Abstract
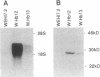
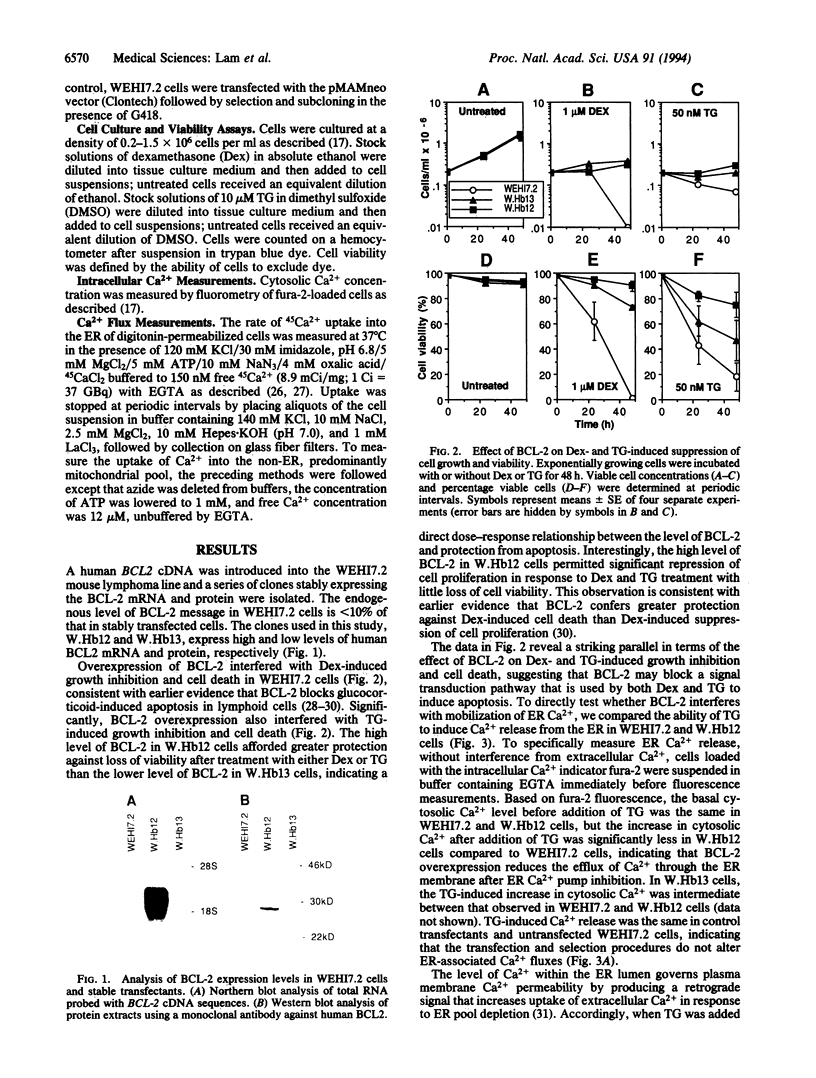
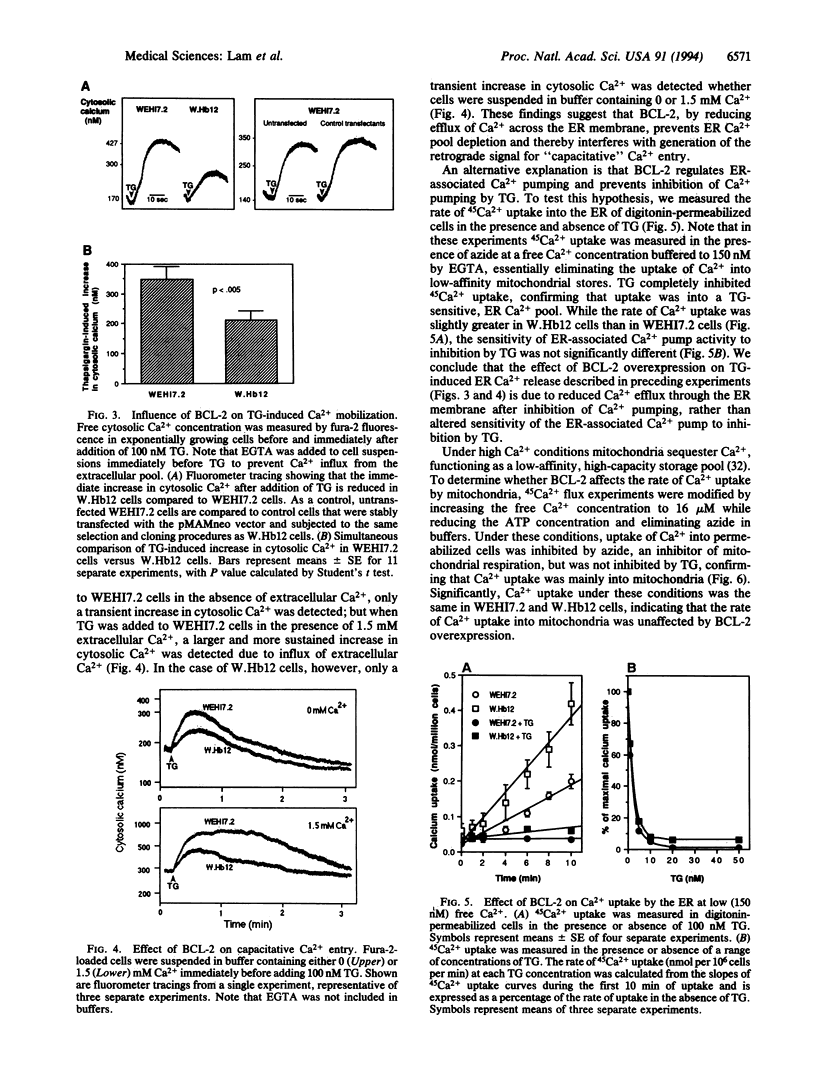
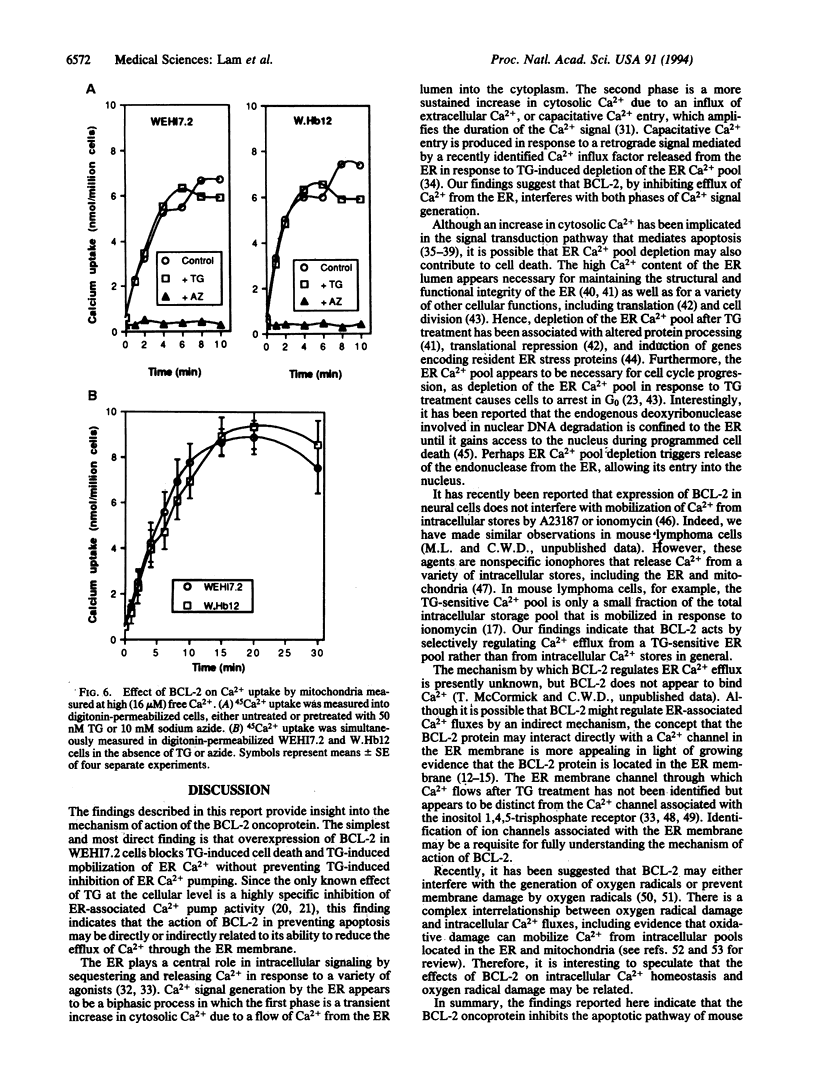
BCL-2 is a 26-kDa integral membrane protein that represses apoptosis by an unknown mechanism. Recent findings indicate that Ca2+ release from the endoplasmic reticulum (ER) mediates apoptosis in mouse lymphoma cells. In view of growing evidence that BCL-2 localizes to the ER, as well as mitochondria and the perinuclear membrane, we investigated the possibility that BCL-2 represses apoptosis by regulating Ca2+ fluxes through the ER membrane. A cDNA encoding BCL-2 was introduced into WEHI7.2 cells and two subclones, W.Hb12 and W.Hb13, which express high and low levels of BCL-2 mRNA and protein, respectively, were isolated. WEHI7.2 cells underwent apoptosis in response to treatment with the glucocorticoid hormone dexamethasone, whereas W.Hb12 and W.Hb13 cells were protected from apoptosis, revealing a direct relationship between the level of BCL-2 expression and the degree of protection. Significantly, BCL-2 also blocked induction of apoptosis by thapsigargin (TG), a highly specific inhibitor of the ER-associated Ca2+ pump. TG completely inhibited ER Ca2+ pumping in both WEHI7.2 and W.Hb12 cells, but the release of Ca2+ into the cytosol after inhibition of ER Ca2+ pumping was significantly less in W.Hb12 cells than in WEHI7.2 cells, indicating that BCL-2 reduces Ca2+ efflux through the ER membrane. By reducing ER Ca2+ efflux, BCL-2 interfered with a signal for "capacitative" entry of extracellular Ca2+, preventing a sustained increase of cytosolic Ca2+ in TG-treated cells. These findings suggest that BCL-2 either directly or indirectly regulates the flux of Ca2+ across the ER membrane, thereby abrogating Ca2+ signaling of apoptosis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert P. R., Tashjian A. H., Jr Ionomycin acts as an ionophore to release TRH-regulated Ca2+ stores from GH4C1 cells. Am J Physiol. 1986 Dec;251(6 Pt 1):C887–C891. doi: 10.1152/ajpcell.1986.251.6.C887. [DOI] [PubMed] [Google Scholar]
- Alnemri E. S., Fernandes T. F., Haldar S., Croce C. M., Litwack G. Involvement of BCL-2 in glucocorticoid-induced apoptosis of human pre-B-leukemias. Cancer Res. 1992 Jan 15;52(2):491–495. [PubMed] [Google Scholar]
- Alnemri E. S., Robertson N. M., Fernandes T. F., Croce C. M., Litwack G. Overexpressed full-length human BCL2 extends the survival of baculovirus-infected Sf9 insect cells. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7295–7299. doi: 10.1073/pnas.89.16.7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baffy G., Miyashita T., Williamson J. R., Reed J. C. Apoptosis induced by withdrawal of interleukin-3 (IL-3) from an IL-3-dependent hematopoietic cell line is associated with repartitioning of intracellular calcium and is blocked by enforced Bcl-2 oncoprotein production. J Biol Chem. 1993 Mar 25;268(9):6511–6519. [PubMed] [Google Scholar]
- Bansal V. S., Majerus P. W. Phosphatidylinositol-derived precursors and signals. Annu Rev Cell Biol. 1990;6:41–67. doi: 10.1146/annurev.cb.06.110190.000353. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Booth C., Koch G. L. Perturbation of cellular calcium induces secretion of luminal ER proteins. Cell. 1989 Nov 17;59(4):729–737. doi: 10.1016/0092-8674(89)90019-6. [DOI] [PubMed] [Google Scholar]
- Brostrom C. O., Brostrom M. A. Calcium-dependent regulation of protein synthesis in intact mammalian cells. Annu Rev Physiol. 1990;52:577–590. doi: 10.1146/annurev.ph.52.030190.003045. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Caron-Leslie L. A., Cidlowski J. A. Similar actions of glucocorticoids and calcium on the regulation of apoptosis in S49 cells. Mol Endocrinol. 1991 Aug;5(8):1169–1179. doi: 10.1210/mend-5-8-1169. [DOI] [PubMed] [Google Scholar]
- Chen-Levy Z., Cleary M. L. Membrane topology of the Bcl-2 proto-oncogenic protein demonstrated in vitro. J Biol Chem. 1990 Mar 25;265(9):4929–4933. [PubMed] [Google Scholar]
- Chen-Levy Z., Nourse J., Cleary M. L. The bcl-2 candidate proto-oncogene product is a 24-kilodalton integral-membrane protein highly expressed in lymphoid cell lines and lymphomas carrying the t(14;18) translocation. Mol Cell Biol. 1989 Feb;9(2):701–710. doi: 10.1128/mcb.9.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chueh S. H., Mullaney J. M., Ghosh T. K., Zachary A. L., Gill D. L. GTP- and inositol 1,4,5-trisphosphate-activated intracellular calcium movements in neuronal and smooth muscle cell lines. J Biol Chem. 1987 Oct 5;262(28):13857–13864. [PubMed] [Google Scholar]
- Cuende E., Alés-Martínez J. E., Ding L., Gónzalez-García M., Martínez C., Nunez G. Programmed cell death by bcl-2-dependent and independent mechanisms in B lymphoma cells. EMBO J. 1993 Apr;12(4):1555–1560. doi: 10.1002/j.1460-2075.1993.tb05799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd D. R., MacDonald P. N., Komm B. S., Haussler M. R., Miesfeld R. L. Stable expression of the calbindin-D28K complementary DNA interferes with the apoptotic pathway in lymphocytes. Mol Endocrinol. 1992 Nov;6(11):1843–1848. doi: 10.1210/mend.6.11.1336124. [DOI] [PubMed] [Google Scholar]
- Dowd D. R., MacDonald P. N., Komm B. S., Haussler M. R., Miesfeld R. Evidence for early induction of calmodulin gene expression in lymphocytes undergoing glucocorticoid-mediated apoptosis. J Biol Chem. 1991 Oct 5;266(28):18423–18426. [PubMed] [Google Scholar]
- Dowd D. R., Miesfeld R. L. Evidence that glucocorticoid- and cyclic AMP-induced apoptotic pathways in lymphocytes share distal events. Mol Cell Biol. 1992 Aug;12(8):3600–3608. doi: 10.1128/mcb.12.8.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. E., Yuan J. Y., Horvitz H. R. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- Ghosh T. K., Bian J. H., Short A. D., Rybak S. L., Gill D. L. Persistent intracellular calcium pool depletion by thapsigargin and its influence on cell growth. J Biol Chem. 1991 Dec 25;266(36):24690–24697. [PubMed] [Google Scholar]
- Hengartner M. O., Ellis R. E., Horvitz H. R. Caenorhabditis elegans gene ced-9 protects cells from programmed cell death. Nature. 1992 Apr 9;356(6369):494–499. doi: 10.1038/356494a0. [DOI] [PubMed] [Google Scholar]
- Hockenbery D. M., Oltvai Z. N., Yin X. M., Milliman C. L., Korsmeyer S. J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993 Oct 22;75(2):241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Jacobson M. D., Burne J. F., King M. P., Miyashita T., Reed J. C., Raff M. C. Bcl-2 blocks apoptosis in cells lacking mitochondrial DNA. Nature. 1993 Jan 28;361(6410):365–369. doi: 10.1038/361365a0. [DOI] [PubMed] [Google Scholar]
- Kaiser N., Edelman I. S. Calcium dependence of glucocorticoid-induced lymphocytolysis. Proc Natl Acad Sci U S A. 1977 Feb;74(2):638–642. doi: 10.1073/pnas.74.2.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane D. J., Sarafian T. A., Anton R., Hahn H., Gralla E. B., Valentine J. S., Ord T., Bredesen D. E. Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science. 1993 Nov 19;262(5137):1274–1277. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- Krajewski S., Tanaka S., Takayama S., Schibler M. J., Fenton W., Reed J. C. Investigation of the subcellular distribution of the bcl-2 oncoprotein: residence in the nuclear envelope, endoplasmic reticulum, and outer mitochondrial membranes. Cancer Res. 1993 Oct 1;53(19):4701–4714. [PubMed] [Google Scholar]
- Lam M., Dubyak G., Distelhorst C. W. Effect of glucocorticosteroid treatment on intracellular calcium homeostasis in mouse lymphoma cells. Mol Endocrinol. 1993 May;7(5):686–693. doi: 10.1210/mend.7.5.8316252. [DOI] [PubMed] [Google Scholar]
- Li W. W., Alexandre S., Cao X., Lee A. S. Transactivation of the grp78 promoter by Ca2+ depletion. A comparative analysis with A23187 and the endoplasmic reticulum Ca(2+)-ATPase inhibitor thapsigargin. J Biol Chem. 1993 Jun 5;268(16):12003–12009. [PubMed] [Google Scholar]
- Lodish H. F., Kong N., Wikström L. Calcium is required for folding of newly made subunits of the asialoglycoprotein receptor within the endoplasmic reticulum. J Biol Chem. 1992 Jun 25;267(18):12753–12760. [PubMed] [Google Scholar]
- Lytton J., MacLennan D. H. Molecular cloning of cDNAs from human kidney coding for two alternatively spliced products of the cardiac Ca2+-ATPase gene. J Biol Chem. 1988 Oct 15;263(29):15024–15031. [PubMed] [Google Scholar]
- Lytton J., Westlin M., Hanley M. R. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem. 1991 Sep 15;266(26):17067–17071. [PubMed] [Google Scholar]
- Mason M. J., Garcia-Rodriguez C., Grinstein S. Coupling between intracellular Ca2+ stores and the Ca2+ permeability of the plasma membrane. Comparison of the effects of thapsigargin, 2,5-di-(tert-butyl)-1,4-hydroquinone, and cyclopiazonic acid in rat thymic lymphocytes. J Biol Chem. 1991 Nov 5;266(31):20856–20862. [PubMed] [Google Scholar]
- McConkey D. J., Nicotera P., Hartzell P., Bellomo G., Wyllie A. H., Orrenius S. Glucocorticoids activate a suicide process in thymocytes through an elevation of cytosolic Ca2+ concentration. Arch Biochem Biophys. 1989 Feb 15;269(1):365–370. doi: 10.1016/0003-9861(89)90119-7. [DOI] [PubMed] [Google Scholar]
- McDonnell T. J., Deane N., Platt F. M., Nunez G., Jaeger U., McKearn J. P., Korsmeyer S. J. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989 Apr 7;57(1):79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- Missiaen L., De Smedt H., Droogmans G., Casteels R. Luminal Ca2+ controls the activation of the inositol 1,4,5-trisphosphate receptor by cytosolic Ca2+. J Biol Chem. 1992 Nov 15;267(32):22961–22966. [PubMed] [Google Scholar]
- Miyashita T., Reed J. C. bcl-2 gene transfer increases relative resistance of S49.1 and WEHI7.2 lymphoid cells to cell death and DNA fragmentation induced by glucocorticoids and multiple chemotherapeutic drugs. Cancer Res. 1992 Oct 1;52(19):5407–5411. [PubMed] [Google Scholar]
- Monaghan P., Robertson D., Amos T. A., Dyer M. J., Mason D. Y., Greaves M. F. Ultrastructural localization of bcl-2 protein. J Histochem Cytochem. 1992 Dec;40(12):1819–1825. doi: 10.1177/40.12.1453000. [DOI] [PubMed] [Google Scholar]
- Nuñez G., London L., Hockenbery D., Alexander M., McKearn J. P., Korsmeyer S. J. Deregulated Bcl-2 gene expression selectively prolongs survival of growth factor-deprived hemopoietic cell lines. J Immunol. 1990 May 1;144(9):3602–3610. [PubMed] [Google Scholar]
- Orrenius S., Burkitt M. J., Kass G. E., Dypbukt J. M., Nicotera P. Calcium ions and oxidative cell injury. Ann Neurol. 1992;32 (Suppl):S33–S42. doi: 10.1002/ana.410320708. [DOI] [PubMed] [Google Scholar]
- Peitsch M. C., Polzar B., Stephan H., Crompton T., MacDonald H. R., Mannherz H. G., Tschopp J. Characterization of the endogenous deoxyribonuclease involved in nuclear DNA degradation during apoptosis (programmed cell death). EMBO J. 1993 Jan;12(1):371–377. doi: 10.1002/j.1460-2075.1993.tb05666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney J. W., Jr, Bird G. S. The signal for capacitative calcium entry. Cell. 1993 Oct 22;75(2):199–201. doi: 10.1016/0092-8674(93)80061-i. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Social controls on cell survival and cell death. Nature. 1992 Apr 2;356(6368):397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- Randriamampita C., Tsien R. Y. Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature. 1993 Aug 26;364(6440):809–814. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]
- Reed D. J. Review of the current status of calcium and thiols in cellular injury. Chem Res Toxicol. 1990 Nov-Dec;3(6):495–502. doi: 10.1021/tx00018a002. [DOI] [PubMed] [Google Scholar]
- Sambrook J. F. The involvement of calcium in transport of secretory proteins from the endoplasmic reticulum. Cell. 1990 Apr 20;61(2):197–199. doi: 10.1016/0092-8674(90)90798-j. [DOI] [PubMed] [Google Scholar]
- Sentman C. L., Shutter J. R., Hockenbery D., Kanagawa O., Korsmeyer S. J. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991 Nov 29;67(5):879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- Short A. D., Bian J., Ghosh T. K., Waldron R. T., Rybak S. L., Gill D. L. Intracellular Ca2+ pool content is linked to control of cell growth. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):4986–4990. doi: 10.1073/pnas.90.11.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y. Stress-resistance conferred by high level of bcl-2 alpha protein in human B lymphoblastoid cell. Oncogene. 1989 Nov;4(11):1331–1336. [PubMed] [Google Scholar]
- Vaux D. L., Cory S., Adams J. M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988 Sep 29;335(6189):440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Vaux D. L. Toward an understanding of the molecular mechanisms of physiological cell death. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):786–789. doi: 10.1073/pnas.90.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. T. Programmed cell death: apoptosis and oncogenesis. Cell. 1991 Jun 28;65(7):1097–1098. doi: 10.1016/0092-8674(91)90002-g. [DOI] [PubMed] [Google Scholar]
- Zhong L. T., Sarafian T., Kane D. J., Charles A. C., Mah S. P., Edwards R. H., Bredesen D. E. bcl-2 inhibits death of central neural cells induced by multiple agents. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4533–4537. doi: 10.1073/pnas.90.10.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]