Abstract
OBJECTIVE:
To evaluate the influence of estrogen therapy and estrogen-progestin therapy on homocysteine and C-reactive protein levels in postmenopausal women.
METHODS:
In total, 99 postmenopausal women were included in this double-blind, randomized clinical trial and divided into three groups: Group A used estrogen therapy alone (2.0 mg of 17β-estradiol), Group B received estrogen-progestin therapy (2.0 mg of 17 β-estradiol +1.0 mg of norethisterone acetate) and Group C received a placebo (control). The length of treatment was six months. Serum measurements of homocysteine and C-reactive protein were carried out prior to the onset of treatment and following six months of therapy.
RESULTS:
After six months of treatment, there was a 20.7% reduction in homocysteine levels and a 100.5% increase in C-reactive protein levels in the group of women who used estrogen therapy. With respect to the estrogen-progestin group, there was a 12.2% decrease in homocysteine levels and a 93.5% increase in C-reactive protein levels.
CONCLUSION:
Our data suggested that hormone therapy (unopposed estrogen or estrogen associated with progestin) may have a positive influence on decreasing cardiovascular risk due to a significant reduction in homocysteine levels.
Keywords: Estrogen-Progestin Therapy, Estrogen, Homocysteine, CRP, Cardiovascular Risk
INTRODUCTION
It is estimated that 25 million women reach menopause each year. Worldwide, there are currently approximately 470 million women over 50 years of age. Data from the World Health Organization show that in 20 years' time, women will live half of their lifespan postmenopause 1.
Cardiovascular disease is the greatest cause of death in industrialized nations and is a problem in developing countries, such as Brazil 2,3. Evidence that estrogen therapy may have a protective effect on the cardiovascular systems of postmenopausal women, reducing morbidity and mortality from cardiovascular disease, was based on various observational studies, such as the “Lipid Research Clinics Study” 4. However, according to Barret-Connor 5, the interpretation of data derived from observational studies should be undertaken cautiously because the possibility of distortions is great. Therefore, to determine the possible benefit of estrogen and estrogen-progestin therapy in reducing ischemic cardiac disease-related mortality, interventional, prospective, randomized, placebo-controlled studies should be carried out in a large number of patients over an extended period of time ,6-10.
The results of interventional studies are also discordant, raising doubts regarding the real benefits of substitutive hormone therapy (HT) in terms of either the primary or secondary prevention of cardiovascular disease postmenopause 7. With respect to secondary prevention, three interventional studies, “Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women” (HERS), “A clinical trial of estrogen-replacement therapy after ischemic stroke” (ERA) and “Effects of estrogen replacement on the progression of coronary-artery atherosclerosis” (WEST), failed to show that postmenopausal HT was able to prevent cardiovascular disease ,8-10. The results of the “Women's Health Initiative” (WHI) study provoked discussions regarding the action of HT in the prevention of cardiovascular disease and raised doubts with respect to pre-established concepts regarding the primary prevention of these diseases6,11. However, the cardiovascular risk associated with unopposed estrogen may be lower than that associated with a progestogen-estrogen combination 12.
Because ischemic cardiovascular disease can surprise women who have no traditional risk factors for the condition, it is important to identify markers that are able to predict the risk of developing atherothrombotic disease 13,14. Studies have therefore been undertaken to find markers that are predictive of the development of cardiovascular events, among which are homocysteine and C-reactive protein (CRP) ,13-15.
Homocysteine is a derivative of methionine, an essential amino acid that is present in meat and dairy products. Homocysteine is re-methylated into methionine through the action of folic acid and vitamins B6 and B12 15. Increased levels of homocysteine are unfavorably associated with coagulation function and with the vasodilatory and antithrombotic action of nitric oxide, thereby increasing the risk of thrombosis and acute myocardial infarction 16. After menopause, the serum levels of homocysteine are higher than those in younger women. Certain investigators have reported that substitutive HT significantly reduces these levels 17.
CRP is a sensitive marker of acute inflammatory reactions. High plasma levels of this protein are associated with a future risk of cardiovascular events. CRP is therefore considered to be an independent risk factor for the development of this type of disease 18. The levels of CRP are higher in apparently healthy women using HT. Certain authors believe that estrogen therapy may stimulate and/or aggravate an inflammatory process that, in predisposed women, could contribute to the progression to atherosclerosis and the thrombotic process 19. However, this effect is not clear for unopposed estrogen 20. Therefore, the aim of this study was to evaluate the effects of estrogen therapy and estrogen-progestin therapy on homocysteine and CRP levels in postmenopausal women.
METHODS
A total of 242 postmenopausal women (last natural menstruation at least 12 months prior to entering the study) were evaluated between December 2, 2000 and January 31, 2002. Of these women, 99 were selected for enrollment into this study. To be considered for inclusion in this study, the women had to have vasomotor complaints (based on the Kupperman Menopausal Index), should not have had a hysterectomy and should not have been using HT in the 60 days preceding admission to the study.
The exclusion criteria consisted of women with a body mass index ≥35, those who were taking vitamin supplements (vitamin B6, vitamin B12 and/or folic acid), those who engaged in physical exercise (with the exception of light walks fewer than three times per week), those who had an endometrial thickness>5 mm according to ultrasonography carried out within the previous six months, those who had any abnormality detected by recent mammography or cervicovaginal oncological colpocytology (carried out in the previous 12 months) and those who presented a personal history of cardiovascular disease or venous or arterial thromboembolism. Women presenting dyslipidemia, diabetes mellitus, or acute or chronic hepatopathies were also excluded as well as those using cholesterol-reducing medication, androgens, raloxifene, tamoxifen, barbiturates, hydantoin, carbamazepine, phenylbutazone, meprobamate or rifampicin and those with hormone-dependent cancer. All subjects voluntarily agreed to participate in the study, which was approved by the institution's Ethics Committee in Research and all patients signed informed consent forms.
This longitudinal clinical trial was a prospective, randomized, double-blind, placebo-controlled study. A total of 99 patients were randomly distributed into three different groups (33 in each): Group A received unopposed estrogen therapy (2.0 mg of 17 β-estradiol), Group B was treated with an estrogen-progestin combination (2.0 mg of 17 β-estradiol +1.0 mg of norethisterone acetate) and Group C received pills containing no active substance (placebo).
Prior to the initiation of treatment, all patients were subjected to general physical and gynecological examinations and their medical history was recorded. The climacteric symptoms were evaluated using the Kupperman Menopausal Index. Blood samples were collected from all patients in the morning, following a 12-hour fast, both at baseline and after six months of treatment for the measurement of the serum levels of homocysteine and CRP (Laboratório Central, UNIFESP, São Paulo, Brazil). The blood sampling was carried out at a maximum of 15 days prior to the initiation of therapy and at the end of six months of treatment.
The Kupperman index is a numerical conversion index that covers 11 menopausal symptoms: hot flushes (vasomotor), paresthesia, insomnia, nervousness, melancholia, vertigo, weakness, arthralgia or myalgia, headache, palpitations and stinging. Each symptom in the Kupperman index is rated on a scale from 0 to 3 for no, slight, moderate and severe complaints. To calculate the Kupperman index 21, the symptoms are weighted as follows: hot flushes (x4), paresthesias (x2), insomnia (x2), nervousness (x2) and all other symptoms (x1). The highest potential score is thus 51. The score for hot flushes was based on the number of complaints per day: slight (more than 5), moderate (5-10), or severe (more than 10).
Homocysteine was measured by high-performance liquid chromatography (HPLC) using a C-R4A Chromatopac Integrator (SHIMADZU), an R-F-10AXL Fluorescent Detector (SHIMADZU), an LC-10AD Pump (SHIMADZU) and a 234 Autoinjector (GILSON). For this method, an intra-test variation level of 4.5% was considered acceptable. Serum CRP was measured by nephelometry using an Array 360 System (Beckman Coulter) with an intra-test variation level established at 5.0%.
Each patient completed four visits (V) during the study: V0, at day 0; V1, 7±3 days after V0; V2, 90±5 days after V1; and V3, 90±5 days after V2.
Statistical analysis
The characteristics of the groups were analyzed by one-way repeated-measures analysis of variance subsequently corrected by a least-significant-difference comparison test (Fisher test). The statistical analysis of the homocysteine and CRP data was based on a non-parametric method and the Kruskal-Wallis test was used to compare the three groups in the study. The rejection of the null hypothesis was established at 5% (p≤0.05). The Dunn test was applied to identify differences between the initial and the final measurements of the serum levels of homocysteine and CRP in each group. The Wilcox test was used to detect any evidence that the variable in question had undergone a change between the beginning and the end of the evaluation period. The results of the Dunn test were used to compare the absolute differences between the means of each pair of groups.
RESULTS
Of a total of 99 women who began this study, 12 discontinued their participation prior to study completion, with 3 in Group A, 2 in Group B and 7 in Group C. In Group A, the three discontinuations occurred due to mastalgia, irregular bleeding and loss to follow-up. Irregular bleeding was the cause of discontinuation for the two patients in Group B. Of the patients in Group C, three abandoned the study because they felt no improvement in vasomotor symptoms, one because of mastalgia, one because of an acute myocardial infarction, one because of a carotid obstruction and one because of loss to follow-up.
At the two evaluative visits (during the 3rd and 6th months), the number of tablets returned to the clinic was proportional to the number of days remaining until the end of treatment in all cases, indicating that compliance was similar in the three groups.
Data on the patient characteristics are shown in Table 1. The statistical analyses showed no difference between the groups for any of the parameters: age, the time since menopause, body mass index, the Kupperman Menopausal Index, systolic blood pressure, diastolic blood pressure and smoking. All participants had normal levels of glucose and no history of diabetes.
Table 1.
-Characteristics of the participants (mean ± standard deviation) in all groups at baseline: A (unopposed estrogen), B (estrogen-progestin combination) and C (placebo).
| Group | A (n = 30) | B (n = 31) | C (n = 24) |
| Age (years) | 50.2±1.2 | 51.3±1.2 | 51.1±1.8 |
| Time since menopause (years) | 3.7±1.5 | 3.8±1.7 | 4.1±1.1 |
| Body mass index (Kg/m2) | 25.4±1.1 | 27.3±1.7 | 26.4±1.6 |
| Kupperman Menopausal Index | 31.8±1.2 | 33.8±2.2 | 32.8±2.1 |
| Systolic blood pressure (mmHg) | 122.1±13.9 | 123.1±15.4 | 118.1±15.1 |
| Diastolic blood pressure (mmHg) | 81.7±10.9 | 82.6±14.3 | 83.9±11.3 |
| Smokers (n) | 3 | 4 | 3 |
The statistical analyses showed no differences between the groups for any parameter.
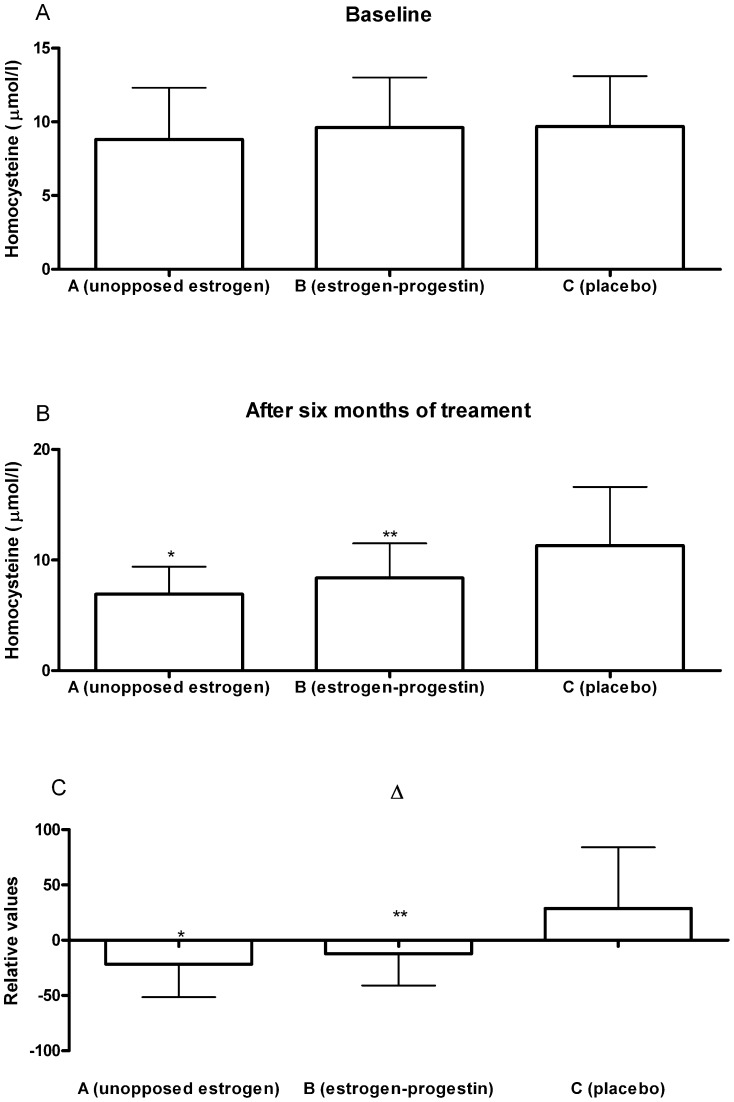
Table 2 and Figure 1 show the distribution of the basal homocysteine and CRP measurements. There were no statistically significant differences in the basal values of these markers between any of the three groups in the study. Additionally, the table shows the percentage of variation in homocysteine levels in the three groups following six months of treatment. A mean reduction of 20.7% was observed in Group A when these levels were compared with basal values (p<0.01). In Group B, there was also a significant reduction (12.2%) when compared with initial values (p<0.01). In Group C, there was a mean increase of 16.5% relative to basal measurements, which was not statistically significant.
Table 2.
-Homocysteine (µmol/l) and C-reactive protein (ng/l) levels of the participants during the study.
| Group | A (unopposed estrogen, n = 30) | B (estrogen-progestin combination, n = 31) | C (placebo, n = 24) | ||||||
| baseline | after* | Δ | baseline | after* | Δ | baseline | after* | Δ | |
| Homocysteine (µmol/l) | 8.8±3.5 | 6.9±2.5a | - 21.6±- 29.8b | 9.6±3.4 | 8.4±3.1a | - 12.2±- 28.9c | 9.7±3.4 | 11.3±5.3 | 16.5±55.1 |
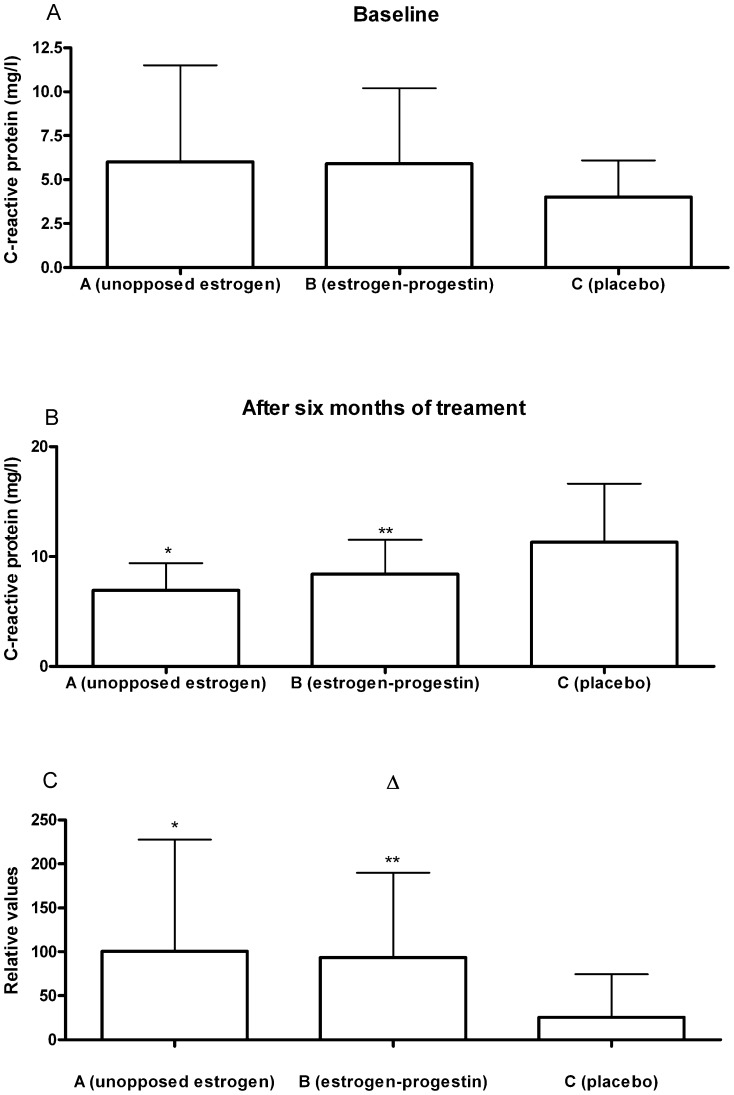
| C-reactive protein (mg/l) | 3.0±2.0 | 6.0±5.5a | 100.5±127.1 b | 3.1±2.2 | 5.9±4.3 a | 93.5±96.4c | 3.2±1.4 | 4.0±2.1 a | 25.5±48.9 |
*After six months of treatment; Δ = [(value after treatment - baseline value)/baseline value * 100]. The statistical analyses showed no difference between the groups' baseline homocysteine and C-reactive protein levels; a – p<0.01 compared with baseline (Wilcox test); b – p<0.01 compared with Δ of the other groups (Kruskal-Wallis and Dunn tests); c – p<0.01 compared with Δ of Group C (Kruskal-Wallis and Dunn tests).
Figure 1.
Graphical representation of homocysteine values during the study: a) baseline; b) after six months of treatment; c) delta {(Δ = [(value after treatment - baseline value)/baseline value * 100]}. *p<0.01 compared with the other groups; **p<0.01 compared with the placebo group.
When the entire sample was examined, the distribution of homocysteine was found to vary between the 3 groups (p<0.01). Dunn's test, applied posteriorly, indicated statistically significant differences between Groups A and C and between Groups B and C but not between the patients receiving estrogen therapy and those receiving estrogen-progestin therapy (Groups A and B, respectively). At the end of treatment, homocysteine levels were significantly lower (p<0.01) in the groups using hormones compared with the placebo group.
The levels of CRP increased in all groups following six months of therapy (Table 2 and Figure 2), but this increase only reached statistical significance in the two groups receiving active medication (estrogen alone or associated with progestin). In Groups A and B, there were increases of 100.5% (p<0.01) and 93.5% (p<0.01), respectively. These values showed statistical significance in relation to the value in the placebo group but were not significantly different from each other. When the sample was considered as a whole, there was evidence that the distribution of CRP showed certain differences between the three groups (p<0.01). Dunn's test, applied posteriorly, showed statistically significant differences between Groups A and C and between Groups B and C.
Figure 2.
Graphical representation of C-reactive protein values during the study: a) baseline; b) after six months of treatment; c) delta {(Δ = [(value after treatment - baseline value)/baseline value * 100]}. *p<0.01 compared with the other groups; **p<0.01 compared with the placebo group.
DISCUSSION
Postmenopausal women have higher blood levels of homocysteine compared with younger women 22. Certain studies have shown that HT is able to significantly reduce these levels. Van der Mooren et al. 23 reported a significant reduction in homocysteine levels following six months of oral sequential combined therapy. Moreover, these reduced levels remained stable during the 24 months of treatment. Twelve months after the end of this therapy, homocysteine levels increased, i.e., they returned to pretreatment levels. Mijatovic et al. 24 followed 135 healthy women who were using oral continuous combined estrogen-progestin therapy. The authors reported a significant reduction (13.5%) in homocysteine levels following six months of treatment. The greatest reduction occurred in those who presented the highest pretreatment levels. Madsen et al. 25 carried out a study in 209 postmenopausal women and showed that homocysteine levels decreased significantly after five years of follow-up in those women using estrogen or estrogen-progestin therapy. Regardless of the estrogen regimen, HT may reduce homocysteine levels.
In our study, we observed a 20.7% reduction in homocysteine levels in women using estrogen therapy after six months of treatment compared with a 12.2% reduction in those using estrogen-progestin therapy. In the women who were taking a placebo, there was an increase of 16.5%. Therefore, estrogen was better at reducing homocysteine levels than combined steroids were. However, another study found a similar difference between the two estrogen regimens (unopposed and combined) 26.
The mechanism through which hyperhomocysteinemia predisposes an individual to atherogenesis and thrombogenesis is still not fully understood. However, it appears that increased levels of homocysteine cause a predisposition to endothelial injury, stimulate HDL oxidation through an increase in the activity of the methionine synthetase enzyme and affect the proliferation of the endothelial smooth muscle cells ,15-17. As a result, 1 mmol/L and 3 mmol/L reductions in the plasma levels of homocysteine are associated with reductions of 10% and 30%, respectively, in the risk of cardiovascular disease 27. The greatest reduction in the levels of this substance was found in the estrogen group in our study.
The relationship between HT and inflammatory response markers, such as CRP, has been widely discussed. In the CARE study, Ridket et al. 28 showed that individuals at greater risk of developing cardiovascular disease had higher basal levels of CRP. It is important to emphasize that the baseline CRP levels may be associated with an increased risk of clinical problems ,28-31. In our study, many patients presented high levels of CRP. Increased levels of CRP are also found in users of HT. Estrogen therapy and estrogen-progestin therapy both appear to stimulate or aggravate an inflammatory process in the endothelium that contributes to the progression to arteriosclerosis and the activation of the coagulation system in high-risk women 29. Evidence indicates that the administration of estrogens may stimulate a pro-inflammatory effect appearing at the beginning of therapy and reverting immediately following the cessation of treatment. However, this phenomenon may be associated with a first-pass hepatic effect rather than a pro-inflammatory response ,30-33.
It appears that the increased hepatic production of CRP is related to the oral administration of HT. In this respect, Sattar et al. 34 found a significant reduction in CRP levels following the transdermal use of 17-β-estradiol combined with norethisterone acetate compared with levels in the placebo group. Bukowka et al. reported 61% and 39% increases in CRP levels in the users of oral and transdermal HT, respectively, after three months of therapy 35.
In a study of 493 postmenopausal women, Ridker et al. 36 showed that CRP levels increased two-fold in those patients who were using estrogen or estrogen-progestin therapy compared with the control group. Moreover, in a re-analysis of the PEPI trial, it was shown that the patients using HT presented an increase in CRP levels. The CRP levels in these patients were 85% higher than those in the placebo group 37. In our study, the mean increases in the levels of CRP after six months of treatment were 100.5% in patients who were taking isolated estrogens, 93.5% in those taking a combination of estrogen and progestin, and 25.5% in the control group. In a recent study, Kwang et al. 38 reported a 70% increase in CRP levels in women using conjugated equine estrogens at 0.625 mg/day after six months of treatment. In the same study, when simvastatin was added to the treatment, the increase in CRP levels was lower (29%). However, in the group of women who were taking only the cholesterol-reducing medication at a dose of 10 mg daily, no change was recorded in CRP levels. Therefore, HT may influence simvastatin's effect.
Another feature of our study was the age of the participants. Certain investigators have suggested a life period called a “window of opportunity” for HT after menopause. In fact, the cardiovascular risk may be low if estrogen or estrogen-progestin therapy is started during this period, in which the time since menopause must be less than 10 years and the age of the woman must not be greater than 60 years old 39. All participants in our study had these features, which may have influenced our results. Regardless of the CRP results, our data suggested that HT and mainly unopposed estrogen, may have a positive effect on the cardiovascular system due to a significant reduction in homocysteine levels.
Footnotes
No potential conflict of interest was reported.
Erratum
Page 107
Include César Eduardo FernandesIII as 4th author.
IIIFaculdade de Medicina do ABC, Department of Gynecology, Santo André/SP, Brazil.
Page 112
AUTHOR CONTRIBUTIONS
Substitute: Lakryc EM, Machado RB and Baracat EC designed the study and wrote the manuscript. Soares Jr JM wrote the manuscript.
For: Lakryc EM, Machado RB, Fernandes CE and Baracat EC designed the study and wrote the manuscript. Soares Jr JM wrote the manuscript.
REFERENCES
- 1.United Nations Department of Economic and Social Affairs Population Division World Population Ageing: 1950–2050. Accessed on November 12, 2014. Available online: http://www.un.org/esa/population/publications/worldageing19502050/ [Google Scholar]
- 2.Bagnoli VR, Fonseca AM, Arie WM, Das Neves EM, Azevedo RS, Sorpreso IC, et al. Metabolic disorder and obesity in 5027 Brazilian postmenopausal women. Gynecol Endocrinol. 2014;30(10):717–20. doi: 10.3109/09513590.2014.925869. [DOI] [PubMed] [Google Scholar]
- 3.Belon AP, Barros MB, Marín-León L. Mortality among adults: gender and socioeconomic differences in a Brazilian city. BMC Public Health. 2012;17:12:39. doi: 10.1186/1471-2458-12-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bush TL, Barrett-Connor E, Cowan LD, Criqui MH, Wallace RB, Suchindran CM, et al. Cardiovascular mortality and noncontraceptive use of estrogen in women: Results from the Lipid Research Clinics Program Follow-up Study. Circulation. 1987;75(6):1102–09. doi: 10.1161/01.cir.75.6.1102. [DOI] [PubMed] [Google Scholar]
- 5.Stamfer MJ, Colditz GA, Willett WC. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the Nurses' Health Study. N Engl J Med. 1991;325(11):756–62. doi: 10.1056/NEJM199109123251102. [DOI] [PubMed] [Google Scholar]
- 6.Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA. 2013;310(13):1353–68. doi: 10.1001/jama.2013.278040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson HD, Humphrey LL, Nygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy: scientific review. JAMA. 2002;288(7):872–81. doi: 10.1001/jama.288.7.872. [DOI] [PubMed] [Google Scholar]
- 8.Lokkegaard E, Pedersen AT, Heitmann BL, Jovanovic Z, Keiding N, Hundrup YA, et al. Relation between hormone replacement therapy and ischaemic heart disease in women: prospective observational study. BMJ. 2003;326(7386):426. doi: 10.1136/bmj.326.7386.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hulley S, Grady D, Bush T. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in post-menopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280(7):605–13. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 10.Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz R. A Clinical Trial of Estrogen-Replacement Therapy after Ischemic Stroke. N Engl J Med. 2001;345(17):1243–9. doi: 10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- 11.Herrington DM. Effects of estrogen replacement on progression of coronary artery atherosclerosis. N Eng J Med. 2000;343(8):52–9. doi: 10.1056/NEJM200008243430801. [DOI] [PubMed] [Google Scholar]
- 12.Cherry N, McNamee R, Heagerty A, Kitchener H, Hannaford P. Long-term safety of unopposed estrogen used by women surviving myocardial infarction: 14-year follow-up of the ESPRIT randomised controlled trial. BJOG. 2014;121(6):700–5. doi: 10.1111/1471-0528.12598. [DOI] [PubMed] [Google Scholar]
- 13.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Hutchinson F, et al. Risks and Benefits of Estrogen plus Progestin in Healthy Postmenopausal Women. JAMA. 2002;228(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 14.Duivenvoorden R, Mani V, Woodward M, Kallend D, Suchankova G, Fuster V, et al. Relationship of serum inflammatory biomarkers with plaque inflammation assessed by FDG PET/CT: the dal-PLAQUE study. JACC Cardiovasc Imaging. 2013;6(10):1087–94. doi: 10.1016/j.jcmg.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Signorello MG, Pascale R, Leoncini G. Effect of homocysteine on arachidonic acid release in human platelets. Eur J Clin Invest. 2002;32(4):279–84. doi: 10.1046/j.1365-2362.2002.00971.x. [DOI] [PubMed] [Google Scholar]
- 16.Antoniades C, Antonopoulos AS, Tousoulis D, Marinou K, Stefanadis C. Homocysteine and coronary atherosclerosis: from folate fortification to the recent clinical trials. Eur Heart J. 2009;30(1):6–15. doi: 10.1093/eurheartj/ehn515. [DOI] [PubMed] [Google Scholar]
- 17.Mijatovic V, van der Mooren MJ. Homocysteine in postmenopausal women and the importance of hormone replacement therapy. Clin Chem Lab Med. 2001;39(8):764–7. doi: 10.1515/CCLM.2001.127. [DOI] [PubMed] [Google Scholar]
- 18.Meng H, Zhang M, Chen P, Wang ZM, Chen B, Yang Z. Comparison of high-sensitivity C-reactive protein level between systemic and coronary circulation in patients with acute myocardial infarction. Acta Biochim Biophys Sin (Shanghai) 2014;46(2):161–2. doi: 10.1093/abbs/gmt129. [DOI] [PubMed] [Google Scholar]
- 19.Kurtz EG, Ridker PM, Rose LM, Cook NR, Everett BM, Buring JE, et al. Oral postmenopausal hormone therapy, C-reactive protein, and cardiovascular outcomes. Menopause. 2011;18(1):23–9. doi: 10.1097/gme.0b013e3181e750dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodis HN, St John JA, Xiang M, Cushman M, Lobo RA, Mack WJ. Inflammatory markers and progression of subclinical atherosclerosis in healthy postmenopausal women (from the Estrogen in the Prevention of Atherosclerosis Trial) Am J Cardiol. 2008;101(8):1131–3. doi: 10.1016/j.amjcard.2007.09.120. [DOI] [PubMed] [Google Scholar]
- 21.Han KK, Soares JM, Jr, Haidar MA, de Lima GR, Baracat EC. Benefits of soy isoflavone therapeutic regimen on menopausal symptoms. Obstet Gynecol. 2002;99(3):389–94. doi: 10.1016/s0029-7844(01)01744-6. [DOI] [PubMed] [Google Scholar]
- 22.Ridker PM, Haughie P. Prospective studies of C-reactive protein as a risk factor for cardiovascular disease. J Invest Med. 1998;46(8):391–5. [PubMed] [Google Scholar]
- 23.Van der Mooren MJ, Wouters MG, Blom HJ, Schellekens LA, Eskes TK, Rolland R. Hormone replacement therapy may reduce high serum homocysteine in postmenopausal women. Eur J Clin Invest. 1994;24(11):733–6. doi: 10.1111/j.1365-2362.1994.tb01069.x. [DOI] [PubMed] [Google Scholar]
- 24.Mijatovic V, Kenemans P, Netelenbos JC, Jacobs C, Popp- Snijders C, Peters-Muller ERA, et al. Postmenopausal oral 17&bgr; oestradiol continuously combined with dydrogesterone reduces fasting serum homocysteine levels. Fertil Steril. 1998;69(5):876–82. doi: 10.1016/s0015-0282(98)00041-7. [DOI] [PubMed] [Google Scholar]
- 25.Madsen JS, Kristensen SR, Klitgaard NA, Bladbjerg EM, Abrahamsen B, Stilgren L, et al. Effect of long-term hormone replacement therapy on plasma homocysteine in postmenopausal women: A randomized controlled study. Am J Obstet Gynecol. 2002;187(1):33–9. doi: 10.1067/mob.2002.123030. [DOI] [PubMed] [Google Scholar]
- 26.Tutuncu L, Ergur AR, Mungen E, Gun I, Ertekin A, Yergok YZ. The effect of hormone therapy on plasma homocysteine levels: a randomized clinical trial. Menopause. 2005;12(2):216–22. doi: 10.1097/00042192-200512020-00017. [DOI] [PubMed] [Google Scholar]
- 27.Boushey CJ, Beresford SAA, Omenn GS, Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. JAMA. 1995;274(13):1049–57. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 28.Ridker PM, Rifai N, Pfeffer MA, Sacks FM, Moye LA, Goldman S, et al. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Circulation. 1998;98(9):839–44. doi: 10.1161/01.cir.98.9.839. for the Cholesterol, and Recurrent Events (CARE) Investigators. [DOI] [PubMed] [Google Scholar]
- 29.Bonduki CE, Lourenço DM, Motta EL, Soares JM, Jr, Haidar MA, Baracat EC. Effect of estrogen-progestin hormonal replacement therapy on blood coagulation and fibrinolysis in postmenopausal women. Clinics. 2007;62(5):553–60. doi: 10.1590/s1807-59322007000500004. [DOI] [PubMed] [Google Scholar]
- 30.Karim R, Stanczyk FZ, Hodis HN, Cushman M, Lobo RA, Hwang J, et al. Associations between markers of inflammation and physiological and pharmacological levels of circulating sex hormones in postmenopausal women. Menopause. 2010;17(4):785–90. [PMC free article] [PubMed] [Google Scholar]
- 31.Patel D, Jhamnani S, Ahmad S, Silverman A, Lindsay J. Discordant association of C-reactive protein with clinical events and coronary luminal narrowing in postmenopausal women: data from the Women's Angiographic Vitamin and Estrogen (WAVE) study. Clin Cardiol. 2013;36(9):535–41. doi: 10.1002/clc.22155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cushman M, Legault C, Barrett-Connor E, Stefanick ML, Kessler C, Judd HL, et al. Effect of Postmenopausal Hormones on Inflammation-Sensitive Proteins. Circulation. 1999;100(7):717–22. doi: 10.1161/01.cir.100.7.717. [DOI] [PubMed] [Google Scholar]
- 33.Van Baal MW, Kenemans P, Van der Moren MJ, Kessel H, Emeis JJ, Stehouwer DAC. Increased C-reactive protein Levels during Short-term Hormone Replacement Therapy in Healthy Postmenopausal Women. Thromb Haemost. 1999;81(6):925–8. [PubMed] [Google Scholar]
- 34.Sattar N, Perera M, Small M, Lumsden MA. Hormone replacement therapy and sensitive C-reactive protein concentrations in women with type-2 diabetes. Lancet. 1997;354(9177):487–8. doi: 10.1016/S0140-6736(99)02079-6. [DOI] [PubMed] [Google Scholar]
- 35.Bulowska H, Stanosz S, Zochowska E, Millo B, Sieja K, Chelstowski K, et al. Does the type of hormone replacement therapy affect lipoprotein (a), homocystein, and C-reactive protein levels in postmenopausal women. Metabolism. 2005;54(1):72–8. doi: 10.1016/j.metabol.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 36.Ridker PM, Hennekens CH, Rifai N, Buring JE, Manson JE. Hormone Replacement Therapy and Increased Plasma Concentration of C-reactive protein. Circulation. 1999;100(7):713–6. doi: 10.1161/01.cir.100.7.713. [DOI] [PubMed] [Google Scholar]
- 37.Cushman M, Legault C, Barrett-Connor E, Stefanick ML, Kessler C, Judd HL, et al. Effect of Postmenopausal Hormones on Inflammation-Sensitive Proteins. Circulation. 1999;100(7):717–22. doi: 10.1161/01.cir.100.7.717. [DOI] [PubMed] [Google Scholar]
- 38.Kwang KK, Schenke WH, Waclawiw MA, Csako G. Combining a statin with oral oestrogen may maximize any cardiovascular benefits in postmenopausal women. Circulation. 2002;105(13):1531–3. doi: 10.1161/01.cir.0000013837.81710.da. [DOI] [PubMed] [Google Scholar]
- 39.Dessapt AL1, Gourdy P. Menopause and cardiovascular risk. J Gynecol Obstet Biol Reprod (Paris) 2012;41(7 Suppl):F13–9. doi: 10.1016/j.jgyn.2012.09.003. [DOI] [PubMed] [Google Scholar]