Key Points
Nonmyeloablative, related HLA-haploidentical BMT utilizing high-dose posttransplantation cyclophosphamide has a favorable safety profile.
Risk-stratified relapse and survival outcomes with this approach are comparable to those of HLA-matched BMT.
Abstract
Related HLA-haploidentical blood or marrow transplantation (BMT) with high-dose posttransplantation cyclophosphamide (PTCy) is being increasingly used because of its acceptable safety profile. To better define outcomes of nonmyeloablative (NMA) HLA-haploidentical BMT with PTCy, 372 consecutive adult hematologic malignancy patients who underwent this procedure were retrospectively studied. Risk-stratified outcomes were evaluated using the refined Disease Risk Index (DRI), developed to stratify disease risk across histologies and allogeneic BMT regimens. Patients received uniform conditioning, T-cell–replete allografting, then PTCy, mycophenolate mofetil, and tacrolimus. Six-month probabilities of nonrelapse mortality and severe acute graft-versus-host disease were 8% and 4%. With 4.1-year median follow-up, 3-year probabilities of relapse, progression-free survival (PFS), and overall survival (OS) were 46%, 40%, and 50%, respectively. By refined DRI group, low (n = 71), intermediate (n = 241), and high/very high (n = 60) risk groups had 3-year PFS estimates of 65%, 37%, and 22% (P < .0001), with corresponding 3-year OS estimates of 71%, 48%, and 35% (P = .0001). On multivariable analyses, the DRI was statistically significantly associated with relapse, PFS, and OS (each P < .001). This analysis demonstrates that the DRI effectively risk stratifies recipients of NMA HLA-haploidentical BMT with PTCy and also suggests that this transplantation platform yields similar survivals to those seen with HLA-matched BMT.
Introduction
Allogeneic blood or marrow transplantation (BMT) is the only potentially curative treatment of many patients with advanced or poor-risk hematologic malignancies. However, the inability to identify or quickly secure an HLA-matched donor has been a major obstacle. Recent advances in alternative-donor transplantation have expanded allogeneic BMT options to patients who lack HLA-matched donors.1 Related, partially HLA-mismatched, or haploidentical (haplo) BMT is available to the vast majority of patients and avoids the potential delays associated with matched unrelated donor (MUD) searches. Unfortunately, haplo BMT has historically been associated with excessive graft failure, graft-versus-host disease (GVHD), and nonrelapse mortality (NRM).2-5 Methods to reduce GHVD and graft failure have centered on T-cell modulation through ex vivo depletion or increased immunosuppression but are associated with increased risks of infection and relapse.6,7 Our group pioneered the use of haplo BMT with high-dose posttransplantation cyclophosphamide (PTCy); when given in a narrow window, PTCy preferentially targets alloreactive proliferating T cells while relatively sparing nonproliferating and regulatory T cells.8-10 Our standard related haplo platform has also exclusively used nonmyeloablative (NMA) conditioning because of concerns that myeloablative conditioning might increase the incidence of GVHD. PTCy-based platforms particularly appear to protect against severe (grade III-IV) acute and chronic GVHD, mitigating GVHD and graft failure even after T-cell–replete haplo allografting.1,10-12
Although numerous groups have now confirmed the acceptable toxicity profile associated with PTCy,1,7,10-13 haplo BMT remains investigational at many centers. This is because of the historically poor outcomes associated with HLA-mismatched BMT and thus the assumption that HLA matching is critically important.2,3 Moreover, concerns have been raised about potentially high relapse rates with PTCy, especially in light of its associated low rates of GVHD. Accordingly, large patient cohorts are still needed to better understand its role, especially with regard to other graft sources. The heterogeneity of patients included in allogeneic transplant studies makes interpretation of outcomes problematic. Using only diagnosis and pretransplantation remission status, the leading predictors of progression-free survival (PFS) after BMT,14 the Disease Risk Index (DRI) stratifies allogeneic transplant recipients into low-risk, intermediate-risk, high-risk, and very high-risk disease groups.15 The DRI has been found to independently risk stratify heterogeneous adult patient cohorts regardless of conditioning intensity and graft source.15 In 2014, a refined and further validated DRI was published.16 Reporting outcomes by risk-stratified groups calibrates outcomes to facilitate interpretation of results across studies. However, few recipients of haplo BMT were included in the DRI study cohorts.15,16 Furthermore, large outcome studies of haplo BMT based on validated risk-stratification schemes have not yet been described. Here, we report the largest cohort of NMA haplo BMT with PTCy to date and analyze risk-stratified outcomes according to the refined DRI.
Patients and methods
Eligibility and treatment
This institutional review board–approved study retrospectively evaluated 372 consecutive hematologic malignancies patients aged ≥18 years who received NMA haplo BMT with high-dose PTCy at Johns Hopkins between 2002 and 2012. Patients were treated in institutional review board–approved prospective clinical trials of this transplantation platform (86%), in most cases phase 2 trials, or similarly off study. Reasons for off-study treatment included insurance restrictions, completed protocol accrual, and rarely protocol ineligibility. Two-hundred fifteen patients (58%) were included in previous manuscripts.1,10,17 Eligibility for NMA BMT included, per institutional standard, age ≤75 years, Eastern Cooperative Oncology Group performance status ≤2, left ventricular ejection fraction ≥35%, forced expiratory volume in the first second and functional vital capacity ≥40% of predicted (≥60% of predicted after thoracic or mantle radiation), not on dialysis, and absence of uncontrolled infection. Complete remission (CR) was standardly required for acute leukemias and partial remission (PR) or better for aggressive lymphomas. Donors were first-degree relatives or half siblings who were HLA-haploidentical based on molecular typing at HLA-A, -B, -Cw, -DRB1, and -DQB1. The study excluded recipients of phenotypically HLA-matched transplants, second allogeneic transplants, or regimens other than the following. All patients received fludarabine (30 mg/m2 IV, days −6 to −2, renally adjusted), Cy (14.5 mg/kg IV, days −6 and −5), total body irradiation (200 cGy, day −1), and T-cell–replete bone marrow grafting (day 0) as previously described.10 GVHD prophylaxis consisted of high-dose PTCy (50 mg/kg IV, days +3 and +4) with mesna, mycophenolate mofetil (days 5-35), and tacrolimus (initiated day 5).1 In the absence of GVHD or graft failure, tacrolimus was stopped without taper at day 90 (n = 45) or day 180 (n = 327). Filgrastim was administered from day 5 until neutrophil recovery to ≥1000/µL. Recipients of posttransplantation consolidative or maintenance therapy (eg, imatinib or rituximab) were included in this study. Supportive care was delivered according to good medical practice.
DRI scoring
The DRI risk group is a composite of disease risk (diagnosis) and stage risk (pretransplantation disease status). The refined DRI was scored as published16 and analyzed by collapsing the initial 4-group index into a 3-group index (low-, intermediate-, and high/very high-risk disease) as validated.16 For example, Hodgkin lymphoma in PR was classified as intermediate risk and aggressive non-Hodgkin lymphoma (NHL) in refractory relapse as very high risk (supplemental Table 1, available on the Blood Web site). Pretransplantation remission status was based on standard response criteria.18-20 For acute leukemia, CR was defined as morphologic CR (<5% blasts in the marrow by CD34+ immunohistochemistry and flow cytometry).20 Disease risk in acute myelogenous leukemia (AML) and myelodysplastic syndrome (MDS) incorporated the cytogenetic risk criteria used in the refined DRI paper.16 For cytogenetic risk in de novo AML, t(8;21), inv(16), or t(15;17) was considered favorable in the absence of a complex karyotype (≥4 abnormalities), complex karyotype was adverse, and all other cytogenetics were intermediate. In MDS, the refined DRI group was based on blast percentage at diagnosis, pretransplantation remission status, and cytogenetic risk, wherein abnormal chromosome 7 or ≥4 abnormalities were considered adverse and normal cytogenetics or any other abnormalities were considered intermediate.16 For AML arising from MDS, this study used MDS cytogenetic risk criteria.
Statistical methods
The primary end point was to evaluate risk-stratified disease and survival outcomes based on the refined DRI. Differences in group characteristics were compared with Fisher exact or Kruskal-Wallis tests. Time-to-event end points were measured from the date of transplantation. PFS was defined as time to progression/relapse, unplanned treatment of disease persistence, or death from any cause. Disease-free survival was defined as time to disease persistence, relapse, or death. Failure-free patients were censored at the time of last evaluation. PFS, disease-free survival, and overall survival (OS) were estimated using the Kaplan-Meier method and compared with stratified log-rank tests or Cox proportional hazards models, with P values stratified by BMT year (2002-2008 vs later). Cumulative incidences (CuIs) of relapse, NRM, count recovery, and GVHD were estimated and compared using competing-risk methods.21-23 All regression models of time-to-event end points were also stratified by BMT year. Multivariable models retained patient age regardless of P value and otherwise used backward stepwise selection with a retention P ≤ .15. All P values are 2 sided and unadjusted for multiple comparisons, with significance based on P ≤ .05. Statistical analysis was performed with R, version 3.0.2.24
Relapse, progression, and unplanned treatment of disease persistence were considered competing risks for NRM and vice versa. Graft failure was defined as ≤5% donor chimerism in peripheral blood and/or bone marrow by ∼day 60 without detected bone marrow disease. Acute GVHD was scored using the modified Keystone Criteria,25 and chronic GVHD was evaluated by National Institutes of Health Consensus Criteria.26
Results
Overall outcomes
Baseline patient and transplant characteristics overall and by refined DRI group are shown in Table 1. The disease types and stages in each DRI group are shown in supplemental Table 1. The median age at BMT was 55 (range 18-75) years, and 131 patients (35%) were aged ≥60 years. Seventy-one patients (19%) had prior autologous BMT. The most common diagnoses were aggressive NHL and AML, followed by indolent B-cell neoplasms. By refined DRI grouping, 71 patients (19%) were categorized as low risk, 241 (65%) as intermediate risk, and 60 patients (16%) as either high risk (13%) or very high risk (3%). In the majority of patients (81%), the risk groups were concordant between the original and refined DRIs.
Table 1.
Patient characteristics overall and by refined DRI group
| Variable | All patients (n = 372) | Low risk (n = 71) | Intermediate risk (n = 241) | High/very high risk* (n = 60) | P† |
|---|---|---|---|---|---|
| Patient age, y | |||||
| Median (range) | 55 (18-75) | 52 (18-72) | 56 (18-75) | 58 (19-72) | .009‡ |
| Age ≥60 y, N | 131 (35%) | 15 (21%) | 93 (39%) | 23 (38%) | |
| Male gender, N | 244 (66%) | 50 (70%) | 154 (64%) | 40 (67%) | .61 |
| Histology, N | |||||
| Myeloid | 120 (32%) | 18 (25%) | 79 (33%) | 23 (38%) | .37 |
| Lymphoid | 247 (66%) | 53 (75%) | 158 (66%) | 36 (60%) | |
| Biphenotypic§ | 5 (1%) | 0 (0%) | 4 (2%) | 1 (2%) | |
| Diagnosis | |||||
| Acute leukemia or lymphoblastic lymphoma | 114 (31%)|| | 11 (15%) | 81 (34%) | 22 (37%) | |
| MDS/MPN | 35 (9%) | 7 (10%) | 19 (8%) | 9 (15%) | |
| Aggressive NHL¶ | 95 (26%) | 0 (0%) | 85 (35%) | 10 (16%) | |
| Mantle cell lymphoma | 29 (8%) | 11 (15%) | 14 (6%) | 4 (7%) | |
| Indolent NHL, CLL, or MM# | 62 (17%) | 32 (45%) | 28 (12%) | 2 (3%) | |
| HL | 37 (10%) | 10 (14%) | 14 (6%) | 13 (22%) | |
| Year of BMT, N | |||||
| 2002-2008 | 154 (41%) | 36 (51%) | 85 (35%) | 33 (55%) | .005 |
| 2009-2012 | 218 (59%) | 35 (49%) | 156 (65%) | 27 (45%) | |
| HCT-CI risk score, N | |||||
| 0 (low risk) | 98 (26%) | 25 (35%) | 56 (23%) | 17 (28%) | .19 |
| 1-2 (intermediate risk) | 137 (37%) | 27 (38%) | 91 (38%) | 19 (32%) | |
| ≥3 (high risk) | 137 (37%) | 19 (27%) | 94 (39%) | 24 (40%) | |
| Comorbidity-age index, N | |||||
| 0-2 (low risk) | 184 (49%) | 44 (61%) | 109 (45%) | 31 (52%) | .04 |
| ≥3 (high risk) | 188 (51%) | 27 (39%) | 132 (55%) | 29 (48%) | |
| Prior autologous BMT, N | 71 (19%) | 13 (18%) | 43 (18%) | 15 (25%) | .43 |
| Patient CMV serostatus, N | |||||
| CMV negative | 198 (53%) | 39 (55%) | 124 (51) | 35 (58%) | .59 |
| CMV positive | 171 (46%) | 30 (42%) | 116 (48) | 25 (42%) | |
| Unknown | 3 (1%) | 2 (3%) | 1 (1%) | 0 (0%) | |
| CMV matching, N | |||||
| Match | 225 (60%) | 40 (56%) | 150 (62%) | 35 (58%) | .68 |
| Mismatch | 143 (38%) | 29 (41%) | 89 (37%) | 25 (42%) | |
| Unknown | 4 (1%) | 2 (3%) | 2 (1%) | 0 (0%) | |
| Donor age (y), median (range) | 41 (13-79) | 42 (17-69) | 40 (13-79) | 41 (22-73) | .53‡ |
| Female donor for male patient, N | 103 (28%) | 19 (27%) | 65 (27%) | 19 (32%) | .76 |
| Cell dose infused, median (IQR) | |||||
| TNC × 108/kg | 4.12 (3.4-4.7) | 4.18 (2.7-4.7) | 4.18 (3.5-4.8) | 3.89 (3.2-4.3) | .07‡ |
| CD34+ cells × 106/kg | 4.05 (3.2-5.2) | 3.55 (3.2-4.8) | 4.29 (3.2-5.3) | 3.86 (3.3-5.3) | .18‡ |
| CD3+ cells × 107/kg | 3.84 (3.1-4.8) | 3.98 (3.2-4.8) | 3.86 (3.1-4.8) | 3.69 (2.8-4.5) | .22‡ |
CLL, chronic lymphocytic leukemia; CMV, cytomegalovirus; HL, Hodgkin lymphoma; IQR, interquartile range; MM, multiple myeloma; MPN, myeloproliferative neoplasm; TNC, total nucleated cells.
Fifty cases (13%) were high-risk, and 10 cases (3%) were very high risk.
P for differences between refined DRI risk groups.
P using nonparametric methods.
Analyzed as AML.
Includes 90 cases (24%) of AML (57 cases de novo) and 24 cases (6%) of acute lymphoblastic leukemia/lymphoma. All percentages are for column-wise comparisons unless otherwise noted.
Includes follicular grade 3 lymphoma and excludes lymphoblastic lymphoma.
Excludes transformed lymphoma.
The median follow-up was 4.1 years overall by the reverse Kaplan-Meier method and 3.7 (range 0.5-11.4) years in surviving patients. On competing-risk analysis, the estimated CuI of neutrophil recovery was 90% (95% confidence interval [CI], 86%-93%) by day 30 (median 17 days). The probability of platelet recovery to 20 000/µL was 88% (95% CI, 84%-91%) by day 60 (median 25 days). Primary or secondary graft failure occurred in 8.2% (95% CI, 5.6%-11.5%) of evaluable patients and was in most cases accompanied by autologous hematopoietic recovery.
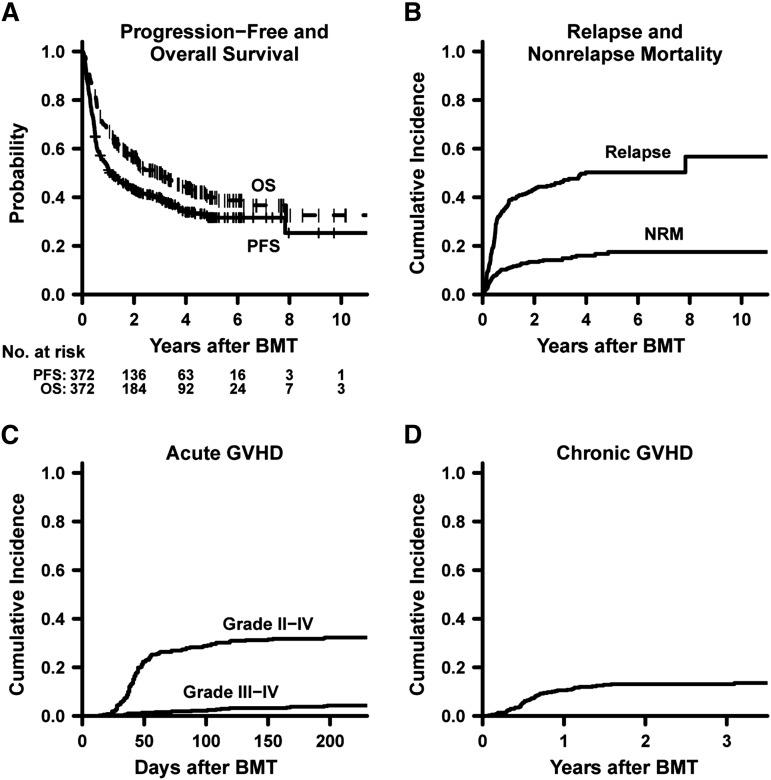
Table 2 summarizes risk-stratified survival, relapse, and NRM estimates with 95% CIs. For the entire cohort, the 3-year probabilities of PFS, OS, and relapse were 40%, 50%, and 46%, respectively (Figure 1A-B). The estimated CuI of NRM was 8% at day 180 (Figure 1B). The estimated 180-day CuIs of grade II-IV and grade III-IV acute GVHD were 32% (95% CI, 27%-36%) and 4% (95% CI, 2%-6%), respectively (Figure 1C). Grade II-IV acute GVHD was first diagnosed at a median of 42 (range 14-236) days posttransplantation, with 3 of the 121 cases occurring after day 180. The estimated 2-year CuI of chronic GVHD was 13% (95% CI, 10%-17%; Figure 1D).
Table 2.
Risk-stratified outcomes after NMA haplo BMT based on refined DRI
| Variable: refined DRI group | Probability (95% CI) | P* | |||
|---|---|---|---|---|---|
| All patients (n = 372) | Low risk (n = 71) | Intermediate risk (n = 241) | High/very high risk (n = 60) | ||
| NRM (CuI) | .60 | ||||
| Day 180 | 0.08 (0.05-0.11) | 0.03 (0-0.07) | 0.10 (0.06-0.14) | 0.05 (0-0.11) | |
| 1 y | 0.11 (0.08-0.14) | 0.10 (0.03-0.17) | 0.13 (0.08-0.17) | 0.05 (0-0.11) | |
| Relapse (CuI) | <.0001 | ||||
| 1 y | 0.37 (0.32-0.42) | 0.17 (0.08-0.26) | 0.38 (0.32-0.44) | 0.58 (0.46-0.71) | |
| 3 y | 0.46 (0.41-0.51) | 0.20 (0.11-0.30) | 0.48 (0.41-0.54) | 0.67 (0.55-0.80) | |
| PFS | <.0001 | ||||
| 1 y | 0.52 (0.47-0.57) | 0.73 (0.63-0.84) | 0.50 (0.44-0.56) | 0.37 (0.26-0.51) | |
| 3 y | 0.40 (0.35-0.45) | 0.65 (0.55-0.78) | 0.37 (0.31-0.44) | 0.22 (0.14-0.36) | |
| DFS | <.0001 | ||||
| 1 y | 0.51 (0.46-0.57) | 0.70 (0.60-0.82) | 0.50 (0.44-0.56) | 0.35 (0.25-0.49) | |
| 3 y | 0.61 (0.57-0.66) | 0.64 (0.54-0.76) | 0.37 (0.31-0.44) | 0.22 (0.14-0.36) | |
| OS | .0001 | ||||
| 1 y | 0.68 (0.63-0.73) | 0.81 (0.73-0.91) | 0.67 (0.61-0.73) | 0.55 (0.44-0.69) | |
| 3 y | 0.50 (0.45-0.56) | 0.71 (0.60-0.82) | 0.48 (0.42-0.55) | 0.35 (0.24-0.49) | |
DFS, disease-free survival.
P for differences between refined DRI risk groups, with stratification by BMT year.
Figure 1.
Overall outcomes of NMA haplo BMT with high-dose posttransplantation cyclophosphamide. (A) Progression-free and overall survival. (B-D) Cumulative incidences by competing-risk analysis of relapse and nonrelapse mortality (B), acute graft-versus-host disease (C), and any chronic graft-versus-host disease (D). Point estimates are provided in Table 2.
Results by refined DRI
The refined DRI groups were well balanced for most patient and transplant characteristics (Table 1). There were no statistically significant differences in histology (lymphoid vs myeloid), hematopoietic cell transplantation–specific comorbidity index (HCT-CI) risk category, median graft doses, or patient cytomegalovirus serostatus. However, patient age was statistically significantly higher in poorer-risk DRI groups (Table 1).
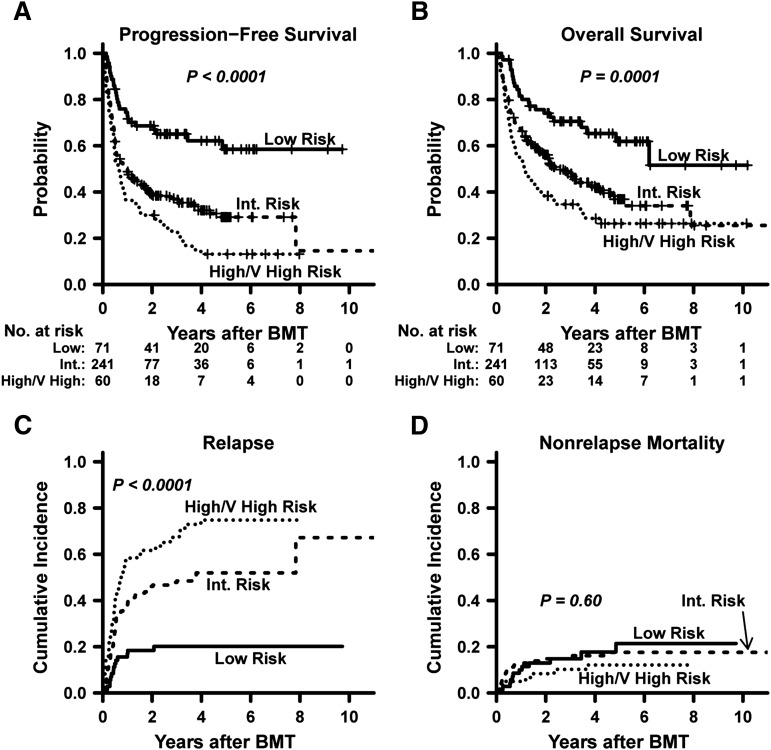
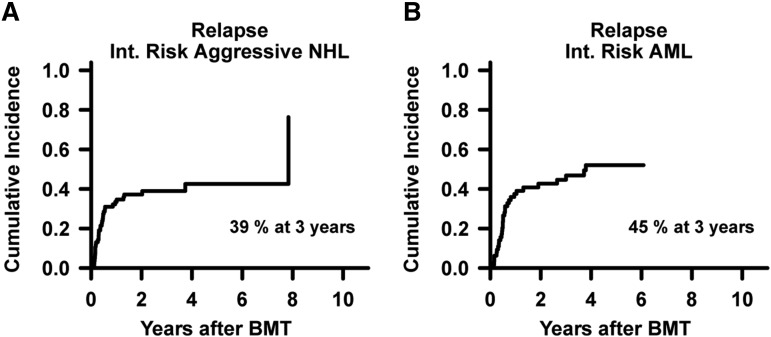
Disease and survival outcomes with 95% CI, grouped by the refined DRI, are shown in Table 2. In low-, intermediate-, and high/very high-risk groups, the 3-year PFS estimates were 65%, 37%, and 22%, and 3-year OS estimates were 71%, 48%, and 35%, respectively (all P values ≤ 0.0001; Figure 2A-B). The probability of relapse differed significantly between the DRI groups (P < .0001; Figure 2C), being greater in both the intermediate- and high/very high-risk groups compared with the low-risk group. There was no statistically significant association between DRI risk group and NRM (P = .60; Figure 2D), suggesting that the PFS and OS differences were attributable primarily to differences in relapse risk. Within a DRI stratum, relapse rates were similar in the 2 groups that are large enough for such a comparison: intermediate-risk aggressive NHL (n = 84) and intermediate-risk AML (n = 64) (Figure 3). In these subsets of NHL and AML, the 3-year probabilities of relapse were 39% (95% CI, 28%-50%) and 45% (95% CI, 32%-57%), respectively, with corresponding 3-year PFS probabilities of 45% (95% CI, 35%-57%) and 41% (95% CI, 31%-56%).
Figure 2.
Risk-stratified outcomes of NMA haplo BMT based on refined DRI group. (A) Progression-free survival. (B) Overall survival. (C) Cumulative incidence of relapse. (D) Cumulative incidence of nonrelapse mortality. Point estimates are provided in Table 2. Int., intermediate; V, very.
Figure 3.
Disease-specific relapse risks. Cumulative incidences by competing-risk analysis of relapse in intermediate-risk aggressive NHL (n = 84) (A) and relapse in intermediate-risk AML (n = 64) (B) based on refined DRI grouping.
Table 3 presents univariable analyses of selected validated prognostic indices for allogeneic BMT outcomes, namely the refined DRI, HCT-CI, and comorbidity-age index.27 In univariable analyses, the refined DRI group was statistically significantly associated with relapse risk, PFS, and OS. In contrast, in this study, neither the HCT-CI risk category nor the comorbidity-age index (<3 vs ≥3) was statistically significantly associated with OS.
Table 3.
Univariable and multivariable analyses of NMA-haplo BMT
| Variables | PFS | OS | Relapse | NRM | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | SDHR (95% CI) | P | HR (95% CI) | P | |
| Univariable* | ||||||||
| Refined DRI group | ||||||||
| Low risk | Ref | Ref | Ref | Ref | ||||
| Intermediate risk | 2.55 (1.67-3.87) | <.0001 | 2.23 (1.44-3.45) | .0004 | 3.32 (1.90-5.78) | <.0001 | 0.92 (0.49-1.74) | .80 |
| High/very high risk | 3.37 (2.10-5.41) | <.0001 | 2.86 (1.74-4.72) | .0001 | 5.29 (2.89-9.66) | <.0001 | 0.66 (0.26-1.65) | .37 |
| HCT-CI score | ||||||||
| 0 (low risk) | NT† | Ref | NT | Ref | ||||
| 1-2 (intermediate risk) | 1.11 (0.77-1.58) | .59 | 1.14 (0.55-2.34) | .73 | ||||
| ≥3 (high risk) | 1.27 (0.88-1.81) | .20 | 1.60 (0.78-3.25) | .20 | ||||
| Comorbidity-age index | ||||||||
| 0-2 (low risk) | NT† | Ref | NT | Ref | ||||
| ≥3 (high risk) | 1.17 (0.89-1.55) | .26 | 1.37 (0.81-2.34) | .24 | ||||
| Multivariable*‡ | ||||||||
| Patient age by decade§ | 1.07 (0.96-1.18) | .24 | 1.10 (0.98-1.23) | .10 | 1.03 (0.92-1.16) | .56 | 1.22 (0.96-1.54) | .10 |
| Refined DRI group | ||||||||
| Low risk | Ref | Ref | Ref | Ref | ||||
| Intermediate risk | 2.34 (1.54-3.57) | .0001 | 2.11 (1.36-3.28) | .0009 | 3.30 (1.89-5.75) | <.0001 | 0.79 (0.42-1.49) | .47 |
| High/very high risk | 3.06 (1.90-4.92) | <.0001 | 2.79 (1.69-4.60) | .0001 | 5.27 (2.90-9.61) | <.0001 | 0.58 (0.23-1.50) | .26 |
| Female donor for male patient (vs other) | 1.26 (0.95-1.68) | .11 | 1.34 (0.99-1.81) | .06 | 1.33 (0.98-1.82) | .07 | NR | |
| Patient CMV positive (vs negative) | 1.34 (1.03-1.74) | .03 | 1.31 (1.00-1.76) | .05 | NR | 1.56 (0.92-2.65) | .10 | |
| CD3+ dose ≥ 3.84 e7 /kg (vs less) | 0.77 (0.59-1.01) | .06 | NR | NR | NR | |||
CMV, cytomegalovirus; NR, not retained in final model; NT, not tested; ref, reference category.
All models were stratified by BMT year (2002-2008 vs later).
Index is validated for NRM and OS only.
All models also considered donor age (continuous), CMV matching, and for NRM and OS, the HCT-CI score.
Increasing age by decade as a continuous variable.
Multivariable analyses adjusted for patient age and other baseline characteristics are shown in Table 3. The refined DRI group was found to be independently associated with relapse risk, PFS, and OS (each P < .001). In multivariable models of relapse, as compared with the low-risk DRI group, the intermediate-risk group had a >3-fold increased relapse risk (hazard ratio [HR], 3.30; P < .0001) and the high/very high-risk group had a >5-fold increased risk (HR, 5.27; P < .0001). The refined DRI group was not statistically significantly associated with NRM on multivariable analysis. The differential relapse risk in DRI groups appeared to translate into marked differences in PFS and OS probabilities. In multivariable models of PFS, as compared with the low-risk DRI group, the HR was >2-fold greater in both the intermediate-risk group (HR, 2.34; P = .0001) and high/very high-risk groups (HR, 3.06; P < .0001). Similarly, in multivariable models of OS, HRs in both the intermediate-risk and high/very high-risk groups were >2-fold greater (HR, 2.11; P = .0009 for intermediate-risk DRI; HR, 2.79; P = .0001 for high/very high-risk DRI).
Discussion
Approximately 70% of patients in need of allogeneic BMT lack an HLA-matched related donor, and the unrelated donor registry match rates can be as low as 16% for certain ethnicities, such as African Americans and Hispanics.28 Furthermore, patients may relapse while awaiting the identification of a MUD. In contrast, haplo donors can be identified for the vast majority of patients, without the at times prohibitive waits associated with unrelated-donor transplants. However, historically haplo BMT has been associated with prohibitive toxicities.2,5 The development of high-dose PTCy has significantly reduced the rates of GVHD, graft failure, and NRM associated with haplo BMT. Nevertheless, haplo BMT remains investigational at many centers. In the largest cohort of NMA haplo BMT with PTCy to date, we demonstrate the favorable safety profile of this transplantation platform. The incidences of severe acute and any chronic GVHD were very low, supporting the role of PTCy in GVHD prevention. Graft failure rates were acceptable and usually accompanied by autologous hematopoietic recovery. The 6-month probability of NRM was 8%, which is comparable to that seen after HLA-matched BMT.29-32
Risk-stratified disease and survival outcomes after NMA haplo BMT with PTCy also appear comparable to those of reduced-intensity conditioned, HLA-matched BMT.15,16 For example, in the original DRI study cohort,15 the 614 recipients of reduced-intensity conditioned, HLA-matched related-donor or MUD BMT had 3-year PFS probabilities of 66%, 31%, and 15% in low-risk, intermediate-risk, and high/very high-risk groups, respectively, with corresponding 3-year OS probabilities of 70%, 47%, and 25% (P. Armand, Dana-Farber Cancer Institute, e-mail, July 24, 2014, personal communication). Similarly, in the present study, NMA haplo BMT patients, when grouped by the original DRI, had 3-year PFS estimates of 65%, 39%, and 25%, respectively, and corresponding 3-year OS estimates of 73%, 49%, and 37%.
Comparisons between retrospective BMT studies are inherently limited because of the complex interplay of many factors, including selection criteria, conditioning regimen and intensity, graft source, GVHD prophylaxis, disease risk, and disease status. Approaches for improving transplantation outcomes, such as modifications to conditioning regimens or GVHD prophylaxis, are usually broadly applicable across diseases and are thus frequently studied in a non–disease-specific manner. Among the strongest determinants of survival after BMT are disease type and pretransplantation disease status, which are often heterogeneously represented in BMT analyses. The DRI was developed to provide a robust tool to improve the interpretation of retrospective as well as prospective data involving a wide range of diseases, disease stages, and transplantation techniques. In the present study, the refined DRI effectively risk stratified a diverse group of patients who received NMA haplo BMT with PTCy. As in the original DRI study cohort,15 the survival differences among risk groups appeared to be driven by differences in relapse risk. The present data suggest that the index is helpful regardless of HLA mismatching and the type of postgrafting immunosuppression. However, the refined DRI categorized 65% of patients in the present study as intermediate risk, indicating the need for further refinement of prognostic tools. Similarly, in the refined DRI validation study, the majority (63%) of patients were categorized as intermediate risk, with 14% of patients being low risk, 20% high risk, and 4% very high risk.16 For disease-specific studies, more detailed risk-stratification tools are needed.
With the greatly expanded ability to safely find donors, disease relapse becomes the major area for improvement, especially in high-risk and very high-risk patients. Evaluation of more intensive conditioning regimens is warranted, and the favorable toxicity profile associated with the PTCy platform also provides an ideal setting to incorporate novel posttransplantation strategies to reduce relapse. For example, emerging data suggest that tyrosine kinase inhibitors can reduce relapse after BMT for both fms-like tyrosine kinase 3 internal tandem duplication AML33 and Philadelphia-chromosome–positive leukemia. Integrating novel immunologic agents with allogeneic BMT also holds promise for relapse reduction and improved survival in patients with poor-risk or advanced hematologic malignancies. However, approaches for reducing relapse, such as more intensive conditioning regimens, could also increase toxicities without improving overall outcomes. Thus, approaches for relapse reduction need to be carefully studied in prospective clinical trials. We initially developed our haplo BMT platform exclusively using NMA conditioning, and it remains our standard for haplo BMT because of toxicity concerns with myeloablative conditioning. More recently, we are testing haplo BMT with more intensive conditioning, but early results suggest that a small improvement in disease control is potentially offset by increased NRM, leading to PFS and OS outcomes similar to those seen with NMA conditioning.34
To the best of our knowledge, this is the largest published study to date of HLA-haplo BMT after NMA or reduced-intensity conditioning. The encouraging results in this series of NMA haplo BMT with PTCy support its role as a viable alternative-donor transplantation platform. The results furthermore suggest that virtually no patient should any longer be denied allogeneic BMT because of lack of an HLA-matched donor. Prospective randomized trials, such as the ongoing BMT Clinical Trials Network comparison of NMA double umbilical cord blood transplantation and haplo BMT,35 are necessary to help prioritize alternative-donor transplantation approaches.
Acknowledgments
The authors thank the Cell Therapy Laboratory at Johns Hopkins for graft data.
This study was supported by National Institutes of Health, National Cancer Institute grants P01 CA015396 (R.J.J.) and P30 CA006973.
Footnotes
Presented in abstract form at the 56th American Society of Hematology Annual Meeting, San Francisco, CA, December 6-9, 2014.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.R.M. and Y.L.K. were involved in the study’s conception and design, data collection, analysis, and interpretation, primary writing of the manuscript, and manuscript revisions; R.J.J. and E.J.F. were also involved in conception and design, data collection, data analysis and interpretation, and manuscript revisions; H.-L.T. and G.L.R. statistically analyzed and interpreted the data and revised the manuscript; J.A.K., M.M.S., J.B.-M., C.G.K., K.P., H.J.S., R.A.B., D.E.G., C.A.H., K.W.P., G.T.P., A.E.D., I.G., W.H.M., I.B., M.A.M., L.J.S., B.D.S., M.J.L., R.F.A., and L.L. were involved in data collection; J.A.K., C.G.K., and R.A.B. also revised the manuscript; and all authors approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Richard J. Jones, CRB I, Room 244, 1650 Orleans St, Baltimore, MD 21287; e-mail: rjjones@jhmi.edu.
References
- 1.Brunstein CG, Fuchs EJ, Carter SL, et al. Blood and Marrow Transplant Clinical Trials Network. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118(2):282–288. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beatty PG, Clift RA, Mickelson EM, et al. Marrow transplantation from related donors other than HLA-identical siblings. N Engl J Med. 1985;313(13):765–771. doi: 10.1056/NEJM198509263131301. [DOI] [PubMed] [Google Scholar]
- 3.Petersdorf EW, Gooley TA, Anasetti C, et al. Optimizing outcome after unrelated marrow transplantation by comprehensive matching of HLA class I and II alleles in the donor and recipient. Blood. 1998;92(10):3515–3520. [PubMed] [Google Scholar]
- 4.Morishima Y, Sasazuki T, Inoko H, et al. The clinical significance of human leukocyte antigen (HLA) allele compatibility in patients receiving a marrow transplant from serologically HLA-A, HLA-B, and HLA-DR matched unrelated donors. Blood. 2002;99(11):4200–4206. doi: 10.1182/blood.v99.11.4200. [DOI] [PubMed] [Google Scholar]
- 5.Szydlo R, Goldman JM, Klein JP, et al. Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. J Clin Oncol. 1997;15(5):1767–1777. doi: 10.1200/JCO.1997.15.5.1767. [DOI] [PubMed] [Google Scholar]
- 6.Bastien JP, Roy J, Roy DC. Selective T-cell depletion for haplotype-mismatched allogeneic stem cell transplantation. Semin Oncol. 2012;39(6):674–682. doi: 10.1053/j.seminoncol.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Ciurea SO, Mulanovich V, Saliba RM, et al. Improved early outcomes using a T cell replete graft compared with T cell depleted haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18(12):1835–1844. doi: 10.1016/j.bbmt.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones RJ. Haploidentical transplantation: repurposing cyclophosphamide. Biol Blood Marrow Transplant. 2012;18(12):1771–1772. doi: 10.1016/j.bbmt.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Luznik L, Bolaños-Meade J, Zahurak M, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115(16):3224–3230. doi: 10.1182/blood-2009-11-251595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raiola A, Dominietto A, Varaldo R, et al. Unmanipulated haploidentical BMT following non-myeloablative conditioning and post-transplantation CY for advanced Hodgkin’s lymphoma. Bone Marrow Transplant. 2014;49(2):190–194. doi: 10.1038/bmt.2013.166. [DOI] [PubMed] [Google Scholar]
- 12.Solomon SR, Sizemore CA, Sanacore M, et al. Haploidentical transplantation using T cell replete peripheral blood stem cells and myeloablative conditioning in patients with high-risk hematologic malignancies who lack conventional donors is well tolerated and produces excellent relapse-free survival: results of a prospective phase II trial. Biol Blood Marrow Transplant. 2012;18(12):1859–1866. doi: 10.1016/j.bbmt.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 13.Kanakry CG, O’Donnell PV, Furlong T, et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol. 2014;32(31):3497–3505. doi: 10.1200/JCO.2013.54.0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sayer HG, Kröger M, Beyer J, et al. Cooperative German Transplant Study Group. Reduced intensity conditioning for allogeneic hematopoietic stem cell transplantation in patients with acute myeloid leukemia: disease status by marrow blasts is the strongest prognostic factor. Bone Marrow Transplant. 2003;31(12):1089–1095. doi: 10.1038/sj.bmt.1704062. [DOI] [PubMed] [Google Scholar]
- 15.Armand P, Gibson CJ, Cutler C, et al. A disease risk index for patients undergoing allogeneic stem cell transplantation. Blood. 2012;120(4):905–913. doi: 10.1182/blood-2012-03-418202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664–3671. doi: 10.1182/blood-2014-01-552984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasamon YL, Luznik L, Leffell MS, et al. Nonmyeloablative HLA-haploidentical bone marrow transplantation with high-dose posttransplantation cyclophosphamide: effect of HLA disparity on outcome. Biol Blood Marrow Transplant. 2010;16(4):482–489. doi: 10.1016/j.bbmt.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hallek M, Cheson BD, Catovsky D, et al. International Workshop on Chronic Lymphocytic Leukemia. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheson BD, Pfistner B, Juweid ME, et al. International Harmonization Project on Lymphoma. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 20.Cheson BD, Bennett JM, Kopecky KJ, et al. International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 21.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141–1154. [Google Scholar]
- 22.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 23.Zhou B, Latouche A, Rocha V, Fine J. Competing risks regression for stratified data. Biometrics. 2011;67(2):661–670. doi: 10.1111/j.1541-0420.2010.01493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ihaka R, Gentleman RR. A language for data analysis and graphics. J Comput Graph Stat. 1996;5:299–314. [Google Scholar]
- 25.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 26.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Sorror ML, Storb RF, Sandmaier BM, et al. Comorbidity-age index: a clinical measure of biologic age before allogeneic hematopoietic cell transplantation. J Clin Oncol. 2014;32(29):3249–3256. doi: 10.1200/JCO.2013.53.8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dehn J, Buck K, Maiers M, et al. 8/8 and 10/10 high-resolution match rate for the Be The Match unrelated donor registry. Biol Blood Marrow Transplant. 2015;21(1):137–141. doi: 10.1016/j.bbmt.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Ho VT, Kim HT, Aldridge J, et al. Use of matched unrelated donors compared with matched related donors is associated with lower relapse and superior progression-free survival after reduced-intensity conditioning hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(8):1196–1204. doi: 10.1016/j.bbmt.2010.12.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sabry W, Le Blanc R, Labbé AC, et al. Graft-versus-host disease prophylaxis with tacrolimus and mycophenolate mofetil in HLA-matched nonmyeloablative transplant recipients is associated with very low incidence of GVHD and nonrelapse mortality. Biol Blood Marrow Transplant. 2009;15(8):919–929. doi: 10.1016/j.bbmt.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Storb R, Gyurkocza B, Storer BE, et al. Graft-versus-host disease and graft-versus-tumor effects after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2013;31(12):1530–1538. doi: 10.1200/JCO.2012.45.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Versluis J, Labopin M, Niederwieser D, et al. Prediction of non-relapse mortality in recipients of reduced intensity conditioning allogeneic stem cell transplantation with AML in first complete remission. Leukemia. 2015;29(1):51-57. [DOI] [PubMed]
- 33.Chen YB, Li S, Lane AA, et al. Phase I trial of maintenance sorafenib after allogeneic hematopoietic stem cell transplantation for fms-like tyrosine kinase 3 internal tandem duplication acute myeloid leukemia. Biol Blood Marrow Transplant. 2014;20(12):2042–2048. doi: 10.1016/j.bbmt.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Symons HJ, Chen A, Gamper C, et al. Haploidentical BMT using fully myeloablative conditioning, T cell replete bone marrow grafts, and post-transplant cyclophosphamide (PT/Cy) has limited toxicity and promising efficacy in largest reported experience with high risk hematologic malignancies [abstract]. Biol Blood Marrow Transplant. 2015;21(2):S29. [Google Scholar]
- 35. A multi-center, phase III, randomized trial of reduced intensity (RIC) conditioning and transplantation of double unrelated umbilical cord blood (dUCB) versus HLA-haploidentical related bone marrow (Haplo-BM) for patients with hematologic malignancies (BMT CTN #1101). https://www.clinicaltrials.gov/ct2/show/NCT01597778. Accessed December 15, 2014.