Abstract
Surveillance involves the collection and analysis of data for the detection and monitoring of threats to public health. Surveillance should also inform as to the epidemiology of the threat and its burden in the population. A further key component of surveillance is the timely feedback of data to stakeholders with a view to generating action aimed at reducing or preventing the public health threat being monitored. Surveillance of antibiotic resistance involves the collection of antibiotic susceptibility test results undertaken by microbiology laboratories on bacteria isolated from clinical samples sent for investigation. Correlation of these data with demographic and clinical data for the patient populations from whom the pathogens were isolated gives insight into the underlying epidemiology and facilitates the formulation of rational interventions aimed at reducing the burden of resistance. This article describes a range of surveillance activities that have been undertaken in the UK over a number of years, together with current interventions being implemented. These activities are not only of national importance but form part of the international response to the global threat posed by antibiotic resistance.
Keywords: antibiotic susceptibility testing, public health threat, interventions
1. Background
Antibiotic resistance poses a major threat to clinical medicine and public health, not only in the UK but internationally [1–3]. Antibiotics are not only essential for the treatment of classical infections such as bacterial pneumonia, sepsis or meningitis, tuberculosis (TB) or gonorrhoea, but also opportunistic infections that may occur in patients predisposed to infection, particularly in hospital. It is an interesting paradox that many advances in medical care have as an unintended consequence, the fact that patients become more prone to contracting infections, often caused by pathogens of low virulence that pose little threat to healthy people. For example, oncology patients may suffer bouts of neutropenia due to the cytotoxic drugs they receive as part of their cancer therapy, whereas organ transplant recipients are immunosuppressed and hence less able to fight off infection due to the drugs they receive to prevent organ rejection. Similarly, many medical procedures such as insertion of intravascular or urinary catheters, intubation or surgery breach the body's natural barriers to infection and allow pathogens direct access to sites such as the bloodstream, urinary tract, lung or abdominal cavity. As an added complication, patients requiring medical care are clustered together in hospital wards, with the result that individual patients who develop infections readily serve as a source of cross-infection to other patients. Hence, many aspects of modern medical care rely on the therapeutic or prophylactic use of antibiotics to minimize the morbidity and mortality associated with opportunistic healthcare-associated infections. The emergence and widespread occurrence of bacteria that are resistant to antibiotics thus threatens not only the treatment of common bacterial infectious diseases but also the management of patients in diverse clinical settings.
2. The need for surveillance of antibiotic resistance
Successful treatment of serious infections requires timely administration of effective chemotherapeutic agents. While some infections (e.g. TB, whooping cough and gonorrhoea) are caused by a single pathogen, the majority of infections, such as those affecting the skin and soft tissues, the upper and lower respiratory tracts, the urinary tract, meningitis and sepsis are caused by a range of pathogens. Hence, clinical decisions about empirical treatment require knowledge of the likely pathogen(s) and the likely susceptibility of these pathogens to antibiotics. Such knowledge is gained in part by clinical experience over time, but more objectively and robustly through surveillance. The US Centers for Disease Control and Prevention (CDC) have defined surveillance as ‘The on-going systematic collection, analysis and interpretation of health data essential to the planning, implementation and evaluation of public health practice, closely integrated with the timely dissemination of these data to those who need to know’ [4, p. 164]. Put more succinctly, surveillance is the generation and timely provision of information to inform decision-making and action. Implicit in these definitions is the fact that undertaking surveillance requires a readily available source of data. For surveillance of antibiotic resistance, the essential core data are generated by microbiology laboratories that routinely identify and determine the susceptibility or resistance of bacteria isolated from clinical specimens. These results are stored in the laboratory computer system and if accessed, collected and analysed, can inform as to the degree of antibiotic resistance seen in different bacterial species or isolates from different types of infection. Changes or variation in antibiotic resistance either geographically or over time can also be monitored. The remainder of this article will focus primarily on examples of surveillance activities undertaken in the UK, and in particular in England, although comparison with data from other parts of the world will be made, as the problem of resistance is one of global dimensions [2].
3. Surveillance of antibiotic resistance in England
The voluntary reporting of microbiological diagnoses by hospital laboratories to Public Health England (PHE) and its predecessors, the Health Protection Agency and the Public Health Laboratory Service, has been a mainstay of infectious disease surveillance in England for many decades. Since 1989, clinical microbiologists working in laboratories in England have been encouraged to report both the identification and antibiotic susceptibility of blood culture isolates to a national database called LabBase2. Initially, reporting was on paper forms but moved to electronic transmission of results in the 1990s [5,6]. The outputs from this surveillance system have tended to focus on national trends in resistance in common pathogens including Staphylococcus aureus, enterococci, Streptococcus pneumoniae, Escherichia coli, Klebsiella pneumoniae, Enterobacter spp. and Pseudomonas aeruginosa [7–13]. Strengths of this surveillance system include wide geographical coverage, the large amount of data collected and the fact that the data are readily available on a continuous basis as routine outputs from laboratories. There are some weaknesses, however, including incomplete data collection as reporting is done on a voluntary basis, variation in laboratory testing methods and the fact that different laboratories may test and report on different antibiotic panels for the same pathogens. With regard to the variation in testing methods, it should be borne in mind, however, that all hospital microbiology laboratories in England must be accredited and that a requirement of accreditation is participation in the assessment scheme run by the UK National External Quality Assessment Service. As part of this scheme, pathogens with defined antibiotic resistance profiles are distributed to laboratories, which must blindly test the isolates and report back their findings. Laboratories that report incorrect results are notified so that they may take remedial action to improve the quality of their testing methodology [14]. Another consideration is that the majority of the data from microbiology laboratories are for isolates from hospitalized patients, as the on-site availability of pathology services means that infections in such patients can be readily investigated. By contrast, infections in the community are commonly treated empirically, with general practitioners (GPs) tending to limit microbiological investigations to more complex patients or those failing antibiotic treatment. As a likely cause of treatment failure may be antibiotic resistance, analysis of test results for isolates referred for investigation by GPs may over-estimate the burden of resistance due to sample bias. Despite these caveats, susceptibility data routinely generated by hospital microbiology laboratories are an informative, valuable and cost-effective source of information, with such surveillance being undertaken not only in the UK, but in many other parts of the world [3,15].
In addition to the collection of routinely generated laboratory results, surveillance systems have also been established that involve the collection of bacterial isolates from sentinel laboratories for testing in a centralized facility, typically a national Reference Laboratory. In the UK, two such schemes, one for respiratory isolates, the other for bacteraemia isolates, are sponsored by the British Society for Antimicrobial Chemotherapy (BSAC) and have been running continuously since 1999 and 2001, respectively [16]. In addition, since 2000, PHE has undertaken surveillance of resistance in Neisseria gonorrhoeae via the Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP), where sentinel genitourinary clinics submit isolates for centralized susceptibility during a three-month period every summer [17]. In contrast to most of the above surveillance schemes that collect data continuously, GRASP is an example of point prevalence surveillance, as data are collected for a fixed time period each year. Regular temporal trend analysis can still however be undertaken as surveillance is repeated each year. Point prevalence surveys can also be undertaken at longer time intervals, an example being two national point prevalence surveys of resistance in S. pneumoniae that were undertaken in 1990 and 1995 by the Public Health Laboratory Service [18]. In these two surveys, all pneumococci isolated during the same two calendar weeks in each survey year were collected from a national network of 52 microbiology laboratories and tested in the national Reference Laboratory. The use of an identical study design in the two surveys meant that the change in the susceptibility of pneumococci over the 5-year period could be assessed. Although these sentinel schemes which involve collection of isolates generally produce smaller datasets than schemes that collect routinely generated test results, they have the advantage that all isolates are tested using the same methods and standardized panels of antibiotics and that resistant isolates can be investigated with regard to the mechanisms of resistance and strain distribution. The two approaches to surveillance should of course be viewed as being complementary to each other, and it is reassuring to note that for bacteraemia, cross tabulation of the data obtained by centralized testing of referred isolates with routinely generated antimicrobial susceptibility test data obtained from hospital laboratories has shown a good correlation in terms of the trends in resistance in individual pathogens [8–11,13].
To provide more detailed insight into the sort of data provided by these national surveillance schemes, two examples, one with a Gram-positive pathogen (S. aureus) and one with Gram-negative pathogens (E. coli and other Enterobacteriaceae) follow below. For both groups of pathogens, the role of strain typing in enhancing our understanding of the underlying epidemiology is also discussed.
(a). Surveillance of resistance in Staphylococcus aureus
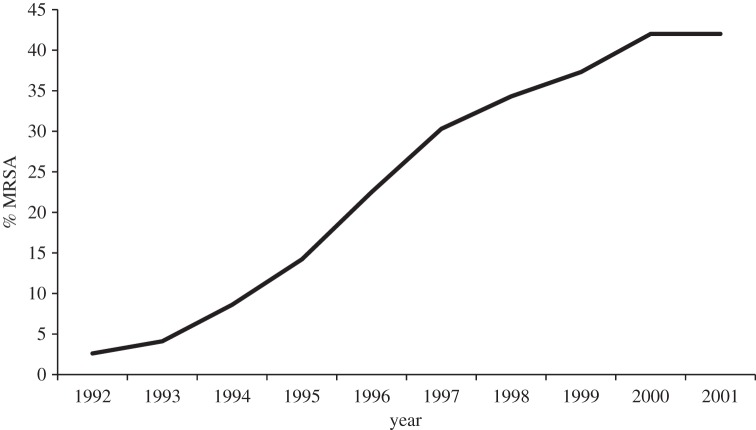
Analysis of blood culture isolates of S. aureus voluntarily reported to LabBase2 showed a dramatic year-on-year increase in the proportion of isolates that were methicillin-resistant during the 1990s, rising from 2% in 1990 to a peak of 43% in 2001 (figure 1) [19]. The problem appeared widespread with increases in methicillin-resistant S. aureus (MRSA) noted in all health regions in England, as well as in Wales and Northern Ireland. In addition, data from the Scottish Centre for Infection and Environmental Health (now part of Health Protection Scotland), indicated a similar situation in Scotland, with 41% of S. aureus being methicillin-resistant in 2000–2001 [19].
Figure 1.
Proportion of Staphylococcus aureus isolated from blood that were methicillin-resistant (MRSA) in England and Wales in 1992–2001.
The dramatic rise in MRSA generated considerable public and media interest, with the result that MRSA became the subject of political debate, with opposition politicians criticizing the government of the day regarding their management of the health service [20]. By way of response, in 2001, the Department of Health in England made a reduction in rates of MRSA a public health priority. It is noteworthy that one of their first actions was to improve the robustness of surveillance by making the reporting of MRSA bacteraemia mandatory for all English acute hospital Trusts [21], which serves to underline the crucial role of surveillance in efforts to improve public health.
An essential component of surveillance is the feedback of analysed data to relevant stakeholders. Hence, the Health Protection Agency (and subsequently PHE) was tasked from the outset with reporting rates of MRSA bacteraemia for individual hospital Trusts (expressed as total cases per 1000 occupied bed days) [21]. A subsequent enhancement of the mandatory surveillance programme required Trusts to report the number of cases where MRSA bacteraemia was likely to have been acquired while patients were in their care, based on positive blood culture being taken more than or equal to 2 days after the date of admission [21].
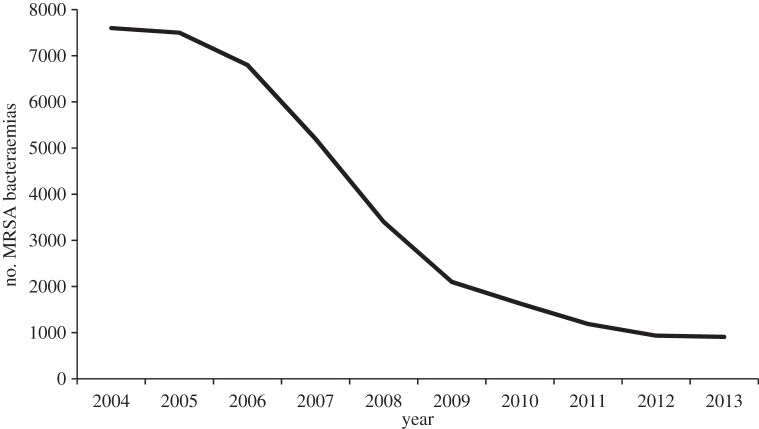
Surveillance is commonly referred to as information for action, and having established a surveillance system for monitoring rates of MRSA bacteraemia in hospital Trusts, the government announced in late 2004 that it was setting Trusts a target of reducing these infections by 50% by 2008 [22]. Although there were some doubts that such a reduction in MRSA rates could be achieved in the designated timescale [23], national data from the mandatory surveillance scheme showed a 56% reduction in the number of reports of MRSA bacteraemia between 2004 and 2008 (figure 2) [24]. Moreover, it is noteworthy that the decline has continued since 2008 with 862 cases reported in the financial year 2013/2014, reflecting an overall reduction of 80.6% from the number of cases (4451) reported in 2007–2008 [25]. Despite this success in reducing the numbers of MRSA bacteraemias, the government has continued to pursue a policy of zero tolerance towards MRSA, with the introduction in April 2013 of Post Infection Reviews for all cases of MRSA bacteraemia, the objective being to identify why an infection occurred and to learn how future cases of infection can be avoided [26,27].
Figure 2.
Cases of MRSA bacteraemia in England reported via the mandatory surveillance scheme between 2004 and 2013.
(i). Enhanced surveillance of methicillin-resistant Staphylococcus aureus bacteraemia in children
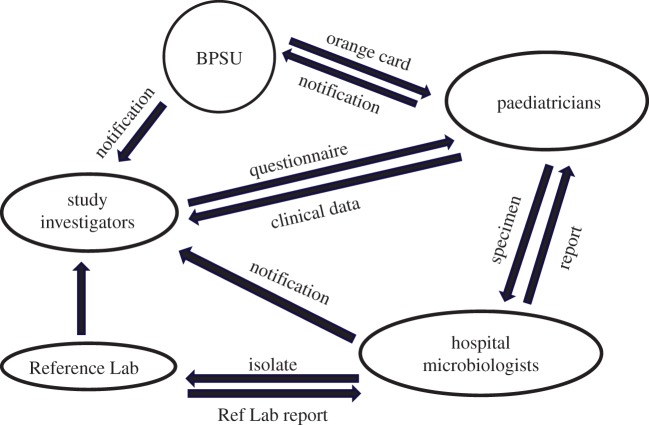
Although MRSA bacteraemia is seen predominantly in older patients, analysis of data in LabBase2 showed an increase in the proportion of such infections that occurred in children between 1990 and 2001 [28]. This trend was viewed with concern by the paediatric subgroup of the Specialist Advisory Committee on Antimicrobial Resistance, whose role was to advise the Chief Medical Officer and government ministers on problems relating to antibiotic resistance [29]. To investigate this issue further, a 2-year programme of enhanced surveillance covering the UK and Ireland was initiated. In this study, which pre-dated the introduction of mandatory surveillance in England, cases of paediatric MRSA bacteraemia were not only ascertained through voluntary laboratory reporting but also through a system of clinical case reporting implemented by the British Paediatric Surveillance Unit (BPSU) via the monthly ‘orange card’ reporting system [30]. This involved requesting paediatricians in the UK and Ireland to report any children with MRSA bacteraemia to the BPSU, with reporting physicians then being sent a questionnaire regarding the patient's demographics and clinical presentation (figure 3) [31]. In addition, MRSA isolates were collected and characterized using molecular and phenotypic techniques.
Figure 3.
Design of enhanced surveillance for cases of MRSA bacteraemia in children. BPSU, British Paediatric Surveillance Unit.
Overall, the study found a low incidence of MRSA bacteraemia in children in the UK (one case per 100 000 children), with little inter-country variation [31]. Paediatric infections were predominantly seen in very young children, often those receiving neonatal or paediatric intensive care. This was subsequently confirmed when mandatory surveillance of MRSA bacteraemia was introduced in England, with only 2% of MRSA bloodstream infections involving children [32]. Molecular investigation of the clinical isolates showed that MRSA bacteraemia in children predominantly involved the well-documented epidemic strains associated with nosocomial infection (described below).
Finally, an important aspect of surveillance is dissemination of accurate information. However, as shown here, this is not always a straightforward process. Although this study found a low incidence of MRSA bacteraemia in children, it is noteworthy that its findings came to the attention of the media, with one national newspaper reporting paediatric MRSA cases as being ‘out of control’ [33].
(ii). Molecular investigation of methicillin-resistant Staphylococcus aureus strains
Complimentary to the surveillance activities outlined above, typing of MRSA isolates has yielded considerable insight into the epidemiology of MRSA infections by elucidating the dynamics of strain transmission. Typing methods have evolved over the years and have moved from phenotypic tests such as phage typing and biochemical profiling to molecular techniques, including multi-locus sequence typing (MLST), spa typing and, most recently, whole genome sequencing (WGS). A finding of particular importance from analysis of MRSA isolates submitted to the national Reference Laboratory was that the year-on-year increase in MRSA among S. aureus isolated from blood during the 1990s coincided with the emergence and spread of two particular epidemic MRSA (EMRSA) strains, designated EMRSA-15 and EMRSA-16, which were subsequently shown by MLST to belong to sequence types (ST) ST22 and ST36, respectively [34,35]. To investigate the epidemiology of MRSA strains in a more structured way, blood culture isolates of MRSA were collected from 26 geographically diverse hospital laboratories between late 1998 and the second quarter of 2000. Analysis of 591 MRSA isolates showed that these two epidemic strains accounted for 95.6% of the isolates; 60.2% were EMRSA-15 and 35.4% were EMRSA-16, with EMRSA-15 found in 25 of the 26 participating hospitals and EMRSA-16 found in 19 [36]. At present, the biological basis underpinning the epidemic potential of EMRSA-15/16 is not understood, although some risk factors for colonization or infection with these strains have been documented. Phenotypically, isolates of both strains were commonly resistant not only to β-lactams but also to ciprofloxacin and macrolides. Investigation of the spread of MRSA in one hospital affected by these epidemic strains showed that exposure of patients to these antibiotics was a risk factor for subsequent colonization or infection [37]. Related to this, pharmacokinetic studies have shown that ciprofloxacin is excreted in sweat onto the skin where it may act to eliminate quinolone-susceptible components of the normal bacterial skin microflora [38]. The resulting reduction in competing skin flora may facilitate the colonization of skin by quinolone-resistant MRSA such as EMRSA-15/16. Further, laboratory studies have shown that exposure of quinolone-resistant isolates of MRSA to sub-inhibitory concentrations of ciprofloxacin results in induction of fibronectin-binding proteins that may result in increased binding to fibronectin in host tissues, and thus promote colonization [39].
Intriguingly, during the 2000s when the rates of MRSA bacteraemia stabilized and then underwent a significant decline, typing of isolates showed that there was a disproportionate decline in isolates of EMRSA16 [40,41]. This led some investigators to argue that it is difficult to determine how much of the decline in MRSA was due to infection control measures versus intrinsic (albeit currently undefined) biological properties of the MRSA strains and that randomized or nested study designs rather than interrupted time-series studies may be better to elucidate the effectiveness of interventions based on improved infection control [42].
(b). Surveillance of resistance in Escherichia coli and other Gram-negative bacteria
The last 50 years have seen the continuous development of resistance to a succession of first-line antibiotics in E. coli. Ampicillin, the first broad-spectrum β-lactam antibiotic with activity encompassing Gram-negative bacteria was introduced in 1961. However, this was followed just 4 years later by the report of an ampicillin-resistant E. coli isolated from the blood of a patient in Greece, the resistance being mediated by production of a β-lactamase enzyme designated TEM-1 (derived from the patient's name, Temoniera) [43]. This was not entirely a surprise as the existence of β-lactamases had been identified some 25 years earlier, with the first β-lactamase in fact having been found in an isolate of E. coli in 1940 [44]. What was striking about the clinical isolate from Greece however, was that the resistance was transferable (due to the resistance gene being located on a mobile plasmid) not only between strains of E. coli but also to other Enterobacteriaceae as well as Haemophilus influenzae, N. gonorrhoeae and P. aeruginosa [45–47]. In the early 1970s, the occurrence of ampicillin resistance due to TEM-1 in H. influenzae type b, which at that time was a significant cause of bacterial meningitis, was a major public health concern, as was the adverse impact of TEM-1 on the use of penicillin for the treatment of gonorrhoea. Over subsequent decades, new classes of antibiotics that were active against ampicillin-resistant E. coli and other bacteria were discovered and introduced into clinical use, but in all cases, this was followed by emergence of resistance.
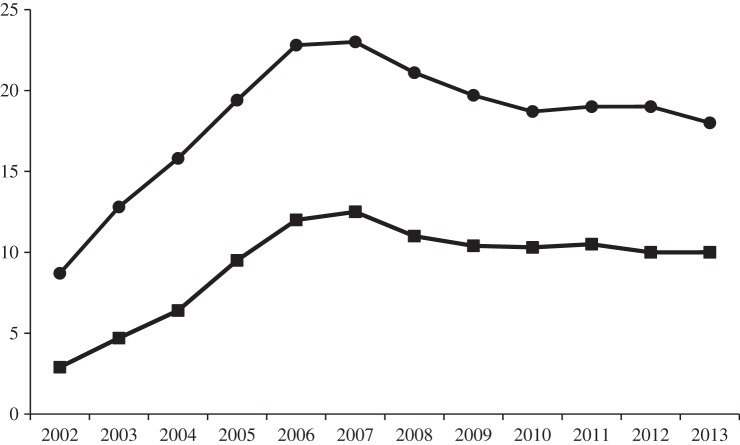
Probably the two most clinically important classes of antibiotics with good activity against E. coli and other genera of Gram-negative bacteria (including strains resistant to ampicillin) were the quinolones, notably ciprofloxacin, and the third-generation cephalosporins, cefotaxime and ceftazidime, which although β-lactams, were resistant to hydrolysis by TEM-1 β-lactamase. However, analysis of national surveillance data reported to LabBase2 by hospital microbiology laboratories during the 1990s and 2000s showed that there were year-on-year increases in the proportion of blood culture isolates of E. coli that were resistant to ciprofloxacin, rising from approximately 1% in 1993 to a peak of 23% in 2006–2007 (figure 4) [13,48,49]. A similar but lower rising trend in resistance to third-generation cephalosporins was also seen over the same time period (approx. 1% in 1993 rising to a peak of 12.5% in 2006–2007). Thereafter, following a slight decline, rates of resistance to ciprofloxacin and third-generation cephalosporins stabilized at approximately 19% and approximately 10%, respectively. These temporal trends were broadly mirrored by data from the national sentinel bacteraemia surveillance programme sponsored by the BSAC [13]. Interestingly, both the LabBase2 data and the sentinel bacteraemia surveillance data showed a similar temporal trend for resistance in Klebsiella and Enterobacter spp. [13]. In Klebsiella spp., resistance to ciprofloxacin and third-generation cephalosporins both peaked at approximately 17% in 2006, and following a decline, stabilized at 8–10% between 2009 and 2013 [50]. In Enterobacter spp., ciprofloxacin resistance fluctuated between 14% and 17% between 2001 and 2006, then fell steadily to 5% by 2012, while cephalosporin resistance peaked at approximately 44% in 2006, then declined to 29% in 2012 [51]. The higher levels of cephalosporin resistance in Enterobacter spp., relative to E. coli and Klebsiella spp., reflects the well-recognized ability of enterobacters to mutate to constitutive production of ampC β-lactamase [52]. It has been suggested that these temporal resistance trends reflect decreased selective pressure for resistant strains following a reduction in hospital and community prescribing of quinolones and cephalosporins, probably due to concerns about the use of these antibiotics promoting infections with Clostridium difficile [13]. It is important to note, however, that although the proportions of isolates of E. coli and K. pneumoniae from blood that were resistant to ciprofloxacin or cephalosporins remained relatively stable between 2010 and 2013, the increasing incidence of bacteraemia caused by these pathogens during this time meant that the total numbers of resistant isolates nonetheless continued to increase [53].
Figure 4.
The proportion of E. coli isolated from blood in England, Wales and Northern Ireland that were resistant to ciprofloxacin (circles) and third-generation cephalosporins (squares) during 2002 to 2013.
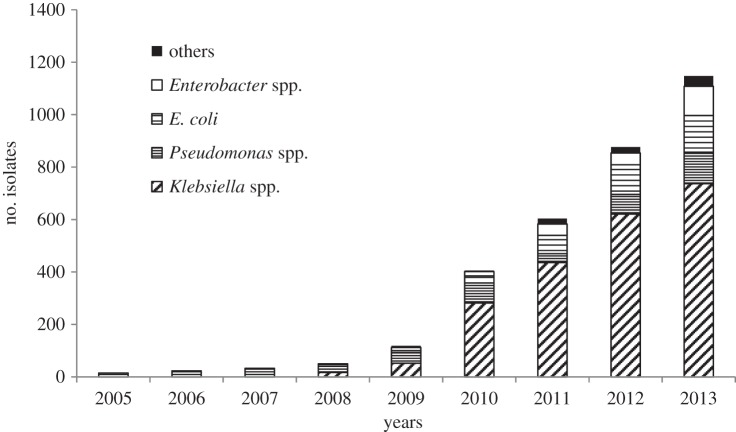
Although rates of resistance to ciprofloxacin and third-generation cephalosporins have stabilized in recent years, the levels of resistance are such that clinicians remain reluctant to use these agents for empirical treatment of suspected Gram-negative sepsis. Hence, they have looked to other antibiotic classes, in particular the carbapenems, to fill this role. Carbapenems are broad-spectrum β-lactams, now commonly referred to as our last resort antibiotics for the treatment of severe infections such as life-threatening sepsis. A national point prevalence survey of healthcare-associated infections and antimicrobial use carried out in England in 2011 showed that meropenem was the ninth most commonly used antibiotic in hospitals in England [54]. A subsequent analysis of trends in antibiotic consumption in England showed that although carbapenems accounted for only 0.3% of total antibiotic consumption in 2013, their use had increased by 31% compared with that seen in 2010 [53]. Given the increased use of carbapenems, there is concern that the associated selective pressure will serve to promote the emergence and spread of carbapenem resistance, particularly in Gram-negative bacteria. These concerns are not limited to the UK as the CDC has highlighted carbapenem resistance as a major threat to healthcare in the USA [3]. Current UK surveillance data have shown that the majority of bloodstream isolates of E. coli and K. pneumoniae remain susceptible to meropenem, with resistance being seen in 0.1% and 0.9%, respectively in 2013 [49,50]. However, there are clear signs that resistance is nonetheless emerging, with the number of isolates of Gram-negative bacteria submitted to the national Reference Laboratory that have been confirmed as carbapenemase producers showing a dramatic increase from between 2005 and 2013 (figure 5). The continuing surveillance of carbapenem resistance in Gram-negative bacteria, particularly Klebsiella spp. and E. coli has been highlighted as a priority area in the UK strategic plan [55].
Figure 5.
Isolates of Gram-negative bacteria confirmed as carbapenemase producers by the Antimicrobial Resistance and Healthcare Associated Infections Reference Unit of PHE, between 2005 and 2013.
(i). Molecular investigation of resistance in Gram-negative bacteria
Molecular studies have shed light on both the underlying mechanisms of resistance, particularly to cephalosporins and carbapenems, and the diversity of strains showing such resistance. Investigation of a number of outbreaks in UK hospitals during the 1990s showed that cephalosporin resistance was for the most part seen in different strains of K. pneumoniae producing different types of extended-spectrum β-lactamases (ESBLs), predominantly variants of the TEM and SHV classes [56–58]. However, a marked epidemiological change was noted from about 2000 onwards, with cephalosporin resistance increasingly being seen in E. coli producing CTX-M type ESBLs, particularly CTX-M-15 [59–61]. In addition, while ESBL-mediated cephalosporin resistance seen during the 1990s had primarily occurred in hospitals, many of the CTX-M-producing isolates of E. coli were initially obtained from patients in the community [60]. Typing undertaken using pulsed-field gel electrophoresis (PFGE) showed that over a third of the isolates of CTX-M-producing E. coli referred to the national Reference Laboratory from hospitals around the UK during 2003 and early 2004 were highly genetically related. These isolates, which were from six different hospitals and a variety of clinical sources (urine, blood, faeces, sputa and wounds), all produced CTX-M-15 and were believed to comprise a single epidemic strain, designated strain A. Four other major PFGE clusters of related E. coli isolates producing CTX-M-15 were also noted and were designated B to E. Representative isolates of each of the five clusters belonged to the same serotype (O25) and given their close relationship by PFGE (78% similarity), the study investigators suggested they may have had a common ancestry [60]. Subsequent analysis using MLST showed that strains A–E all belonged to an extremely successful clone of E. coli of ST131 which is now known to have disseminated globally [62,63]. It is also of note that E. coli ST131 is commonly resistant to quinolones and was a likely contributor to the increase in ciprofloxacin resistance seen in E. coli during the 2000s [63]. Hence the increase in resistance to ciprofloxacin and third-generation cephalosporins in E. coli during this time is probably explained in large part by clonal expansion following the emergence of ST131 in the UK. The dynamics of spread still require further investigation as available data suggest a complex epidemiology not only involving hospitalized patients but also patients in nursing homes and international travellers returning to the UK, as well as possible water-borne transmission [64–66]. As an added complexity, plasmid-mediated spread of CTX-M ESBLs has also been demonstrated [67], although the contribution of this means of spread of cephalosporin resistance is difficult to quantify.
Although isolates of Gram-negative bacteria that produce ESBLs may also show some degree of reduced susceptibility to carbapenems if they also have reduced permeability due to loss of outer membrane porins [68], the major mechanism of resistance to carbapenems involves the production of carbapenem-hydrolysing β-lactamases called carbapenemases. A range of carbapenemases have been identified to date and include representatives of Ambler β-lactamase classes A, B (metallo-enzymes) and D (OXA enzymes) [69]. In terms of the carbapenemases seen in the UK, analysis of carbapenemase-producing Gram-negative isolates referred on a voluntary basis to the national Reference Laboratory (figure 5) shows that a range of enzymes have been seen including NDM-1, KPC, IMP, VIM and OXA-48 [70,71].
4. Making use of surveillance data
Surveillance of antibiotic resistance plays a major role in patient management by providing data that influence clinical decision-making, particularly the choice of antibiotics to be used both for empirical treatment of patients with suspected infections or for prophylaxis in patients at enhanced risk of infection. Commonly, data on rates of resistance in specific pathogens contribute to the evidence base used for formulation of national treatment guidelines for different types of infections. For example, while guidelines for empirical treatment of suspected staphylococcal endocarditis published in the mid-1980s recommended a combination of penicillin, flucloxacillin and gentamicin [72], subsequent updated guidelines have recommended the inclusion of vancomycin in recognition of the increase in MRSA seen since the initial guidance was published [73,74]. Similarly, guidelines for the management of community-acquired pneumonia in adults, published by the British Thoracic Society, specifically took account of rates of resistance of pneumococci (the commonest cause of community-acquired pneumonia) to various antibiotics [75]. Surveillance of susceptibility of particular pathogens has also been used to evaluate the continuing appropriateness of published guidelines for management of infections in particular patient groups, for example neonates presenting with sepsis [76]. In some instances, detection of rapid changes in resistance of pathogens to critical antibiotics has resulted in rapid changes to national treatment guidelines, a key example being the change in the national guidance for the treatment of gonorrhoea from ciprofloxacin to either oral cefixime or intra-muscular ceftriaxone following a marked increase in ciprofloxacin resistance in gonococci [77,78]. Following reports of isolation of gonococci with reduced susceptibility to cefixime and ceftriaxone, the guidelines were subsequently further amended, with a recommendation to use a higher dose of ceftriaxone combined with azithromycin [79].
Surveillance also has a key role to play in detecting the emergence and spread of previously uncommon or completely novel types of resistance. Surveillance should provide information for action, and in the UK a national alert system has been established for notifying clinical microbiologists of the emergence of new types of antibiotic resistance. Alerts typically describe the nature of the resistance, the extent of its known spread (if any) and how the resistance can be detected in diagnostic laboratories. Alerts issued to date have highlighted carbapenem-resistant Enterobacteriaceae (2005), high-level azithromycin resistance in N. gonorrhoeae (2008), carbapenemase-producing Enterobacteriaceae in the UK (2009), New Delhi metallo-β-lactamase imported into the UK from the Indian sub-continent (2009) and potentially transferable linezolid resistance in Enterococcus faecium in the UK (2012) [80]. In addition, detailed guidance and advice on tackling specific antibiotic resistance problems have been produced, a recent example being the production and dissemination of an acute Trust toolkit for the early detection, management and control of carbapenemase-producing Enterobacteriaceae [81]. In order to maximize awareness of both the problem posed by carbapenem-resistant bacteria and availability of the toolkit, a letter was sent to the CEOs of all acute care Trusts in England by the Director of Health Protection at PHE and the NHS England Medical Director, with the message being reinforced by NHS England who additionally issued a related national Patient Safety Alert [82].
Two crucial and inter-related roles of surveillance are to enhance our understanding of the epidemiology of resistance and the factors that influence its emergence and spread, with a view to devising interventions aimed at reducing its burden, and then to assess the effectiveness of interventions by monitoring rates of resistance following their implementation. In 2013, the Department of Health on behalf of the UK government published a 5-year strategic plan comprising seven areas for action aimed at controlling and ideally reducing the burden of resistance (figure 6) [55]. It is noteworthy that the Department of Health subsequently issued a further document outlining a number of measures to be used to assess the effectiveness of the strategy [83]. These included: (i) the list of key human and veterinary pathogens to be monitored for changes in resistance to critical antibiotics; (ii) monitoring the levels of antibiotic usage in humans and animals, both in terms of total usage and use of specific antibiotic classes over time; (iii) monitoring unintended consequences of reduced antibiotic prescribing, such as increases in hospital admissions for suppurative complications of upper respiratory tract infections; (iv) assessing the attitudes and knowledge of the public towards appropriate antibiotic use; (v) assessing the level of professional engagement of healthcare professionals by monitoring the uptake of the ‘TARGET Antibiotics' toolkit by GPs and the ‘Start Smart Then Focus' toolkit by hospital doctors, as well as an annual evaluation of the impact of European Antibiotic Awareness Day activities in the UK; and (vi) reviewing the actions taken by the UK to garner increased international collaboration to minimize the global spread of AMR. Monitoring of a number of these outcome measures is being undertaken as part of the English Surveillance Programme for Antimicrobial Utilisation and Resistance [53].
Figure 6.

The seven key areas for action outlined in the UK 5-year Antimicrobial Resistance (AMR) Strategy.
5. Current and future developments in surveillance
(a). Data linkage
One of the limitations of surveillance based on collection of routinely generated antibiotic susceptibility test results from hospital microbiology laboratories is that although basic patient demographic data are commonly available, other important data such as clinical details, dates of hospital admission and/or discharge, treatment received and clinical outcomes are often not available. Although such data can be collected through the provision of paper or online questionnaires, this approach is labour intensive and often requires hard-pressed hospital staff to spend time and effort trawling through patient case notes or other records and then manually enter the data. An alternative tactic that is increasingly being recognized as an important and cost-effective approach involves linkage of routine microbiology data with other existing datasets that contain relevant data [84]. At an international level, data on antibiotic prescribing across Europe produced by the European Surveillance of Antimicrobial Consumption Network (ESACNet) have been cross tabulated with rates of antibiotic resistance in different European countries produced by the European Antimicrobial Resistance Surveillance Network (EARSNet), with this ecological analysis showing a strong correlation between high levels of prescribing and high levels of resistance [85]. Datasets available in the UK that have been used for data linkage to investigate the epidemiology of antibiotic resistance, healthcare-associated infections and clinical outcomes include national Hospital Episode Statistics (HES) [86,87], death registrations from the Office of National Statistics [88] and clinical data from the Paediatric Intensive Care Audit Network (PICANet) [89]. In contrast to the ecological approach used by ESACNet and EARSNet, which can show strong correlations between data, but cannot prove a causal relationship, efforts are increasingly being made to link individual patient records in different datasets to provide large integrated patient-level datasets. Application of this approach of course requires stringent information governance to ensure there are no breaches of patient confidentiality or that surveillance outputs do not allow deductive disclosure of patient identity. However, once appropriate governance is put in place, this approach allows important public health issues such as antibiotic resistance to be investigated in a cost-effective manner that should yield more robust and informative data than are obtained from current surveillance systems.
In a pilot study to investigate the incidence and aetiology of hospital-acquired bloodstream infections in children and the antibiotic resistance of the causative pathogens, probabilistic matching methods were used to link records for children with bacteraemia stored in LabBase2 with hospital admission data from HES, which contains demographic and clinical data, dates of hospital admission and discharge and in-hospital mortality with date of death [86]. This study established that about 23% of paediatric bacteraemias were hospital-acquired, equating to a rate of 4.74 infections per 1000 hospital admissions lasting 2 or more days. It also provided important insight into the pathogens associated with hospital-acquired infections and their susceptibility to recommended first-line antibiotics. A subsequent study using the same linked dataset quantified the increased length of stay and excess mortality associated with hospital-acquired bacteraemia [87]. A complementary approach involving linkage of LabBase2 data with clinical and demographic data from PICANet has also been used for monitoring of risk-adjusted bacteraemia trends in paediatric intensive care [89]. Having established the methodology using data for paediatric patients, further work could be undertaken with other patient populations.
(b). Local surveillance
The systems for undertaking surveillance of antibiotic resistance outlined in this article have frequently focused on the national picture. In keeping with one of the action points in the UK 5-year strategy for controlling resistance, namely better access to and use of surveillance data [55], systems are being put in place in England to enhance local and regional access to resistance data. At the time of writing, PHE is launching a new surveillance system and database called Second Generation Surveillance System (SGSS), which will incorporate previously developed resistance surveillance software called amsurv, together with an interactive web tool called AmWeb [90,91]. This system will allow microbiology laboratories that submit data to SGSS timely access to their data using user-configurable reporting tools that will allow a range of outputs that will meet the needs of their local communities.
(c). Using whole-genome sequencing to investigate transmission of resistant bacteria
Another key action point in the UK 5-year strategy for controlling antibiotic resistance involves improving infection prevention and control practices [55]. The rational design of interventions to reduce the incidence of infections caused by resistant bacteria requires a detailed understanding of the mode and dynamics of pathogen spread. As outlined earlier in this article, the application of molecular techniques such as MLST to characterize the relatedness of clinical isolates of bacterial pathogens has led to the detection and recognition of successful epidemic clones of resistant bacteria, such as EMRSA-15/16 [35] and the ST131 clone of E. coli [62,63]. It is becoming increasingly clear, however, that the discriminatory power of WGS to differentiate between epidemiologically related and unrelated isolates within these clones is greatly enhancing our ability to investigate clusters and putative outbreaks of antibiotic-resistant infections. For example, using conventional outbreak investigation methods, a hospital infection control team noted a putative outbreak of MRSA on a special care baby unit (SCBU), where 12 infants colonized with MRSA over a six-month period were suspected but not categorically proved to be linked. WGS analysis of MRSA isolates from the SCBU and elsewhere not only confirmed that the cluster did indeed comprise an outbreak, but that it was more extensive than originally envisioned, with transmission of the outbreak strain between mothers on a postnatal ward and in the community also being found [92]. Conversely, in another study, apparent instances of patient-to-patient transmission of MRSA in an adult intensive care unit identified by spa typing of isolates from patients with overlapping stays were found to have been incorrectly identified when subsequent WGS analysis showed that the isolates from ‘epidemiologically linked’ patients were in fact genetically distinct [93].
Understanding the epidemiology of colonization and infection with antibiotic-resistant Gram-negative bacteria is particularly challenging as the spread of resistance is not limited to the transmission of bacterial cells but may also involve the inter-species spread of the genes encoding resistance on mobile genetic elements such as plasmids. However, studies are already being published showing that WGS has the potential to unravel the complex epidemiology of such infections, including the emerging and critically important problem of infections caused by carbapenem-resistant organisms [94,95]. With the cost of WGS falling, some investigators have argued that this methodology has the potential to transform diagnostic and public health microbiology and is now positioned to become an essential tool in the control of antibiotic resistance [96,97].
(d). Surveillance of the impact of antibiotic resistance on morbidity
Analysis of data on patients hospitalized with bloodstream infections has shown that antibiotic resistance, for example methicillin resistance in S. aureus and cephalosporin resistance in E. coli, are associated with increased morbidity, manifest as extended lengths of hospital stay, as well as increased mortality [98]. However, while the clinical impact of resistance in the hospital setting is well documented, there is a relative paucity of data on the impact of resistance on morbidity in patients presenting with infections in the community. The main problem is the lack of routinely available data on the aetiology of community infections and the antibiotic susceptibility or resistance of the causative pathogens, as most infections managed by GPs are treated empirically, based on the clinical history. As the majority of antibiotic prescribing occurs in the community [53], this is an important gap in our knowledge of the epidemiology and public health impact of resistance. The principal barrier to investigating the prevalence of antibiotic resistance in the community and the associated clinical and economic burden is that such studies are extremely resource intensive and thus very expensive. However, such studies are nonetheless feasible, an example being an ongoing clinical trial of a point-of-care test (POCT) to guide the management of uncomplicated urinary tract infection (UTI) in primary care [99]. The POCT in this study is a simple culture procedure that provides both a microbiological diagnosis of bacterial UTI within 24 h and the susceptibility of any identified pathogen to the antibiotics most commonly prescribed for UTI in primary care. The objective is to assess if the test helps GPs to decide more effectively whether or not to prescribe antibiotics, and if so, to select the most appropriate drug. The trial also aims to provide data on patient morbidity including symptoms and rates of recurrence within a three-month period. The trial should also inform as to the prevalence of antibiotic resistance in pathogens isolated from urine (and stool) samples at both initial presentation and at two-week follow-up. In addition, the findings from the study will be analysed to assess if this approach is cost effective, and should this be the case, there would be an argument for its widespread introduction, which would in turn provide a source of routinely available data for ongoing surveillance. With the current impetus to develop rapid diagnostics for detection of antibiotic resistance [100] (which is one of the action points in the 5-year UK strategy for controlling resistance [55]) the development of surveillance systems for monitoring antibiotic resistance in the community is becoming a realistic option. Such surveillance, coupled with collection of data on patient consultations from sources such as the Clinical Practice Research Datalink [101] or the Health Improvement Network [102] and admissions to hospital (from HES) would inform as to trends in resistance, antibiotic prescribing and associated patient morbidity and treatment outcomes.
6. Conclusion
Surveillance programmes not only in the UK, but across the world, have shown that antibiotic resistance is a major threat to global health. Many initiatives are being launched in efforts to reduce, or at least slow down the rate of increase of resistance. Having served to identify the threat posed by antibiotic resistance, existing and new surveillance systems must now be used to assess the effectiveness and impacts of these initiatives and interventions. Timely and targeted dissemination of surveillance data will continue to be an essential component of efforts to combat the threat of resistance. Crucially, dissemination of surveillance data should not be restricted to the scientific and medical community but must include all major stakeholders including the public as well as policy-makers and governments.
Acknowledgements
I thank Neil Woodford, Antimicrobial Resistance and Healthcare Associated Infections Reference Unit, Public Health England, for providing the data shown in figure 5.
References
- 1.Chief Medical Officer. 2013. Chief Medical Officer annual report 2011, volume 2: Infections and the rise of antimicrobial resistance. https://www.gov.uk/government/publications/chief-medical-officer-annual-report-volume-2.
- 2.World Health Organisation. 2012. The evolving threat of antimicrobial resistance: options for action. http://www.who.int/patientsafety/implementation/amr/publication/en/.
- 3.Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States. http://www.cdc.gov/drugresistance/threat-report-2013/.
- 4.Thacker SB, Berkelman RL. 1988. Public health surveillance in the United States. Epidemiol. Rev. 10, 164–190. [DOI] [PubMed] [Google Scholar]
- 5.Henry R. 1996. CoSurv: a regional computing strategy for communicable disease surveillance. PHLS Microbiol. Digest 13, 26–28. [Google Scholar]
- 6.Grant AD, Eke B. 1993. Application of information technology to the laboratory reporting of communicable disease in England and Wales. Commun. Dis. Rep. CDR Rev. 3, R75–R78. [PubMed] [Google Scholar]
- 7.Reacher MH, et al. 2000. Bacteraemia and antibiotic resistance of its pathogens reported in England and Wales between 1990 and 1998: trend analysis. Br. Med. J. 320, 213–216. ( 10.1136/bmj.320.7229.213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hope R, Livermore DM, Brick G, Lillie M, Reynolds R. 2008. Non-susceptibility trends among staphylococci from bacteraemias in the UK and Ireland, 2001–06. J. Antimicrob. Chemother. 62(Suppl. 2), ii65–ii74. ( 10.1093/jac/dkn353) [DOI] [PubMed] [Google Scholar]
- 9.Brown DF, Hope R, Livermore DM, Brick G, Broughton K, George RC, Reynolds R. 2008. Non-susceptibility trends among enterococci and non-pneumococcal streptococci from bacteraemias in the UK and Ireland, 2001–06. J. Antimicrob. Chemother. 62(Suppl. 2), ii75–ii85. ( 10.1093/jac/dkn354) [DOI] [PubMed] [Google Scholar]
- 10.Livermore DM, Hope R, Brick G, Lillie M, Reynolds R. 2008. Non-susceptibility trends among Enterobacteriaceae from bacteraemias in the UK and Ireland, 2001–06. J. Antimicrob. Chemother. 62(Suppl. 2), ii41–ii54. ( 10.1093/jac/dkn351) [DOI] [PubMed] [Google Scholar]
- 11.Livermore DM, Hope R, Brick G, Lillie M, Reynolds R. 2008. Non-susceptibility trends among Pseudomonas aeruginosa and other non-fermentative Gram-negative bacteria from bacteraemias in the UK and Ireland, 2001–06. J. Antimicrob. Chemother. 62(Suppl. 2), ii55–ii63. ( 10.1093/jac/dkn352) [DOI] [PubMed] [Google Scholar]
- 12.Henderson KL, Muller-Pebody B, Blackburn RM, Johnson AP. 2010. Reduction in erythromycin resistance in invasive pneumococci from young children in England and Wales. J. Antimicrob. Chemother. 65, 369–370. ( 10.1093/jac/dkp442) [DOI] [PubMed] [Google Scholar]
- 13.Livermore DM, Hope R, Reynolds R, Blackburn R, Johnson AP, Woodford N. 2013. Declining cephalosporin and fluoroquinolone non-susceptibility among bloodstream Enterobacteriaceae from the UK: links to prescribing change? J. Antimicrob. Chemother. 68, 2667–2674. ( 10.1093/jac/dkt212) [DOI] [PubMed] [Google Scholar]
- 14.United Kingdom National External Quality Assessment Service. See http://www.ukneqas.org.uk/content/PageServer.asp?S=412800196&C=1252&Type=G&ID=62.
- 15.European Centre for Disease Prevention and Control. Antimicrobial resistance and healthcare-associated infections. http://www.ecdc.europa.eu/en/activities/surveillance/EARS-Net/documentsx/Pages/documents.aspx.
- 16.White AR. 2008. The British Society for Antimicrobial Chemotherapy Resistance Surveillance Project: a successful collaborative model. J. Antimicrob. Chemother. 62(Suppl. 2), ii3–ii14. ( 10.1093/jac/dkn348) [DOI] [PubMed] [Google Scholar]
- 17.Public Health England. 2013. Gonococcal resistance to antimicrobials surveillance programme (GRASP) report. https://www.gov.uk/government/publications/gonococcal-resistance-to-antimicrobials-surveillance-programme-grasp-report.
- 18.Johnson AP, Speller DC, George RC, Warner M, Domingue G, Efstratiou A. 1996. Prevalence of antibiotic resistance and serotypes in pneumococci in England and Wales: results of observational surveys in 1990 and 1995. Br. Med. J. 312, 1454–1456. ( 10.1136/bmj.312.7044.1454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson AP, Pearson A, Duckworth G. 2005. Surveillance and epidemiology of MRSA bacteraemia in the UK. J. Antimicrob. Chemother. 56, 455–462. ( 10.1093/jac/dki266) [DOI] [PubMed] [Google Scholar]
- 20.Flynn P. HANSARD 1803–2005 Methicillin-resistant Staphylococcus aureus. Written Answers—March 11, 1997. http://hansard.millbanksystems.com/search/mrsa%20nhs?page=4.
- 21.Johnson AP, Davies J, Guy R, Abernethy JK, Sheridan EA, Pearson A, Duckworth G. 2012. Mandatory surveillance of methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia in England: the first 10 years. J. Antimicrob. Chemother. 67, 802–809. ( 10.1093/jac/dkr561) [DOI] [PubMed] [Google Scholar]
- 22.Health Protection Agency. 2004. Health secretary announces MRSA target. Commun. Dis. Rep. CDR Wkly 14, 46 http://webarchive.nationalarchives.gov.uk/20140714084352/http://www.hpa.org.uk/cdr/archives/2004/cdr4604.pdf. [Google Scholar]
- 23.BBC News. 2007. MRSA target ‘likely to be missed’. http://news.bbc.co.uk/1/hi/health/6249149.stm.
- 24.Pearson A, Chronias A, Murray M. 2009. Voluntary and mandatory surveillance for methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-susceptible S. aureus (MSSA) bacteraemia in England. J. Antimicrob. Chemother. 64(Suppl. 1), i11–i17. ( 10.1093/jac/dkp260) [DOI] [PubMed] [Google Scholar]
- 25.Public Health England. 2014. Annual Epidemiological Commentary: mandatory MRSA, MSSA and E. coli bacteraemia and C. difficile infection data. 2013/14. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/330529/HCAI_mandatory_surveillance_annual_epidemiological_commentary_2013_14.pdf.
- 26.NHS Commissioning Board. 2013. Everyone counts: planning for patients 2013/14. http://www.england.nhs.uk/everyonecounts/.
- 27.NHS England. 2014. Guidance on the reporting and monitoring arrangements and post infection review process for MRSA bloodstream infections from April 2014 (version 2). http://www.england.nhs.uk/wp-content/uploads/2014/04/mrsa-pir-guid-april14.pdf.
- 28.Khairulddin N, Bishop L, Lamagni TL, Sharland M, Duckworth G. 2004. Emergence of methicillin resistant Staphylococcus aureus (MRSA) bacteraemia among children in England and Wales, 1990–2001. Arch. Dis. Child. 89, 378–379. ( 10.1136/adc.2003.028712) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharland M. 2007. The use of antibacterials in children: a report of the Specialist Advisory Committee on Antimicrobial Resistance (SACAR) Paediatric Subgroup. J. Antimicrob. Chemother. 60(Suppl. 1), i15–i26. ( 10.1093/jac/dkm153) [DOI] [PubMed] [Google Scholar]
- 30.Nicoll A, Lynn R, Rahi J, Verity C, Haines L. 2000. Public health outputs from the British Paediatric Surveillance Unit and similar clinician-based systems. J. R. Soc. Med. 93, 580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson AP, et al. 2010. Enhanced surveillance of methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia in children in the UK and Ireland. Arch. Dis. Child. 95, 781–785. ( 10.1136/adc.2010.162537) [DOI] [PubMed] [Google Scholar]
- 32.Health Protection Agency. 2007. Surveillance of Healthcare Associated Infections Report 2007. http://webarchive.nationalarchives.gov.uk/20070205105506/http://hpa.org.uk/webw/HPAweb&HPAwebStandard/HPAweb_C/1196942166935?p=1158945066450.
- 33.Marsh B. 2006. Hospital superbug ‘out of control’ as child MRSA cases rise to 150. The Telegraph, 10 September 2006. http://www.telegraph.co.uk/news/1528475/Hospital-superbug-out-of-control-as-child-MRSA-cases-rise-to-150.html.
- 34.Combined working party of the British Society for Antimicrobial Chemotherapy, the Hospital Infection Society and the Infection Control Nurses Association. 1998. Revised guidelines for the control of methicillin-resistant Staphlococcus aureus infection in hospitals. J. Hosp. Infect. 39, 253–290. ( 10.1016/S0195-6701(98)90293-6) [DOI] [PubMed] [Google Scholar]
- 35.Enright MC, Robinson DA, Randle G, Feil EJ, Grundmann H, Spratt BG. 2002. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA). Proc. Natl Acad. Sci. USA 99, 7687–7692. ( 10.1073/pnas.122108599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson AP, Aucken HM, Cavendish S, Ganner M, Wale MC, Warner M, Livermore DM, Cookson BD. 2001. Dominance of EMRSA-15 and -16 among MRSA causing nosocomial bacteraemia in the UK: analysis of isolates from the European Antimicrobial Resistance Surveillance System (EARSS). J. Antimicrob. Chemother. 48, 143–144. ( 10.1093/jac/48.1.143) [DOI] [PubMed] [Google Scholar]
- 37.Monnet DL, MacKenzie FM, Lopez-Lozano JM, Beyaert A, Camacho M, Wilson R, Stuart D, Gould IM. 2004. Antimicrobial drug use and methicillin-resistant Staphylococcus aureus, Aberdeen, 1996–2000. Emerg. Infect. Dis. 10, 1432–1441. ( 10.3201/eid1008.020694) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoiby N, Jarlov JO, Kemp M, Tvede M, Bangsborg JM, Kjerulf A, Pers C, Hansen H. 1997. Excretion of ciprofloxacin in sweat and multiresistant Staphylococcus epidermidis. Lancet 349, 167–169. ( 10.1016/S0140-6736(96)09229-X) [DOI] [PubMed] [Google Scholar]
- 39.Bisognano C, Vaudaux P, Rohner P, Lew DP, Hooper DC. 2000. Induction of fibronectin-binding proteins and increased adhesion of quinolone-resistant Staphylococcus aureus by subinhibitory levels of ciprofloxacin. Antimicrob. Agents Chemother. 44, 1428–1437. ( 10.1128/AAC.44.6.1428-1437.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ellington MJ, Hope R, Livermore DM, Kearns AM, Henderson K, Cookson BD, Pearson A, Johnson AP. 2010. Decline of EMRSA-16 amongst methicillin-resistant Staphylococcus aureus causing bacteraemias in the UK between 2001 and 2007. J. Antimicrob. Chemother. 65, 446–448. ( 10.1093/jac/dkp448) [DOI] [PubMed] [Google Scholar]
- 41.Wyllie D, et al. 2011. Decline of meticillin-resistant Staphylococcus aureus in Oxfordshire hospitals is strain-specfic and preceded infection-control intensification. Br. Med. J. Open 1, e000160 ( 10.1136/bmjopen-2011-000160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wyllie D, Paul J, Crook D. 2011. Waves of trouble: MRSA strain dynamics and assessment of the impact of infection control. J. Antimicrob. Chemother. 66, 2685–2688. ( 10.1093/jac/dkr392) [DOI] [PubMed] [Google Scholar]
- 43.Datta N, Kontomichalou P. 1965. Penicillinase synthesis controlled by infectious R factors in Enterobacteriaceae. Nature 208, 239–241. ( 10.1038/208239a0) [DOI] [PubMed] [Google Scholar]
- 44.Abraham EP, Chain E. 1940. An enzyme from bacteria able to destroy penicillin. Nature 146, 837 ( 10.1038/146837a0) [DOI] [PubMed] [Google Scholar]
- 45.Brunton J, Clare D, Meier MA. 1986. Molecular epidemiology of antibiotic resistance plasmids of Haemophilus species and Neisseria gonorrhoeae. Rev. Infect. Dis. 8, 713–724. ( 10.1093/clinids/8.5.713) [DOI] [PubMed] [Google Scholar]
- 46.Turner PJ. 2005. Extended-spectrum beta-lactamases. Clin. Infect. Dis. 41(Suppl. 4), S273–S275. ( 10.1086/430789) [DOI] [PubMed] [Google Scholar]
- 47.Sykes R. 2010. The 2009 Garrod lecture: the evolution of antimicrobial resistance: a Darwinian perspective. J. Antimicrob. Chemother. 65, 1842–1852. ( 10.1093/jac/dkq217) [DOI] [PubMed] [Google Scholar]
- 48.Livermore DM, Nichols T, Lamagni TL, Potz N, Reynolds R, Duckworth G. 2003. Ciprofloxacin-resistant Escherichia coli from bacteraemias in England; increasingly prevalent and mostly from men. J. Antimicrob. Chemother. 52, 1040–1042. ( 10.1093/jac/dkg479) [DOI] [PubMed] [Google Scholar]
- 49.Public Health England. 2014. Voluntary surveillance of Escherichia coli bacteraemia in England, Wales and Northern Ireland: 2009–2013. https://www.gov.uk/government/publications/escherichia-coli-bacteraemia-annual-trends-in-voluntary-surveillance.
- 50.Public Health England. 2014. Voluntary surveillance of Klebsiella spp. bacteraemia in England, Wales and Northern Ireland: 2009–2013. https://www.gov.uk/government/publications/klebsiella-spp-bacteraemia-voluntary-surveillance-2009-to-2013.
- 51.Public Health England. 2014. Voluntary surveillance of bacteraemia caused by Enterobacter spp., Serratia spp. and Citrobacter spp. in England, Wales and Northern Ireland: 2009–2013. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/364824/4014_enterobacter_final_v2.pdf.
- 52.Livermore DM. 1995. Beta-lactamases in laboratory and clinical resistance. Clin. Microbiol. Rev. 8, 557–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Public Health England. 2014. English surveillance programme for antimicrobial utilisation and resistance (ESPAUR) report 2014. https://www.gov.uk/government/publications/english-surveillance-programme-antimicrobial-utilisation-and-resistance-espaur-report.
- 54.Health Protection Agency. 2012. English national point prevalence survey on healthcare-associated infections and antimicrobial use, 2011: preliminary data. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/331871/English_National_Point_Prevalence_Survey_on_Healthcare_associated_Infections_and_Antimicrobial_Use_2011.pdf.
- 55.Department of Health. 2013. UK five year antimicrobial resistance strategy 2013 to 2018. https://www.gov.uk/government/publications/uk-5-year-antimicrobial-resistance-strategy-2013-to-2018.
- 56.Johnson AP, Weinbren MJ, Ayling-Smith B, Du Bois SK, Amyes SG, George RC. 1992. Outbreak of infection in two UK hospitals caused by a strain of Klebsiella pneumoniae resistant to cefotaxime and ceftazidime. J. Hosp. Infect. 20, 97–103. ( 10.1016/0195-6701(92)90111-X) [DOI] [PubMed] [Google Scholar]
- 57.Cookson B, et al. 1995. International inter- and intrahospital patient spread of a multiple antibiotic-resistant strain of Klebsiella pneumoniae. J. Infect. Dis. 171, 511–513. ( 10.1093/infdis/171.2.511) [DOI] [PubMed] [Google Scholar]
- 58.Shannon K, Stapleton P, Xiang X, Johnson A, Beattie H, El Bakri F, Cookson B, French G. 1998. Extended-spectrum beta-lactamase-producing Klebsiella pneumoniae strains causing nosocomial outbreaks of infection in the United Kingdom. J. Clin. Microbiol. 36, 3105–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mushtaq S, Woodford N, Potz N, Livermore DM. 2003. Detection of CTX-M-15 extended-spectrum beta-lactamase in the United Kingdom. J. Antimicrob. Chemother. 52, 528–529. ( 10.1093/jac/dkg353) [DOI] [PubMed] [Google Scholar]
- 60.Woodford N, et al. 2004. Community and hospital spread of Escherichia coli producing CTX-M extended-spectrum beta-lactamases in the UK. J. Antimicrob. Chemother. 54, 735–743. ( 10.1093/jac/dkh424) [DOI] [PubMed] [Google Scholar]
- 61.Potz NA, Hope R, Warner M, Johnson AP, Livermore DM. 2006. Prevalence and mechanisms of cephalosporin resistance in Enterobacteriaceae in London and South-East England. J. Antimicrob. Chemother. 58, 320–326. ( 10.1093/jac/dkl217) [DOI] [PubMed] [Google Scholar]
- 62.Lau SH, et al. 2008. UK epidemic Escherichia coli strains A-E, with CTX-M-15 beta-lactamase, all belong to the international O25:H4-ST131 clone. J. Antimicrob. Chemother. 62, 1241–1244. ( 10.1093/jac/dkn380) [DOI] [PubMed] [Google Scholar]
- 63.Rogers BA, Sidjabat HE, Paterson DL. 2011. Escherichia coli O25b-ST131: a pandemic, multiresistant, community-associated strain. J. Antimicrob. Chemother. 66, 1–14. ( 10.1093/jac/dkq415) [DOI] [PubMed] [Google Scholar]
- 64.Dhanji H, Doumith M, Rooney PJ, O'Leary MC, Loughrey AC, Hope R, Woodford N, Livermore DM. 2011. Molecular epidemiology of fluoroquinolone-resistant ST131 Escherichia coli producing CTX-M extended-spectrum beta-lactamases in nursing homes in Belfast, UK. J. Antimicrob. Chemother. 66, 297–303. ( 10.1093/jac/dkq463) [DOI] [PubMed] [Google Scholar]
- 65.Dhanji H, Patel R, Wall R, Doumith M, Patel B, Hope R, Livermore DM, Woodford N. 2011. Variation in the genetic environments of blaCTX-M-15 in Escherichia coli from the faeces of travellers returning to the United Kingdom. J. Antimicrob. Chemother. 66, 1005–1012. ( 10.1093/jac/dkr041) [DOI] [PubMed] [Google Scholar]
- 66.Dhanji H, Murphy NM, Akhigbe C, Doumith M, Hope R, Livermore DM, Woodford N. 2011. Isolation of fluoroquinolone-resistant O25b:H4-ST131 Escherichia coli with CTX-M-14 extended-spectrum beta-lactamase from UK river water. J. Antimicrob. Chemother. 66, 512–516. ( 10.1093/jac/dkq472) [DOI] [PubMed] [Google Scholar]
- 67.Woerther PL, Burdet C, Chachaty E, Andremont A. 2013. Trends in human fecal carriage of extended-spectrum beta-lactamases in the community: toward the globalization of CTX-M. Clin. Microbiol. Rev. 26, 744–758. ( 10.1128/CMR.00023-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tangden T, Adler M, Cars O, Sandegren L, Lowdin E. 2013. Frequent emergence of porin-deficient subpopulations with reduced carbapenem susceptibility in ESBL-producing Escherichia coli during exposure to ertapenem in an in vitro pharmacokinetic model. J. Antimicrob. Chemother. 68, 1319–1326. ( 10.1093/jac/dkt044) [DOI] [PubMed] [Google Scholar]
- 69.Nordmann P, Dortet L, Poirel L. 2012. Carbapenem resistance in Enterobacteriaceae: here is the storm!. Trends Mol. Med. 18, 263–272. ( 10.1016/j.molmed.2012.03.003) [DOI] [PubMed] [Google Scholar]
- 70.Woodford N, Warner M, Pike R, Zhang J. 2011. Evaluation of a commercial microarray to detect carbapenemase-producing Enterobacteriaceae. J. Antimicrob. Chemother. 66, 2887–2890. ( 10.1093/jac/dkr374) [DOI] [PubMed] [Google Scholar]
- 71.Jain A, et al. 2014. NDM carbapenemases in the United Kingdom: an analysis of the first 250 cases. J. Antimicrob. Chemother. 69, 1777–1784. ( 10.1093/jac/dku084) [DOI] [PubMed] [Google Scholar]
- 72.Working Party of the British Society for Antimicrobial Chemotherapy. 1985. Antibiotic treatment of streptococcal and staphylococcal endocarditis. Lancet 326, 815–817. ( 10.1016/S0140-6736(85)90803-7) [DOI] [PubMed] [Google Scholar]
- 73.Shanson DC. 1998. New guidelines for the antibiotic treatment of streptococcal, enterococcal and staphylococcal endocarditis. J. Antimicrob. Chemother. 42, 292–296. ( 10.1093/jac/42.3.292) [DOI] [PubMed] [Google Scholar]
- 74.Gould FK, Denning DW, Elliott TS, Foweraker J, Perry JD, Prendergast BD, Sandoe JA, Spry MJ, Watkin RW. 2012. Guidelines for the diagnosis and antibiotic treatment of endocarditis in adults: a report of the Working Party of the British Society for Antimicrobial Chemotherapy. J. Antimicrob. Chemother. 67, 269–289. ( 10.1093/jac/dkr450) [DOI] [PubMed] [Google Scholar]
- 75.Lim WS, et al. 2009. British Thoracic Society guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax 64(Suppl. 3), 1–55. ( 10.1136/thx.2009.121434) [DOI] [PubMed] [Google Scholar]
- 76.Muller-Pebody B, Johnson AP, Heath PT, Gilbert RE, Henderson KL, Sharland M. 2011. Empirical treatment of neonatal sepsis: are the current guidelines adequate? Arch. Dis. Child. Fetal Neonatal. Ed. 96, F4–F8. ( 10.1136/adc.2009.178483) [DOI] [PubMed] [Google Scholar]
- 77.Bignell CJ. 2004. BASHH guideline for gonorrhoea. Sex. Transm. Infect. 80, 330–331. ( 10.1136/sti.2004.012781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fenton KA, Ison C, Johnson AP, Rudd E, Soltani M, Martin I, Nichols T, Livermore DM. 2003. Ciprofloxacin resistance in Neisseria gonorrhoeae in England and Wales in 2002. Lancet 361, 1867–1869. ( 10.1016/S0140-6736(03)13489-7) [DOI] [PubMed] [Google Scholar]
- 79.Bignell C, FitzGerald M. 2011. UK national guideline for the management of gonorrhoea in adults, 2011. Int. J. STD AIDS 22, 541–547. ( 10.1258/ijsa.2011.011267) [DOI] [PubMed] [Google Scholar]
- 80.Public Health England. The resistance alert system. http://webarchive.nationalarchives.gov.uk/20140714084352/http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/AntimicrobialResistance/Guidelines/antimTheResistanceAlertSystem/.
- 81.Public Health England. 2013. Acute trust toolkit for the early detection, management and control of carbapenemase-producing Enterobacteriaceae. https://www.gov.uk/government/publications/carbapenemase-producing-enterobacteriaceae-early-detection-management-and-control-toolkit-for-acute-trusts.
- 82.NHS England. 2014. Patient Safety Alert. Addressing rising trends and outbreaks in carbapenemase- producing Enterobacteriaceae. http://www.england.nhs.uk/wp-content/uploads/2014/03/psa-carbapenemase.pdf.
- 83.Department of Health. 2014. UK 5 year antimicrobial resistance (AMR) strategy 2013–2018: measuring success. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/322358/Outcome_measures.pdf.
- 84.Garcia AL, Aylin P, Tian J, King C, Catchpole M, Hassall S, Whittaker-Axon K, Holmes A. 2011. Data linkage between existing healthcare databases to support hospital epidemiology. J. Hosp. Infect. 79, 231–235. ( 10.1016/j.jhin.2011.06.016) [DOI] [PubMed] [Google Scholar]
- 85.Goossens H, Ferech M, Vander SR, Elseviers M. 2005. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 365, 579–587. ( 10.1016/S0140-6736(05)17907-0) [DOI] [PubMed] [Google Scholar]
- 86.Blackburn RM, Henderson KL, Minaji M, Muller-Pebody B, Johnson AP, Sharland M. 2012. Exploring the epidemiology of hospital-acquired bloodstream infections in children in England (January 2009-March 2010) by linkage of national hospital admissions and microbiological databases. Pediatr. Infect. Dis. J. 1, 284–294. ( 10.1093/jpids/pis084) [DOI] [PubMed] [Google Scholar]
- 87.Green N, Johnson AP, Henderson KL, Muller-Pebody B, Thelwall S, Robotham JV, Sharland M, Wolkewitz M, Deeny SR. In press. Quantifying the burden of hospital-acquired bloodstream infection in children in England by estimating excess length of hospital stay and mortality using a multistate analysis of linked, routinely collected data. J. Pediatr. Infect. Dis. Soc. ( 10.1093/jpids/piu073) [DOI] [PubMed] [Google Scholar]
- 88.Abernethy JK, Johnson AP, Guy R, Hinton N, Sheridan EA, Hope RJ. In press. Thirty day all-cause mortality in patients with Escherichia coli bacteraemia in England. Clin. Microbiol. Infect. ( 10.1016/j.cmi.2015.01.001) [DOI] [PubMed] [Google Scholar]
- 89.Harron K, Goldstein H, Wade A, Muller-Pebody B, Parslow R, Gilbert R. 2013. Linkage, evaluation and analysis of national electronic healthcare data: application to providing enhanced blood-stream infection surveillance in paediatric intensive care. PLoS ONE 8, e85278 ( 10.1371/journal.pone.0085278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ironmonger D, Edeghere O, Gossain S, Bains A, Hawkey PM. 2013. AmWeb: a novel interactive web tool for antimicrobial resistance surveillance, applicable to both community and hospital patients. J. Antimicrob. Chemother. 68, 2406–2413. ( 10.1093/jac/dkt181) [DOI] [PubMed] [Google Scholar]
- 91.Johnson AP. 2013. Improving antimicrobial stewardship: AmWeb, a tool for helping microbiologists in England to ‘Start Smart’ when advising on antibiotic treatment. J. Antimicrob. Chemother. 68, 2181–2182. ( 10.1093/jac/dkt158) [DOI] [PubMed] [Google Scholar]
- 92.Harris SR, et al. 2013. Whole-genome sequencing for analysis of meticillin-resistant Staphylococcus aureus: a descriptive study. Lancet 13, 130–136. ( 10.1016/S1473-3099(12)70268-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Price JR, et al. 2014. Whole-genome sequencing shows that patient-to-patient transmission rarely accounts for acquisition of Staphylococcus aureus in an intensive care unit. Clin. Infect. Dis. 58, 609–618. ( 10.1093/cid/cit807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stoesser N, et al. 2014. Genome sequencing of an extended series of NDM-producing Klebsiella pneumoniae isolates from neonatal infections in a Nepali hospital characterizes the extent of community- versus hospital-associated transmission in an endemic setting. Antimicrob. Agents. Chemother. 58, 7347–7357. ( 10.1128/AAC.03900-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mathers AJ, et al. 2015. Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae at a single institution: insights into endemicity from whole genome sequencing. Antimicrob. Agents Chemother. 59, 1656–1663. ( 10.1128/AAC.04292-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Torok ME, Peacock SJ. 2012. Rapid whole-genome sequencing of bacterial pathogens in the clinical microbiology laboratory—pipe dream or reality? J. Antimicrob. Chemother. 67, 2307–2308. ( 10.1093/jac/dks247) [DOI] [PubMed] [Google Scholar]
- 97.Koser CU, Ellington MJ, Peacock SJ. 2014. Whole-genome sequencing to control antimicrobial resistance. Trends Genet. 30, 401–407. ( 10.1016/j.tig.2014.07.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.de Kraker ME, Davey PG, Grundmann H. 2011. Mortality and hospital stay associated with resistant Staphylococcus aureus and Escherichia coli bacteremia: estimating the burden of antibiotic resistance in Europe. PLoS Med. 8, e1001104 ( 10.1371/journal.pmed.1001104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bates J, et al. 2014. Point of care testing for urinary tract infection in primary care (POETIC): protocol for a randomised controlled trial of the clinical and cost effectiveness of FLEXICULT informed management of uncomplicated UTI in primary care. BMC Fam. Pract. 15, 187 ( 10.1186/s12875-014-0187-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Longitude Prize, 2014. https://longitudeprize.org.
- 101.The Clinical Practice Research Datalink. http://www.cprd.com/home/.
- 102.CSD Health Research. The Health Improvement Network. http://www.thin-uk.com.