OspC peptide in all three biopsies. However, the signal above the LLOQ in only one biopsy (H1). Using the AQUA peptide, we estimated a quantity of around 20 fmol of OspC/mg in this biopsy (Fig. 4). Flagellin was detected with two peptides in the biopsy H1 but was undetected in biopsies H2 and H3.
The second LC-SRM method (M2) was designed to target only the four proteins that had been detected in mouse and/or human skin biopsies but was extended for more OspC and flagellin peptides (76 monitored peptides) to increase the sensitivity and the assay specificity for these two proteins. We included all OspC tryptic peptides available on NCBInr for the different Borrelia species and analyzed three other human skin biopsies (H4, H5, and H6). This led to the successful detection of nine Borrelia peptides in one biopsy (H4): five OspC peptides and four flagellin peptides. Using GPNLTEISK (OspC) and ANLGAFQNR (flagellin) AQUA peptides, we estimated quantities of around 7 fmol of OspC and 14 fmol of flagellin/mg of biopsy H4. None Borrelia peptides were detected in biopsies H5 and H6.
Overall, Borrelia proteins were detected in four of the six tick-infected human biopsies. These results constitute the first demonstration of the detection of Borrelia in human skin samples by an SRM mass spectrometry approach.
Targeted Analyses of Infected Skin Biopsies by Using a Simplified Gel-free LC-SRM Strategy
To simplify the workflow, we investigated a gel-free strategy based on protein extraction, liquid digestion, and LC-SRM. Thus, we evaluated using infected mouse skin biopsies six protocols of extraction compatible with a liquid digestion. We selected either an “MS-compatible” detergent or detergents that can be removed before LC-SRM analysis. As shown in Table II, SDS-based extraction provided the best yield with 160–180 μg of proteins/mg of skin biopsy. RapiGest, DOC, and urea protocols showed an intermediary yield of nearly 100 μg/mg of mouse skin biopsy. Protein extraction with octyl glucoside was less efficient with a yield 5 times lower than that of SDS. Although the yield with SDS-based extraction was important, the sample recovery was also extremely low (15%). The DOC protocol also induced a high sample loss (36% recovery). The sample loss for the RapiGest and urea protocols was quite reasonable (>80% recovery). Finally, we detected OspC and flagellin with four of the six protocols tested (Table II). Despite the high extraction yields, we did not detect any Borrelia peptide with the SDS-based protocols. This shows the negative influence of sample loss during the preparation steps. Interestingly, we observed that an octyl glucoside-based extraction followed by a protein precipitation allowed the detection of three OspC peptides, whereas no detection was obtained with this detergent without precipitation (not shown). OspC and flagellin were also detected using RapiGest, DOC, and urea protocols.
Table II. Evaluation of the six extraction protocols compatible with liquid digestion and mass spectrometry in terms of yield, sample recovery, detection of flagellin, and OspC peptides.
The detection was performed in infected mouse skin biopsies using the first SRM method (M1). The number of crosses (+) indicates the number of detected peptides per protein. − indicates an absence of detection. FASP, filter-aided sample preparation.
| Yield | Sample recovery | OspC detection | Flagellin detection | |
|---|---|---|---|---|
| μg protein/mg skin biopsy | % | |||
| RapiGest | 109 | 84 | +++ | + |
| DOC | 96 | 36 | +++ | + |
| Urea | 100 | 85 | ++ | + |
| Octyl glucoside with a precipitation step | 34 | 61 | +++ | − |
| FASP | 162 | Not evaluated | − | − |
| SDS with a precipitation step | 186 | 15 | − | − |
Taken together, our results indicate that 1% RapiGest is a good compromise for the gel-free strategy with detection of both flagellin and OspC peptides. In particular, the GPNLTEISK peptide, which is the only highly conserved peptide among different Borrelia strains, is detected in these conditions. This is important in view of a diagnosis regardless of the Borrelia strain responsible of infection. In addition, when we compared the amount of OspC detected in two fragments of the same infected mouse biopsy, we estimated a quantity of around 337 fmol of OspC/mg of biopsy using the gel-based approach and 305 fmol of OspC/mg of biopsy using the gel-free (RapiGest) approach. These values are consistent considering the results we obtained for the intramouse variability study.
Therefore, we used the RapiGest-based protocol to analyze a remaining fragment of the human biopsy H1 without gel fractionation. We detected one flagellin and one OspC peptide and estimated a quantity of around 36 fmol of OspC/mg of biopsy. As for the mouse sample, considering the intramouse variability, this result is consistent with the OspC quantity determined from the gel-based approach (20 fmol). Because of a lack of material, we were not able to analyze more human biopsies using the gel-free strategy. However, this preliminary result shows the feasibility of Borrelia detection in a cutaneous naturally infected human sample by using a targeted mass spectrometry approach without gel prefractionation.
DISCUSSION
In arthropod-borne diseases, the skin constitutes a key interface where pathogens are inoculated and can persist (31, 32). Local cutaneous manifestations are often the first clinical signs to appear before dissemination to extracutaneous sites occurs (3). Tools allowing reliable early diagnosis at that stage are necessary to treat patients early and to cure the disease. Using a mouse model, we set up the best conditions to monitor the development of the disease in the skin. We have previously shown that Borrelia multiplies actively in the skin whether inoculated via a syringe or after natural inoculation via infected ticks (29). For this reason, we selected the intradermal inoculation of Borrelia to increase our chances to detect a sufficient amount of Borrelia, and analyses were performed on day 7, which corresponds to the peak of multiplication in the skin. After shotgun/discovery analyses using a Ge-LC-MS/MS strategy, we designed a targeted proteomic approach and detected four Borrelia proteins in mouse skin biopsies. Among them, flagellin is a very conserved periplasmic flagellar protein and is crucial for bacterial motility (33). The surface lipoprotein OspC is essential in the early transmission of Borrelia to the vertebrate host (34, 35), DbpA is also critical for the virulence of Borrelia (36), and RpoN is related to the RpoN-RpoS pathway, which plays an important role in Borrelia pathogenicity and survival (37). We then evaluated the capacity of the SRM technology to be an efficient tool for the detection of Borrelia proteins in cutaneous samples of patients with EM. Despite the low number of Borrelia expected in tick-infected biopsies, we demonstrated the applicability of our targeted approach to human skin biopsies from patients harboring an EM. The use of a nano-LC setup might have improved the sensitivity of the assay for the analyses of the gel-fractionated samples for which the system was not used at its maximum loading capacity. The micro-LC setup was chosen because of the available sample quantity (>100 μg in average) and the higher robustness of the system, which is a crucial requirement for clinical use, when compared with a nano-LC setup (38).
We first developed an SDS-based strategy that allowed the extraction of whole proteins present in the skin. SDS is indeed the most widely used detergent because of its solubilizing and denaturing capacity and its ability to extract membrane proteins (39, 40). However, SDS use requires sample dilution to get a concentration below 0.1% for an efficient protein digestion (41). Moreover, the major drawback of SDS is its incompatibility with MS due to high ion suppression (42, 43). To solve this problem, SDS removal using ion exchange chromatography (44), dialysis (45), ethyl acetate extraction (46), protein precipitation, use of a spin column removal kit (47), SDS precipitation (48), and filter-aided sample preparation have been tested (24). Alternatively, detergents with properties similar to SDS but that are MS-compatible have been used. Among them, RapiGest SF is an acid-labile surfactant (49). It is hydrolyzed at low pH and thus minimizes interference with reverse-phase columns and mass spectrometry. It has been successfully tested in several studies, showing good performance for membrane proteins (50). Overall, we tested four protocols with MS-compatible detergents and two SDS removal protocols. The four MS-compatible protocols allowed the detection of two Borrelia proteins, whereas no protein was detected with the SDS removal protocols. A bottleneck of these removal protocols is sample recovery, which in our case was very low.
SRM is now recognized as a “gold standard” for the quantification of protein biomarkers in clinical studies (51). After identification of protein targets by Ge-LC-MS/MS, this technique is also well suited to validate new disease biomarkers for diagnosis (15). In addition, SRM presents major advantages over other classical diagnostic techniques (antibody-based assays) such as multiplexing (over 100 analytes/run in scheduled mode), reproducibility (52), and higher specificity (19). However, the sensitivity is still not sufficient to quantify the whole dynamic range of a complex sample particularly when the order of magnitude is above 8–9 as in mammalian cell extracts or in blood (19). To attempt to address both of these issues, the mass spectrometry field is still rapidly growing, and new instruments with better resolution, selectivity, and sensitivity are being developed (53 –57).
Most experiments in this study were performed using an SRM assay monitoring 33 peptides including one OspC peptide, GPNLTEISK, which is conserved between different Borrelia species and strains. Indeed, this protein is essential in the early transmission and survival of Borrelia in the skin of the vertebrate host. However, this protein is known to be one of the most variable Borrelia proteins (58, 59). Thus, in an extended method, we targeted all OspC tryptic peptides available in NCBInr for the different Borrelia species. As compared with the first method, we detected four supplemental OspC peptides, reinforcing the presence of the protein. This study provides the proof of feasibility of LC-SRM to detect a few Borrelia proteins in the skin of mammals, mice and human, infected with different Borrelia species, B. burgdorferi s.s. and B. afzelii. These preliminary results are very promising and suggest that LC-SRM could become a diagnostic tool allowing the detection of different species of B. burgdorferi sensu lato infecting humans. In addition, if specific peptides are detected, the SRM technique has the potential to be used to discriminate the different species and strains of B. burgdorferi sensu lato. Indeed, for epidemiological studies, it is essential to know how the different Borrelia species circulate in humans and animal reservoirs. In addition, from a clinical point of view, B. afzelii tends to show more skin manifestations, B. garinii tends to show more neurological manifestations, and B. burgdorferi s.s. tends to show more articular manifestations (3). As a next step, the ability of LC-SRM to be a sensitive and efficient tool for the diagnosis of Lyme disease as compared with the classical techniques (e.g. PCR assay) has to be confirmed on a large cohort of patients. This is currently in progress. It will also be very challenging to use this LC-SRM approach on other biological fluids such as cerebrospinal fluid or synovial fluid. Indeed, at this stage, the detection of active infection is always difficult in patients due to the large polymorphism of the clinical manifestations especially in Europe. Finally, the multiplexing capacity offered by the SRM technique using different target proteins from the different borrelial species should help to detect the bacteria that are present and persist at a very low level in the skin.
Supplementary Material
Acknowledgments
We are grateful to Laurence Zilliox for technical assistance in Borrelia culture.
Footnotes
Author contributions: G.S., A.B., B.J., N.B., and L.E. designed research; G.S., A.B., and B.W. performed research; C.C. contributed new reagents or analytic tools; G.S., A.B., B.W., C.C., and L.E. analyzed data; G.S., A.B., B.J., C.C., N.B., and L.E. wrote the paper; D.L. provided human skin biopsies.
* This work was supported by the French Proteomics Infrastructure (ProFi) Grant ANR-10-INSB-08-03, the Centre National de Reference des Borrelia (CNR Borrelia), and the Société Française de Dermatologie.
 This article contains supplemental Tables S1 and S2.
This article contains supplemental Tables S1 and S2.
1 The abbreviations used are:
- EM
- erythema migrans
- DOC
- sodium deoxycholate
- DbpA
- decorin-binding protein A
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- Ge-LC-MS/MS
- gel prefractionation followed by liquid chromatography and tandem mass spectrometry
- HSP 90
- heat shock protein 90
- LC-SRM
- liquid chromatography hyphenated to selected reaction monitoring mass spectrometry
- LLOD
- low limit of detection
- LLOQ
- low limit of quantification
- OspC
- outer surface protein C
- RpoN
- RNA polymerase σ factor σ54
- SRM
- selected reaction monitoring
- s.s.
- sensu stricto
- OMSSA
- Open Mass Spectrometry Search Algorithm.
REFERENCES
- 1. Hubálek Z. (2009) Epidemiology of lyme borreliosis. Curr. Probl. Dermatol. 37, 31–50 [DOI] [PubMed] [Google Scholar]
- 2. Lindgren E., Jaensson T. G. T. (2006) Lyme Borreliosis in Europe: Influences of Climate and Climate Change, Epidemiology, Ecology and Adaptation Measures, World Health Organization Europe, Copenhagen, Denmark [Google Scholar]
- 3. Stanek G., Wormser G. P., Gray J., Strle F. (2012) Lyme borreliosis. Lancet 379, 461–473 [DOI] [PubMed] [Google Scholar]
- 4. Radolf J. D., Caimano M. J., Stevenson B., Hu L. T. (2012) Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat. Rev. Microbiol. 10, 87–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Strle F., Stanek G. (2009) Clinical manifestations and diagnosis of lyme borreliosis. Curr. Probl. Dermatol. 37, 51–110 [DOI] [PubMed] [Google Scholar]
- 6. Aguero-Rosenfeld M. E., Wang G., Schwartz I., Wormser G. P. (2005) Diagnosis of lyme borreliosis. Clin. Microbiol. Rev. 18, 484–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benach J. L., Bosler E. M., Hanrahan J. P., Coleman J. L., Habicht G. S., Bast T. F., Cameron D. J., Ziegler J. L., Barbour A. G., Burgdorfer W., Edelman R., Kaslow R. A. (1983) Spirochetes isolated from the blood of two patients with Lyme disease. N. Engl. J. Med. 308, 740–742 [DOI] [PubMed] [Google Scholar]
- 8. Asbrink E., Hovmark A. (1985) Successful cultivation of spirochetes from skin lesions of patients with erythema chronicum migrans Afzelius and acrodermatitis chronica atrophicans. Acta Pathol. Microbiol. Immunol. Scand. B 93, 161–163 [DOI] [PubMed] [Google Scholar]
- 9. Nocton J. J., Dressler F., Rutledge B. J., Rys P. N., Persing D. H., Steere A. C. (1994) Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N. Engl. J. Med. 330, 229–234 [DOI] [PubMed] [Google Scholar]
- 10. Jaulhac B., Chary-Valckenaere I., Sibilia J., Javier R. M., Piémont Y., Kuntz J. L., Monteil H., Pourel J. (1996) Detection of Borrelia burgdorferi by DNA amplification in synovial tissue samples from patients with Lyme arthritis. Arthritis Rheum. 39, 736–745 [DOI] [PubMed] [Google Scholar]
- 11. Wilske B., Fingerle V., Schulte-Spechtel U. (2007) Microbiological and serological diagnosis of Lyme borreliosis. FEMS Immunol. Med. Microbiol. 49, 13–21 [DOI] [PubMed] [Google Scholar]
- 12. Nowakowski J., Schwartz I., Liveris D., Wang G., Aguero-Rosenfeld M. E., Girao G., McKenna D., Nadelman R. B., Cavaliere L. F., Wormser G. P., and Lyme Disease Study Group (2001) Laboratory diagnostic techniques for patients with early Lyme disease associated with erythema migrans: a comparison of different techniques. Clin. Infect. Dis. 33, 2023–2027 [DOI] [PubMed] [Google Scholar]
- 13. Marques A. R. (2010) Lyme disease: a review. Curr. Allergy Asthma Rep. 10, 13–20 [DOI] [PubMed] [Google Scholar]
- 14. Kuhn E., Whiteaker J. R., Mani D. R., Jackson A. M., Zhao L., Pope M. E., Smith D., Rivera K. D., Anderson N. L., Skates S. J., Pearson T. W., Paulovich A. G., Carr S. A. (2012) Interlaboratory evaluation of automated, multiplexed peptide immunoaffinity enrichment coupled to multiple reaction monitoring mass spectrometry for quantifying proteins in plasma. Mol. Cell. Proteomics 11, M111.013854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rifai N., Gillette M. A., Carr S. A. (2006) Protein biomarker discovery and validation: the long and uncertain path to clinical utility. Nat. Biotechnol. 24, 971–983 [DOI] [PubMed] [Google Scholar]
- 16. Hüttenhain R., Soste M., Selevsek N., Röst H., Sethi A., Carapito C., Farrah T., Deutsch E. W., Kusebauch U., Moritz R. L., Niméus-Malmström E., Rinner O., Aebersold R. (2012) Reproducible quantification of cancer-associated proteins in body fluids using targeted proteomics. Sci. Transl. Med. 4, 142ra94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Percy A. J., Chambers A. G., Yang J., Borchers C. H. (2013) Multiplexed MRM-based quantitation of candidate cancer biomarker proteins in undepleted and non-enriched human plasma. Proteomics 13, 2202–2215 [DOI] [PubMed] [Google Scholar]
- 18. Kennedy J. J., Abbatiello S. E., Kim K., Yan P., Whiteaker J. R., Lin C., Kim J. S., Zhang Y., Wang X., Ivey R. G., Zhao L., Min H., Lee Y., Yu M.-H., Yang E. G., Lee C., Wang P., Rodriguez H., Kim Y., Carr S. A., Paulovich A. G. (2014) Demonstrating the feasibility of large-scale development of standardized assays to quantify human proteins. Nat. Methods 11, 149–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Picotti P., Aebersold R. (2012) Selected reaction monitoring-based proteomics: workflows, potential, pitfalls and future directions. Nat. Methods 9, 555–566 [DOI] [PubMed] [Google Scholar]
- 20. Williamson J. C., Scheipers P., Schwämmle V., Zibert J. R., Beck H. C., Jensen O. N. (2013) A proteomics approach to the identification of biomarkers for psoriasis utilising keratome biopsy. J. Proteomics 94, 176–185 [DOI] [PubMed] [Google Scholar]
- 21. Hidri N., Barraud O., de Martino S., Garnier F., Paraf F., Martin C., Sekkal S., Laskar M., Jaulhac B., Ploy M.-C. (2012) Lyme endocarditis. Clin. Microbiol. Infect. 18, E531–E532 [DOI] [PubMed] [Google Scholar]
- 22. Candiano G., Bruschi M., Musante L., Santucci L., Ghiggeri G. M., Carnemolla B., Orecchia P., Zardi L., Righetti P. G. (2004) Blue silver: a very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis 25, 1327–1333 [DOI] [PubMed] [Google Scholar]
- 23. Villiers C., Chevallet M., Diemer H., Couderc R., Freitas H., Van Dorsselaer A., Marche P. N., Rabilloud T. (2009) From secretome analysis to immunology: chitosan induces major alterations in the activation of dendritic cells via a TLR4-dependent mechanism. Mol. Cell. Proteomics 8, 1252–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wiśniewski J. R., Zougman A., Nagaraj N., Mann M. (2009) Universal sample preparation method for proteome analysis. Nat. Methods 6, 359–362 [DOI] [PubMed] [Google Scholar]
- 25. Geer L. Y., Markey S. P., Kowalak J. A., Wagner L., Xu M., Maynard D. M., Yang X., Shi W., Bryant S. H. (2004) Open mass spectrometry search algorithm. J. Proteome Res. 3, 958–964 [DOI] [PubMed] [Google Scholar]
- 26. Carapito C., Burel A., Guterl P., Walter A., Varrier F., Bertile F., Van Dorsselaer A. (2014) MSDA, a proteomics software suite for in-depth mass spectrometry data analysis using grid computing. Proteomics 14, 1014–1019 [DOI] [PubMed] [Google Scholar]
- 27. Vizcaíno J. A., Côté R. G., Csordas A., Dianes J. A., Fabregat A., Foster J. M., Griss J., Alpi E., Birim M., Contell J., O'Kelly G., Schoenegger A., Ovelleiro D., Pérez-Riverol Y., Reisinger F., Ríos D., Wang R., Hermjakob H. (2013) The PRoteomics IDEntifications (PRIDE) database and associated tools: status in 2013. Nucleic Acids Res. 41, D1063–D1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. MacLean B., Tomazela D. M., Shulman N., Chambers M., Finney G. L., Frewen B., Kern R., Tabb D. L., Liebler D. C., MacCoss M. J. (2010) Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 26, 966–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kern A., Collin E., Barthel C., Michel C., Jaulhac B., Boulanger N. (2011) Tick saliva represses innate immunity and cutaneous inflammation in a murine model of lyme disease. Vector Borne Zoonotic Dis. 11, 1343–1350 [DOI] [PubMed] [Google Scholar]
- 30. Domanski D., Percy A. J., Yang J., Chambers A. G., Hill J. S., Freue G. V., Borchers C. H. (2012) MRM-based multiplexed quantitation of 67 putative cardiovascular disease biomarkers in human plasma. Proteomics 12, 1222–1243 [DOI] [PubMed] [Google Scholar]
- 31. Bernard Q., Jaulhac B., Boulanger N. (2014) Smuggling across the border: how arthropod-borne pathogens evade and exploit the host defense system of the skin. J. Invest. Dermatol. 134, 1211–1219 [DOI] [PubMed] [Google Scholar]
- 32. Ménard R., Tavares J., Cockburn I., Markus M., Zavala F., Amino R. (2013) Looking under the skin: the first steps in malarial infection and immunity. Nat. Rev. Microbiol. 11, 701–712 [DOI] [PubMed] [Google Scholar]
- 33. Panelius J., Lahdenne P., Saxen H., Heikkilä T., Seppälä I. (2001) Recombinant flagellin A proteins from Borrelia burgdorferi sensu stricto, B. afzelii, and B. garinii in serodiagnosis of Lyme borreliosis. J. Clin. Microbiol. 39, 4013–4019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grimm D., Tilly K., Byram R., Stewart P. E., Krum J. G., Bueschel D. M., Schwan T. G., Policastro P. F., Elias A. F., Rosa P. A. (2004) Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc. Natl. Acad. Sci. U.S.A. 101, 3142–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tilly K., Krum J. G., Bestor A., Jewett M. W., Grimm D., Bueschel D., Byram R., Dorward D., Vanraden M. J., Stewart P., Rosa P. (2006) Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect. Immun. 74, 3554–3564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shi Y., Xu Q., McShan K., Liang F. T. (2008) Both decorin-binding proteins A and B are critical for the overall virulence of Borrelia burgdorferi. Infect. Immun. 76, 1239–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ouyang Z., Narasimhan S., Neelakanta G., Kumar M., Pal U., Fikrig E., Norgard M. V. (2012) Activation of the RpoN-RpoS regulatory pathway during the enzootic life cycle of Borrelia burgdorferi. BMC Microbiol. 12, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Percy A. J., Chambers A. G., Yang J., Domanski D., Borchers C. H. (2012) Comparison of standard- and nano-flow liquid chromatography platforms for MRM-based quantitation of putative plasma biomarker proteins. Anal. Bioanal. Chem. 404, 1089–1101 [DOI] [PubMed] [Google Scholar]
- 39. le Maire M., Champeil P., Moller J. V. (2000) Interaction of membrane proteins and lipids with solubilizing detergents. Biochim. Biophys. Acta 1508, 86–111 [DOI] [PubMed] [Google Scholar]
- 40. Speers A. E., Wu C. C. (2007) Proteomics of integral membrane proteins—theory and application. Chem. Rev. 107, 3687–3714 [DOI] [PubMed] [Google Scholar]
- 41. Yu Y.-Q., Gilar M., Lee P. J., Bouvier E. S., Gebler J. C. (2003) Enzyme-friendly, mass spectrometry-compatible surfactant for in-solution enzymatic digestion of proteins. Anal. Chem. 75, 6023–6028 [DOI] [PubMed] [Google Scholar]
- 42. Rundlett K. L., Armstrong D. W. (1996) Mechanism of signal suppression by anionic surfactants in capillary electrophoresis-electrospray ionization mass spectrometry. Anal. Chem. 68, 3493–3497 [DOI] [PubMed] [Google Scholar]
- 43. Beavis R. C., Chait B. T. (1990) Rapid, sensitive analysis of protein mixtures by mass spectrometry. Proc. Natl. Acad. Sci. U.S.A. 87, 6873–6877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weber K., Kuter D. J. (1971) Reversible denaturation of enzymes by sodium dodecyl sulfate. J. Biol. Chem. 246, 4504–4509 [PubMed] [Google Scholar]
- 45. Hjelmeland L. M. (1990) Removal of detergents from membrane proteins. Methods Enzymol. 182, 277–282 [DOI] [PubMed] [Google Scholar]
- 46. Yeung Y. G., Nieves E., Angeletti R. H., Stanley E. R. (2008) Removal of detergents from protein digests for mass spectrometry analysis. Anal. Biochem. 382, 135–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Antharavally B. S., Mallia K. A., Rosenblatt M. M., Salunkhe A. M., Rogers J. C., Haney P., Haghdoost N. (2011) Efficient removal of detergents from proteins and peptides in a spin column format. Anal. Biochem. 416, 39–44 [DOI] [PubMed] [Google Scholar]
- 48. Zhou J.-Y., Dann G. P., Shi T., Wang L., Gao X., Su D., Nicora C. D., Shukla A. K., Moore R. J., Liu T., Camp D. G., 2nd, Smith R. D., Qian W.-J. (2012) Simple sodium dodecyl sulfate-assisted sample preparation method for LC-MS-based proteomics applications. Anal. Chem. 84, 2862–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Meng F., Cargile B. J., Patrie S. M., Johnson J. R., McLoughlin S. M., Kelleher N. L. (2002) Processing complex mixtures of intact proteins for direct analysis by mass spectrometry. Anal. Chem. 74, 2923–2929 [DOI] [PubMed] [Google Scholar]
- 50. Vowinckel J., Capuano F., Campbell K., Deery M. J., Lilley K. S., Ralser M. (2013) The beauty of being (label)-free: sample preparation methods for SWATH-MS and next-generation targeted proteomics. F1000Res 2, 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang P., Whiteaker J. R., Paulovich A. G. (2009) The evolving role of mass spectrometry in cancer biomarker discovery. Cancer Biol. Ther. 8, 1083–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Addona T. A., Abbatiello S. E., Schilling B., Skates S. J., Mani D. R., Bunk D. M., Spiegelman C. H., Zimmerman L. J., Ham A.-J., Keshishian H., Hall S. C., Allen S., Blackman R. K., Borchers C. H., Buck C., Cardasis H. L., Cusack M. P., Dodder N. G., Gibson B. W., Held J. M., Hiltke T., Jackson A., Johansen E. B., Kinsinger C. R., Li J., Mesri M., Neubert T. A., Niles R. K., Pulsipher T. C., Ransohoff D., Rodriguez H., Rudnick P. A., Smith D., Tabb D. L., Tegeler T. J., Variyath A. M., Vega-Montoto L. J., Wahlander A., Waldemarson S., Wang M., Whiteaker J. R., Zhao L., Anderson N. L., Fisher S. J., Liebler D. C., Paulovich A. G., Regnier F. E., Tempst P., Carr S. A. (2009) Multi-site assessment of the precision and reproducibility of multiple reaction monitoring-based measurements of proteins in plasma. Nat. Biotechnol. 27, 633–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Andrews G. L., Simons B. L., Young J. B., Hawkridge A. M., Muddiman D. C. (2011) Performance characteristics of a new hybrid quadrupole time-of-flight tandem mass spectrometer (TripleTOF 5600). Anal. Chem. 83, 5442–5446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Michalski A., Damoc E., Hauschild J.-P., Lange O., Wieghaus A., Makarov A., Nagaraj N., Cox J., Mann M., Horning S. (2011) Mass spectrometry-based proteomics using Q Exactive, a high-performance benchtop quadrupole Orbitrap mass spectrometer. Mol. Cell. Proteomics 10, M111.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kelstrup C. D., Young C., Lavallee R., Nielsen M. L., Olsen J. V. (2012) Optimized fast and sensitive acquisition methods for shotgun proteomics on a quadrupole orbitrap mass spectrometer. J. Proteome Res. 11, 3487–3497 [DOI] [PubMed] [Google Scholar]
- 56. Collins B. C., Gillet L. C., Rosenberger G., Röst H. L., Vichalkovski A., Gstaiger M., Aebersold R. (2013) Quantifying protein interaction dynamics by SWATH mass spectrometry: application to the 14-3-3 system. Nat. Methods 10, 1246–1253 [DOI] [PubMed] [Google Scholar]
- 57. Liu Y., Hüttenhain R., Collins B., Aebersold R. (2013) Mass spectrometric protein maps for biomarker discovery and clinical research. Expert Rev. Mol. Diagn. 13, 811–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Seinost G., Dykhuizen D. E., Dattwyler R. J., Golde W. T., Dunn J. J., Wang I. N., Wormser G. P., Schriefer M. E., Luft B. J. (1999) Four clones of Borrelia burgdorferi sensu stricto cause invasive infection in humans. Infect. Immun. 67, 3518–3524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Baranton G., Seinost G., Theodore G., Postic D., Dykhuizen D. (2001) Distinct levels of genetic diversity of Borrelia burgdorferi are associated with different aspects of pathogenicity. Res. Microbiol. 152, 149–156 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.