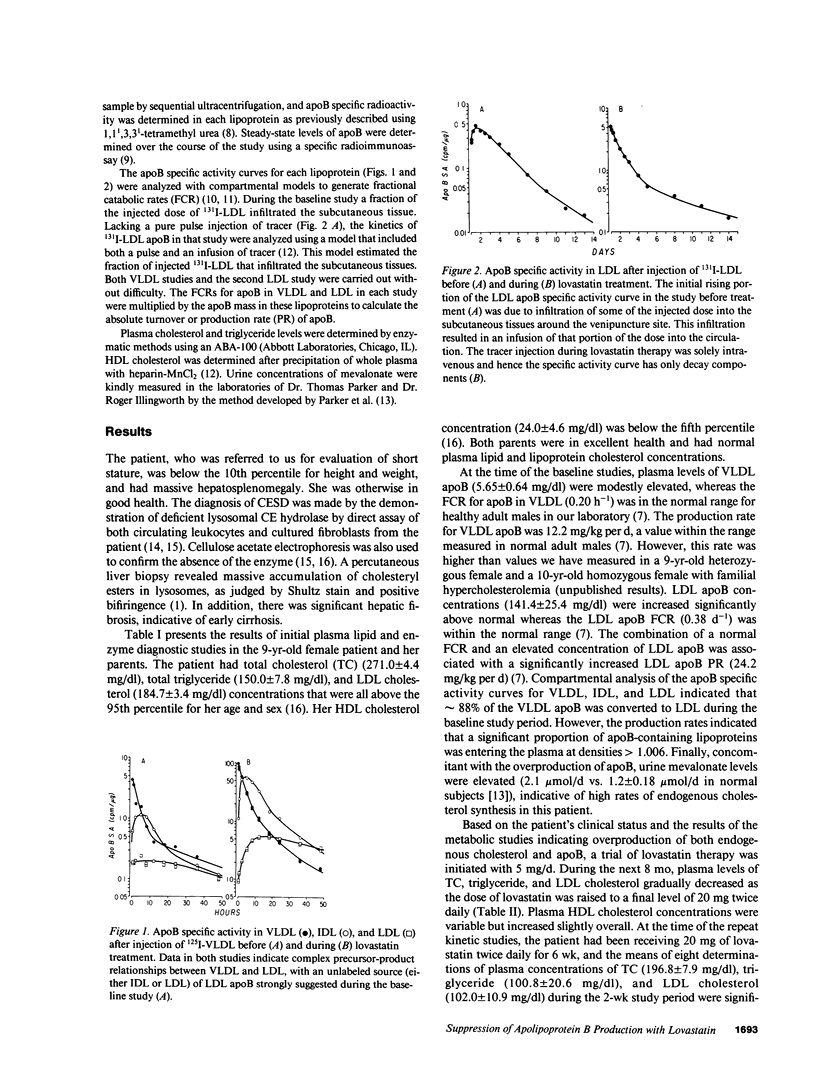
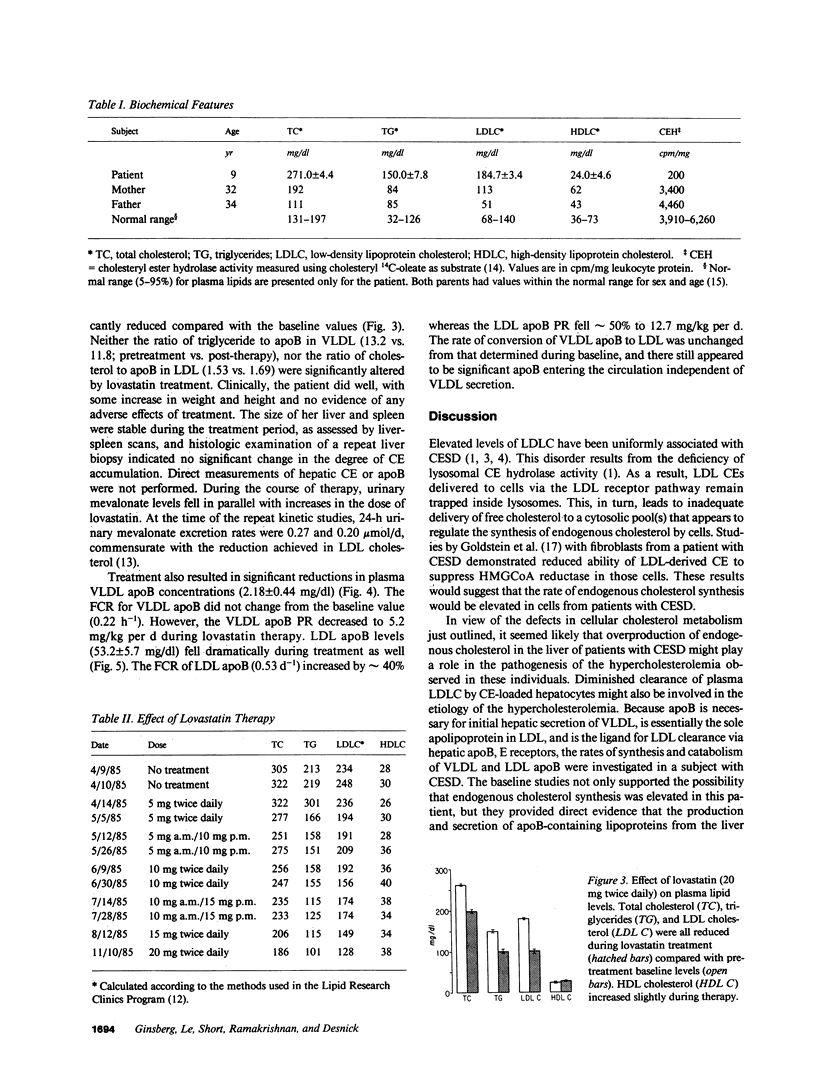
Abstract
Cholesteryl ester storage disease (CESD) is characterized by the deficient activity of lysosomal cholesteryl ester (CE) hydrolase, accumulation of LDL-derived CE in lysosomes, and hyperlipidemia. We studied the kinetics of VLDL and LDL apolipoprotein B (apoB), using 125I-VLDL and 131I-LDL, in a 9-yr-old female with CESD and elevated total cholesterol (TC) (271.0 +/- 4.4 mg/dl), triglyceride (TG) (150.0 +/- 7.8 mg/dl), and LDL cholesterol (184.7 +/- 3.4 mg/dl). These studies demonstrated a markedly elevated production rate (PR) of apoB, primarily in LDL, with normal fractional catabolism of apoB in VLDL and LDL. Urine mevalonate levels were elevated, indicative of increased synthesis of endogenous cholesterol. Treatment with lovastatin, a competitive inhibitor of hydroxymethylglutaryl coenzyme A reductase, resulted in significant reductions in TC (196.8 +/- 7.9 mg/dl), TG (100.8 +/- 20.6 mg/dl), and LDL cholesterol (102.0 +/- 10.9 mg/dl). Therapy reduced VLDL apoB PR (5.2 vs. 12.2 mg/kg per d pretreatment) and LDL apoB PR (12.7 vs. 24.2 mg/kg per d pretreatment). Urine mevalonate levels also decreased during therapy. These results indicate that, in CESD, the inability to release free cholesterol from lysosomal CE resulted in elevated synthesis of endogenous cholesterol and increased production of apoB-containing lipoproteins. Lovastatin reduced both the rate of cholesterol synthesis and the secretion of apoB-containing lipoproteins.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMOV A., SCHORR S., WOLMAN M. Generalized xanthomatosis with calcified adrenals. AMA J Dis Child. 1956 Mar;91(3):282–286. doi: 10.1001/archpedi.1956.02060020284010. [DOI] [PubMed] [Google Scholar]
- Alberts A. W., Chen J., Kuron G., Hunt V., Huff J., Hoffman C., Rothrock J., Lopez M., Joshua H., Harris E. Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3957–3961. doi: 10.1073/pnas.77.7.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelin B., Einarsson K., Hellström K., Leijd B. Effects of cholestyramine and chenodeoxycholic acid on the metabolism of endogenous triglyceride in hyperlipoproteinemia. J Lipid Res. 1978 Nov;19(8):1017–1024. [PubMed] [Google Scholar]
- Beltz W. F., Kesäniemi Y. A., Howard B. V., Grundy S. M. Development of an integrated model for analysis of the kinetics of apolipoprotein B in plasma very low density lipoproteins, intermediate density lipoproteins, and low density lipoproteins. J Clin Invest. 1985 Aug;76(2):575–585. doi: 10.1172/JCI112009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilheimer D. W., Goldstein J. L., Grundy S. M., Brown M. S. Reduction in cholesterol and low density lipoprotein synthesis after portacaval shunt surgery in a patient with homozygous familial hypercholesterolemia. J Clin Invest. 1975 Dec;56(6):1420–1430. doi: 10.1172/JCI108223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J. C., Rubinstein A., Bukberg P. R., Brown W. V. Apolipoprotein E-enriched lipoprotein subclasses in normolipidemic subjects. J Lipid Res. 1983 Jul;24(7):886–898. [PubMed] [Google Scholar]
- Ginsberg H. N., Le N. A., Gibson J. C. Regulation of the production and catabolism of plasma low density lipoproteins in hypertriglyceridemic subjects. Effect of weight loss. J Clin Invest. 1985 Feb;75(2):614–623. doi: 10.1172/JCI111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Dana S. E., Faust J. R., Beaudet A. L., Brown M. S. Role of lysosomal acid lipase in the metabolism of plasma low density lipoprotein. Observations in cultured fibroblasts from a patient with cholesteryl ester storage disease. J Biol Chem. 1975 Nov 10;250(21):8487–8495. [PubMed] [Google Scholar]
- Grundy S. M., Vega G. L. Influence of mevinolin on metabolism of low density lipoproteins in primary moderate hypercholesterolemia. J Lipid Res. 1985 Dec;26(12):1464–1475. [PubMed] [Google Scholar]
- Heiss G., Tamir I., Davis C. E., Tyroler H. A., Rifkand B. M., Schonfeld G., Jacobs D., Frantz I. D., Jr Lipoprotein-cholesterol distributions in selected North American populations: the lipid research clinics program prevalence study. Circulation. 1980 Feb;61(2):302–315. doi: 10.1161/01.cir.61.2.302. [DOI] [PubMed] [Google Scholar]
- Huff M. W., Telford D. E., Woodcroft K., Strong W. L. Mevinolin and cholestyramine inhibit the direct synthesis of low density lipoprotein apolipoprotein B in miniature pigs. J Lipid Res. 1985 Oct;26(10):1175–1186. [PubMed] [Google Scholar]
- Janus E. D., Nicoll A. M., Turner P. R., Magill P., Lewis B. Kinetic bases of the primary hyperlipidaemias: studies of apolipoprotein B turnover in genetically defined subjects. Eur J Clin Invest. 1980 Apr;10(2 Pt 1):161–172. doi: 10.1111/j.1365-2362.1980.tb02076.x. [DOI] [PubMed] [Google Scholar]
- Kelly D. R., Hoeg J. M., Demosky S. J., Jr, Brewer H. B., Jr Characterization of plasma lipids and lipoproteins in cholesteryl ester storage disease. Biochem Med. 1985 Feb;33(1):29–37. doi: 10.1016/0006-2944(85)90123-1. [DOI] [PubMed] [Google Scholar]
- Kostner G. M., Hadorn B., Roscher A., Zechner R. Plasma lipids and lipoproteins of a patient with cholesteryl ester storage disease. J Inherit Metab Dis. 1985;8(1):9–12. doi: 10.1007/BF01805475. [DOI] [PubMed] [Google Scholar]
- Kovanen P. T., Bilheimer D. W., Goldstein J. L., Jaramillo J. J., Brown M. S. Regulatory role for hepatic low density lipoprotein receptors in vivo in the dog. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1194–1198. doi: 10.1073/pnas.78.2.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Ville A., Moshy R., Turner P. R., Miller N. E., Lewis B. Inhibition of cholesterol synthesis reduces low-density-lipoprotein apoprotein B production without decreasing very-low-density-lipoprotein apoprotein B synthesis in rabbits. Biochem J. 1984 Apr 1;219(1):321–323. doi: 10.1042/bj2190321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer T., Strober W., Levy R. I. The metabolism of low density lipoprotein in familial type II hyperlipoproteinemia. J Clin Invest. 1972 Jun;51(6):1528–1536. doi: 10.1172/JCI106949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le N. A., Melish J. S., Roach B. C., Ginsberg H. N., Brown W. V. Direct measurement of apoprotein B specific activity in 125I-labeled lipoproteins. J Lipid Res. 1978 Jul;19(5):578–584. [PubMed] [Google Scholar]
- Melish J., Le N. A., Ginsberg H., Steinberg D., Brown W. V. Dissociation of apoprotein B and triglyceride production in very-low-density lipoproteins. Am J Physiol. 1980 Nov;239(5):E354–E362. doi: 10.1152/ajpendo.1980.239.5.E354. [DOI] [PubMed] [Google Scholar]
- Parker T. S., McNamara D. J., Brown C. D., Kolb R., Ahrens E. H., Jr, Alberts A. W., Tobert J., Chen J., De Schepper P. J. Plasma mevalonate as a measure of cholesterol synthesis in man. J Clin Invest. 1984 Sep;74(3):795–804. doi: 10.1172/JCI111495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan R., Dell R. B., Goodman D. S. On determining the extent of side-pool synthesis in a three-pool model for whole body cholesterol kinetics. J Lipid Res. 1981 Nov;22(8):1174–1180. [PMC free article] [PubMed] [Google Scholar]
- Ruderman N. B., Jones A. L., Krauss R. M., Shafrir E. A biochemical and morphologic study of very low density lipoproteins in carbohydrate-induced hypertriglyceridemia. J Clin Invest. 1971 Jun;50(6):1355–1368. doi: 10.1172/JCI106615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons L. A., Reichl D., Myant N. B., Mancini M. The metabolism of the apoprotein of plasma low density lipoprotein in familial hyperbetalipoproteinaemia in the homozygous form. Atherosclerosis. 1975 Mar-Apr;21(2):283–298. doi: 10.1016/0021-9150(75)90087-8. [DOI] [PubMed] [Google Scholar]
- Soutar A. K., Myant N. B., Thompson G. R. Simultaneous measurement of apolipoprotein B turnover in very-low-and low-density lipoproteins in familial hypercholesterolaemia. Atherosclerosis. 1977 Nov;28(3):247–256. doi: 10.1016/0021-9150(77)90174-5. [DOI] [PubMed] [Google Scholar]



