To the editor:
We were very interested to read in Blood the recent papers by Meng et al, “Defective release of α granule and lysosome content from platelets in mouse Hermansky-Pudlak syndrome models” and by Sharda et al, “Defective PDI release from platelets and endothelial cells impairs thrombus formation in Hermansky-Pudlak syndrome.”1,2 In these papers, the authors show that platelet adenosine diphosphate (ADP) secretion from dense granules is an important autocrine regulator of α-granule secretion. We have reached essentially the same conclusion, however, from a different perspective. We believe that, together, our data show that experimental manipulations or conditions that disrupt dense granule secretion may indirectly affect α-granule secretion and should be taken into account when investigating the mechanisms of α-granule secretion.
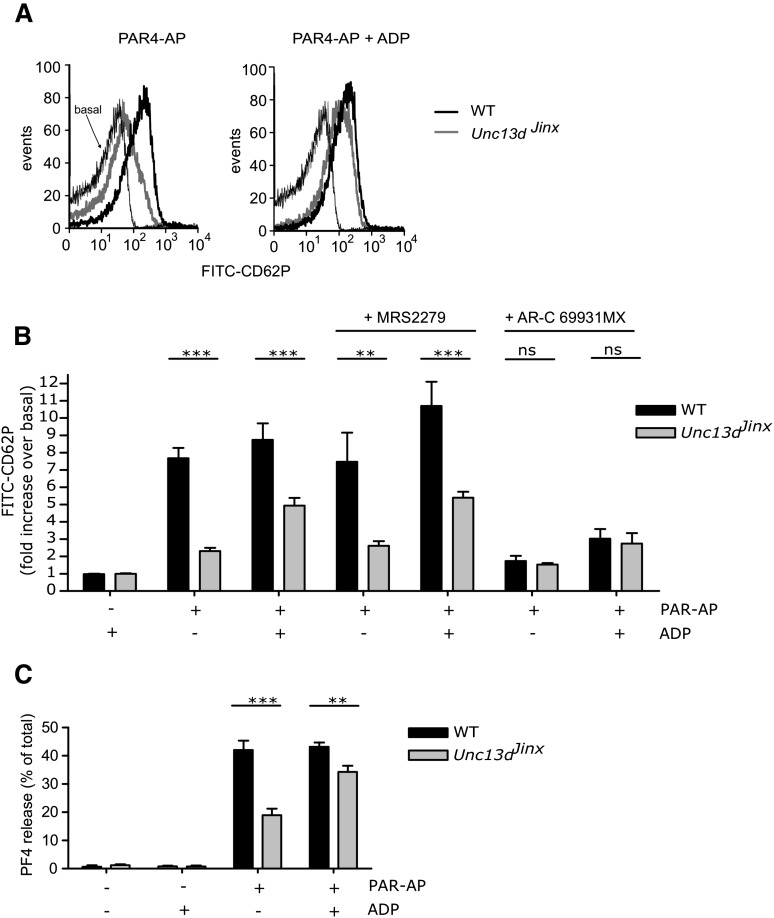
We are interested in the molecular machinery that controls platelet granule secretion. The SNARE regulator, Munc13-4, was shown to be an essential factor for dense granule secretion.3 Consistent with this, Unc13dJinx mice (which lack Munc13-4) have prolonged tail bleeding and are protected from arterial thrombosis and cerebral infarct progression.3-5 It has also been previously shown that Unc13dJinx platelets have a substantial, but incomplete, suppression of α-granule secretion,3 an effect that we also observe. Washed platelets were stimulated with PAR4-activating peptide (PAR4-AP) (300 µM) and surface expression of CD62P was used as a marker of α-granule secretion. PAR4-AP–induced CD62P expression was significantly less in Unc13dJinx platelets compared with wild-type (WT) platelets (Figure 1A). This suggests that almost all α-granule secretion is dependent on Munc13-4. However, the block to α-granule secretion in the absence of functional Munc13-4 was substantially rescued by the addition of ADP (Figure 1A-B). Therefore, there must also be a Munc13-4–independent pathway that is capable of inducing substantial α-granule secretion.
Figure 1.
The role of Munc13-4 and ADP/P2Y12 signaling in platelet α-granule secretion. Washed platelets were prepared from WT and Unc13dJinx mice. Indomethacin (10 µM) was added to remove the effect of P2Y12-dependent regulation of thromboxane A2 synthesis. Platelets were stimulated with PAR4-AP (AYPGKF-NH2 / PAR4-AP; 300 µM) for 10 minutes in the presence of fluorescein isothiocyanate-labeled anti-CDP62P antibodies. CD62P surface expression was determined by flow cytometry. (A) Shows representative histograms of platelets stimulated with PAR4-AP in the absence or presence of exogenous ADP (10 µM). (B) Shows mean data (±S.E.). In some experiments, platelets were pretreated with the P2Y1 antagonist, MRS2279 (10 µM), or the P2Y12 antagonist, AR-C69913MX (1 µM), prior to stimulation. n = 5-14; **P < .01; ***P < .001 (2-way analysis of variance, Bonferroni posttest). In (C), platelets were stimulated with PAR4-AP and/or ADP. Platelets were pelleted by centrifugation and released PF4 in the supernatant was quantified by enzyme-linked immunosorbent assay. Released PF4 is expressed as the percentage of total PF4 in unstimulated platelets. n = 4; **P < .01; ***P < .001 (2-way analysis of variance, Bonferroni posttest).
ADP alone was not able to induce detectable CD62P expression under these conditions, suggesting that ADP acts synergistically to enhance PAR4-AP–induced α-granule secretion. In the presence of the P2Y12 antagonist AR-C69931MX, there was a marked suppression of CD62P surface expression in both WT and Unc13dJinx platelets, and the ADP-mediated rescue of secretion was ablated (Figure 1B). The P2Y1 antagonist, MRS2279, had no effect. In addition, we found that 5-HT was not able to induce α-granule secretion, and neither did it synergise with PAR4-AP to rescue the response in the Unc13dJinx platelets (data not shown), suggesting that 5-HT has no role in the regulation of secretion under these conditions. Therefore, secreted ADP, acting through the P2Y12 receptor, synergizes with PAR4-AP to induce Munc13-4–independent α-granule secretion, but the presence of this pathway is obscured by the complete loss of dense granule secretion in Unc13dJinx platelets.
To confirm that secreted ADP regulates PAR4-AP–induced α-granule secretion, we also investigated the role of ADP in the release of the α-granule cargo, PF4 (Figure 1C). PAR4-AP–stimulated Unc13dJinx platelets released significantly less PF4 than WT platelets. Co-stimulation with ADP induced more PF4 release in Unc13dJinx platelets than PAR4-AP alone (34.3 ± 2.2% of total PF4 with PAR4-AP+ADP compared with 19.0 ± 2.3% with PAR4-AP alone), again suggesting that loss of secreted ADP contributes to the α-granule secretion defect in these platelets.
These data demonstrate that the disruption of dense granule secretion can alter the apparent contribution of different proteins to α-granule secretion. For example, under our stimulation conditions, we would have concluded that Munc13-4 is a major regulator of PF4 release, whereas it may be released in a largely Munc13-4–independent manner that is enhanced by secreted ADP. There was still a small, statistically significant, difference between PF4 release from WT and Unc13dJinx platelets in the presence of ADP, suggesting that there may be a small direct contribution of Munc13-4 under these conditions. Alternatively, this may reflect a small contribution of other dense granule components, or that exogenous ADP does not fully mimic locally high concentrations of secreted ADP. The relative contribution of the Munc13-4–dependent pathway and the Munc13-4–independent pathway may depend on the precise conditions of platelet stimulation. However, we believe that our data raise the more general question: How much do we know about the molecular mechanisms of α-granule release in platelets? It will be particularly interesting to re-evaluate knockout mice studies that show defects in dense and α-granule secretion, to determine which proteins regulate α-granule secretion directly, and which act indirectly through dense granule secretion.
Authorship
Acknowledgment: This work was supported by the British Heart Foundation (programme grant RG/10/006/28299).
Contribution: M.T.H. designed and performed experiments, analysed data, and wrote the manuscript; M.T.v.d.B. and I.H. designed experiments and edited the manuscript; and A.W.P. designed experiments and wrote the manuscript.
The current affiliation for M.T.H. is Department of Pharmacology, University of Cambridge, Cambridge, United Kingdom.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Matthew Harper, Department of Pharmacology, Tennis Court Road, Cambridge CB2 1PD, United Kingdom; e-mail: mth29@cam.ac.uk; or Alastair Poole, School of Physiology and Pharmacology, Medical Sciences Building, University Walk, Bristol BS8 1TD, United Kingdom; e-mail: a.poole@bristol.ac.uk.
References
- 1.Meng R, Wu J, Harper DC, et al. Defective release of α granule and lysosome contents from platelets in mouse Hermansky-Pudlak syndrome models. Blood. 2015;125(10):1623–1632. doi: 10.1182/blood-2014-07-586727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharda A, Kim SH, Jasuja R, et al. Defective PDI release from platelets and endothelial cells impairs thrombus formation in Hermansky-Pudlak syndrome. Blood. 2015;125(10):1633–1642. doi: 10.1182/blood-2014-08-597419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ren Q, Wimmer C, Chicka MC, et al. Munc13-4 is a limiting factor in the pathway required for platelet granule release and hemostasis. Blood. 2010;116(6):869–877. doi: 10.1182/blood-2010-02-270934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savage JS, Williams CM, Konopatskaya O, Hers I, Harper MT, Poole AW. Munc13-4 is critical for thrombosis through regulating release of ADP from platelets. J Thromb Haemost. 2013;11(4):771–775. doi: 10.1111/jth.12138. [DOI] [PubMed] [Google Scholar]
- 5.Stegner D, Deppermann C, Kraft P, et al. Munc13-4-mediated secretion is essential for infarct progression but not intracranial hemostasis in acute stroke. J Thromb Haemost. 2013;11(7):1430–1433. doi: 10.1111/jth.12293. [DOI] [PubMed] [Google Scholar]