Abstract
The purpose of this review was to provide an updated overview on the use of antimicrobial agents in livestock, the associated problems for humans and current knowledge on the effects of reducing resistance in the livestock reservoir on both human health and animal production. There is still limiting data on both use of antimicrobial agents, occurrence and spread of resistance as well as impact on human health. However, in recent years, emerging issues related to methicillin-resistant Staphylococcus aureus, Clostridium difficile, Escherichia coli and horizontally transferred genes indicates that the livestock reservoir has a more significant impact on human health than was estimated 10 years ago, where the focus was mainly on resistance in Campylobacter and Salmonella. Studies have indicated that there might only be a marginal if any benefit from the regular use of antibiotics and have shown that it is possible to substantially reduce the use of antimicrobial agents in livestock production without compromising animal welfare or health or production. In some cases, this should be done in combination with other measures such as biosecurity and use of vaccines. To enable better studies on both the global burden and the effect of interventions, there is a need for global harmonized integrated and continuous surveillance of antimicrobial usage and antimicrobial resistance, preferably associated with data on production and animal diseases to determine the positive and negative impact of reducing antimicrobial use in livestock.
Keywords: antimicrobial resistance, antimicrobial usage, interventions, food animals, productivity
1. Introduction
The introduction of antimicrobial agents in human and veterinary clinical medicine has been one of the most significant achievements of the twentieth century. The first antimicrobial agents were introduced in the 1930s, and a large number of new compounds were discovered in the following decades. Antimicrobial agents have for the past 80 years literally changed the entire medical profession and our way of living. Not only can we now treat infections that previously were considered lethal, but without the prophylactic use of antimicrobial agents even simple operations such as bowel surgery, tooth removal or hip replacements would be impossible or associated with very high mortality rates.
However, shortly after the introduction of antimicrobials, resistance began to emerge, and in all known cases, emergence of antimicrobial resistance has sooner or later followed the introduction of new antimicrobial compounds [1]. It has now become clear that antimicrobial resistance poses a threat to the continued use of antimicrobial agents in both human and veterinary medicine. Antimicrobial resistance is an increasing problem and has limited the effective lifespan of newly developed antimicrobial compounds to only 10–20 years. In addition, new effective antimicrobials are unlikely to be developed at a sufficient rate [2]. Therefore, we are faced with a major challenge to find alternative ways of combating bacterial pathogens—or find ways to delay resistance development.
In modern food animal production, large amounts of antibiotics are used for disease control. In many places, antimicrobial agents are an integrated and routinely used management tool, and in many cases, farmers might not even be aware that they are using them or for what purpose. The huge use provides favourable conditions for selection, spread and persistence of antimicrobial-resistant bacteria capable of causing infections in animals and humans. During the past two decades, there has been an increased awareness of the potential problems that selection of antimicrobial resistance among food producing animals could have on human health. In addition, food animals and food of animal origin are traded worldwide. Thus, the selection of antimicrobial resistance in one country is today a problem for all countries. This has emphasized the need for global initiatives, control and monitoring systems for containment of antimicrobial resistance in all countries [3,4].
The speed with which antimicrobial resistance has emerged, evolved and spread worldwide has been without comparison and clearly shows the great adaptive ability of bacteria. Recent studies have shown a huge reservoir of novel potential resistance genes of different environmental origins [5,6]. Bacteria also harbour multiple genetic mechanisms that may facilitate spread of resistance genes once acquired. Besides a large number of different genetic mechanisms that facilitate gene transfer, the modern globalized world has provided efficient ways for bacteria and resistance genes to rapidly disseminate worldwide through trade of food products or live animals, and people travelling. Further globalization will only increase trade and international contacts in the future. Besides the many benefits thereof, this can also create serious health problems. Therefore, how we handle the control of antimicrobial resistance in human pathogens, including those of animal origin, veterinary pathogens and the non-pathogenic reservoirs will have important public health consequences for the future. This emphasizes the need for global interventions based on global standards.
This review provides an overview of the use of antimicrobial agents for livestock, excluding aquaculture, and of the importance of the livestock reservoir for current and emerging antimicrobial resistance in human pathogens. In addition, an overview is given of the different attempts to limit the selection and spread of antimicrobial resistance, as well as the positive and negative consequences thereof.
2. Antimicrobial usage in livestock production
The first observations on the inhibitory effect of penicillium mould on bacteria seem to have been made by Sir John Burdon–Sanderson in 1871 and Joseph Lister in 1872 [7,8]. In 1928, Alexander Fleming made similar observations [9], and when it later became possible to purify penicillin [10], the way was opened for its therapeutic use. Since then, a large number of other antimicrobial agents have been discovered and introduced for human and veterinary use, and in the course of the past 50 years, antimicrobial agents have become the keystone of bacterial infection treatments in humans and animals.
(a). Purposes of using antimicrobial agents
The introduction of antimicrobial agents in agriculture soon after the Second World War caused a revolution in the treatment of many infectious diseases in livestock, as well as attempts to reduce or eradicate specific diseases, such as infections with Streptococcus agalactiae in dairy cattle [11]. In these ‘wonder years’, antimicrobial agents were introduced and used with a minimum of controlled experiments and, as in human medicine, early dosages were largely empiric. It took four to five decades before the use and dosages of the most commonly used agents for the most important diseases were more scientifically determined and based on an understanding of the actual interaction with the target microorganism. In modern food-animal production, as in human medicine, antimicrobial agents are used therapeutically for specific treatment of infections in clinically sick animals, preferably with a bacteriological diagnosis. However, in addition to this, antimicrobial agents are now used in a number of ways which are unique to livestock production. As metaphylactics: this is the treatment of clinically healthy animals belonging to the same flock or pen as animals with clinical signs. In this way, infections may be treated before they become clinically visible, and the entire treatment period for all animals may thereby be shortened. Because of the modern productions systems, this can often be the only way to treat large groups of animals with water medication, such as tens of thousands of chickens in a single house. As prophylactics: this is the treatment of healthy animals in a period where they are either stressed or in other ways susceptible to prevent disease. Examples include medicated early weaning of piglets or dry cow treatment of dairy cattle between lactations. In these cases, antimicrobial agents may be used in low concentrations, similar to the use for growth promotion. This use of antimicrobial agents can be a sign of management problems, and in many countries it is not considered legal or prudent. For eradication: the use of antimicrobial agents for a defined time-period to eradicate a specific pathogen. This was widely used against contagious bovine mastitis in the 1950s, but in most countries is not considered prudent or legal.
One of the most controversial modes of using antimicrobial agents for livestock has been for growth promotion: inclusion of antimicrobial agents continuously in animal feed to improve growth. Whereas the use of antimicrobial agents for therapy, prophylaxis or metaphylaxis, in some instances can seem logical and scientifically based, the usage of antimicrobial agents for growth promotion has been a question of intensive debate for the past decades. Soon after the introduction of antimicrobial agents for therapy, the first discoveries of the growth-promoting effect of antimicrobial agents were made [12]. In the 1940s, deficiencies in the basal feed ratio in swine led to failure in reproductive performance and growth. This was corrected by the addition of different animal by-products altogether designated as ‘animal protein factor’ [13]. The addition of waste products from tetracycline production to chicken feed was found to have a similar effect. The growth-promoting effect was soon found to be due to residual tetracycline (aureomycin). Besides sulfonamides, streptomycin and tetracycline, other antibiotics including penicillin were also found to have a growth-promoting effect [14,15]. Antimicrobial agents were soon approved for growth promotion and have been commonly used in the USA since 1949 and since 1953 in the UK [16]. In the following years, a large number of substances with antimicrobial activity have been introduced into modern agricultural production, and during the past decades, industrialized animal production has continuously used supplements of antibiotics in feed. This introduction is supported by a large number of experimental studies showing a benefit to growth [17], but there is a lack of good long-term epidemiological studies under field conditions also conducted under modern circumstances with improved feeding regimes to compare with the situation 40–60 years ago. It has been argued that this use is essential to feed the world with animal proteins but more recent data suggest that the importance of antimicrobial growth promoters (AGPs) might be over-estimated [18–21].
(b). The ethical dimension
The choice of whether or not to use antimicrobial agents for treatment of a diagnosed infectious disease might seem reasonably straightforward. When faced with a sick patient or animal, it seems appropriate to use any possible means to cure the infection and potentially save the life or ensure the well-being of animals and humans. However, even such simple choices face the prescriber with a number of difficult ethical questions [22]. Thus, in human medicine, most antimicrobial treatments are started empirically before any microbiological diagnosis is available, and in many cases, the recommended empiric treatment is narrow spectrum to avoid broad selection for resistance. Even this simple situation offers several ethical considerations. Thus, a restrictive treatment strategy may, in some cases, lead to increased fatality rates in the individual patient, but may benefit both the patient and future patient by avoiding broad development of resistance in the microbiome, and thereby reducing the risk of acquiring an infection with a resistant bacterium later in life.
When dealing with use of antimicrobial agents in livestock production, an entire new set of dilemmas are introduced, making this even more complicated and ethically challenging. In table 1, a number of factors that in my personal opinion may influence the question of whether or not to use antimicrobial agents for livestock are listed. It is immediately clear that most factors leading to increased and more broad-spectrum use are located on the individual farm, whereas any negative consequences are almost exclusively related to consequences which do not or only to a limited degree influence the individual farmer or the veterinarian who, in many cases, do the actual prescription. Thus, it may not be strange that farmers, veterinarians, the consumers, politicians and medical physicians do not necessarily agree on all aspects regarding whether to use, restrict or ban the use of antimicrobial agents for livestock. This debate becomes even more difficult when issues related to the modes of use and which compounds to use for which purposes are included in the discussion. Furthermore, in livestock, production data are often not collected in the same systematic way as sometimes happens in human medicine, and while mortality and morbidity are the main outcomes looked at in human medicine, parameters related to overall production might be more important for farmers.
Table 1.
Selected factors which in the author's opinion may influence the decision of using more or less antibiotics for animals at a single farm. The first column lists positive effects for the farmer and the society of using more, as well as other drivers for using more. The second column lists negative effects as well as other drivers for using less.
| reasons for using antimicrobial agents | |
|---|---|
| more | less |
positive effects of using antimicrobials
|
negative effects of using antimicrobials
|
other drivers for using more
|
other drivers for using less
|
3. Importance of the livestock reservoir for human health—changing patterns
(a). Amounts of antimicrobial agents used
Exact figures for worldwide usage of antimicrobial agents are not available. It has generally been very difficult to obtain good information about the consumption of antimicrobial agents in human and veterinary medicine as well as the amounts added to feed for growth-promoting purposes. Exact figures are rare, and estimates were until recently only available for a few countries.
In the USA, the total consumption of antimicrobial agents increased tremendously from 1950 to 1978. In 1951, a total of 110 tonnes were produced for addition to animal feed and other application, whereas 580 tonnes were produced for medical use in humans and animals [23]. In 1978, this had increased to 5580 tonnes as feed additives, and 6080 tonnes for medical use. Thus, a 50 and 10 times increase, respectively. Until recently, no official data on the usage of antimicrobial agents in the USA have been available. However, from 2010, the US Food and Drug Administration has reported on the total domestic consumption. In 2011, the total domestic usage was reported at 13 542 tonnes [24], or more than the combined animal and human usage in 1978. Total meat production in the USA was 42 452 759 000 kg in 2011 [25] and this would amount to 319 mg antibiotics per kg produced meat. This is considerably higher than for any country in Europe [26]. For humans in the USA, a total of 3290 tonnes of antibiotics were sold in 2011 [27]. Direct comparison with the amounts used for animals is difficult because it is not the same drug classes. However, in total amounts, the use for animals is approximately four times higher than for human use.
In Australia, in the 1990s, almost two-thirds of all antibiotics imported were for use in animals [28]. In 1999, before the ban on AGPs, the European Medicines Agency estimated the consumption for growth promotion and therapeutic indications for 1996 and compared this value with food animal production in 15 European countries during 1997. Mean consumption was 98 mg of antimicrobials per kg of meat and poultry produced (range, 24–184 mg kg−1). More recently, a mandatory surveillance for antimicrobial consumption was implemented in the European Union from 2010. In 2011, data from 25 member states were available, and a total of 8481 tonnes of antimicrobials were used in these 25 countries [26].
Global estimates are currently not available. However, from these estimates, it seems likely that the global consumption in livestock out-ranks the consumption for humans. In addition, most antimicrobial agents used for food animals are given orally, thus directly exposing the microbiome in the gastrointestinal tract, whereas in human medicine, the parenteral route is much more used. In addition, feed additives are often sold as large volume purchases that do not require pharmaceutical formulations or high purity. Thus, antimicrobial agents used for livestock are likely administered to a very large number of animals, and thereby affect a larger part of the global microbiome than might be expected from the total sales figures.
(b). Selection for and transmission of resistance in the livestock reservoir
When dealing with the human health risks associated with antimicrobial consumption in livestock, it is important to recognize that it is not the selection of resistance in the bacteria causing infections in the animals we primarily are concerned about, even though this also is a point to consider. It is the selection and mobilization of antimicrobial resistance genes in the microbiome of treated animals and the potential subsequent spread into human pathogenic bacteria that is the issue of concern.
(i). Origin of antimicrobial resistance
Bacteria may acquire resistance by mutations in their DNA, changing the target, by hyper-production of already present genes or by acquisition of heterologous resistance genes. The actual origin of most antimicrobial resistance genes in pathogenic bacteria is unknown, but environmental microbes, including the natural antimicrobial producers, are believed to be important primary sources [29]. Our knowledge is still incomplete since most DNA sequences are from various clinical and pathogenic isolates, with comparatively little from environmental species. Recently, functional cloning studies have shown that there is a large reservoir of novel potential resistance genes that still have not found their way into pathogenic strains [5,6]. It must thus, be expected that even if novel antibiotics are developed, resistance will develop in pathogenic strains sooner or later. Studies have shown that exposure to antimicrobial agents may increase mobilization of genes between bacteria and thus increase the chances that resistance genes in one perhaps non-pathogenic strain are acquired by a pathogenic strain [5,6]. Various housekeeping genes are also a likely origin for several resistance determinants. This is especially the case for the different efflux genes, where these transmembrane transport proteins also can play a role in protecting bacteria from antimicrobials.
(ii). Selection for resistance
The single most important driver for the emergence and increase in antimicrobial resistance in the microbiome as well as pathogenic strains is the use of antimicrobial agents. In some cases, such as with methicillin-resistant Staphylococcus aureus (MRSA) and Salmonella Typhimurium DT104, transmission might be very important once the resistance gene has become established in the bacterium. However, in general, there is a very close association between use and occurrence of resistance, which has been well documented in multiple experimental, epidemiological and ecological studies [30].
(iii). Genetic transmission of resistance
Horizontal acquisition of genes from other organisms provides an efficient way to acquire new traits and adapt to new or changing environments. While the exact origin of the resistance determinants we see among pathogenic bacteria today may be obscure, there can be no doubt that the rapid dissemination of resistance genes among different commensal and pathogenic bacteria is a very recent evolutionary event. This dissemination has occurred within the antibiotic era as a consequence of horizontal transfer mediated by different mobile DNA elements such as plasmids, transposons, genomic islands, integrons, natural transformation, etc., which together constitute a widely diverse assembly of mobile DNA elements [31,32].
(iv). Macrotransmission of resistance
Multiple resistant bacterial clones have in several cases spread worldwide. In animal populations, examples have included the international dissemination of different Salmonella clones, such as DT104 [33], the global spread of MRSA CC398 [34] and recently the vertical transmission of cephalosporin-resistant Escherichia coli from grandparent poultry flocks in the UK, though parent poultry flocks in Sweden and into the conventional broiler production in Denmark [35]. In the latter case, selection in one country has consequences for at least two other countries and interventions reducing selection have to take place at the origin. In most cases, the modes of transmission are not definitively known but are in the authors opinion probably mainly related to trade in breeding animals, travelling and the international sale of food products. Antimicrobial-resistant bacteria and resistance genes may also disseminate via a large number of other routes, including between farms, though the environment, animal waste containing resistance genes, migrating animals, imported food and feed products, etc., as has been highlighted in several reviews over the past decades [30]. The importance of the multiple routes is not easily quantified. It also depends very much on the individual countries, animal species and production system. Thus, when performing interventions in this area, it is necessary to have very specific local knowledge.
Animal-to-human transmission can also easily occur through multiple routes, of which the direct transmission via fresh food products, such as meat and eggs, probably is the most important [29,30,32,36]. Antimicrobial-resistant bacteria may also transfer from animals to humans though direct contact. This is, for example, the case for MRSA CC398 where transmission frequently occurs from the animals to the farmers or other people in contact with the animals [37].
(c). Importance of the animal reservoir for human health
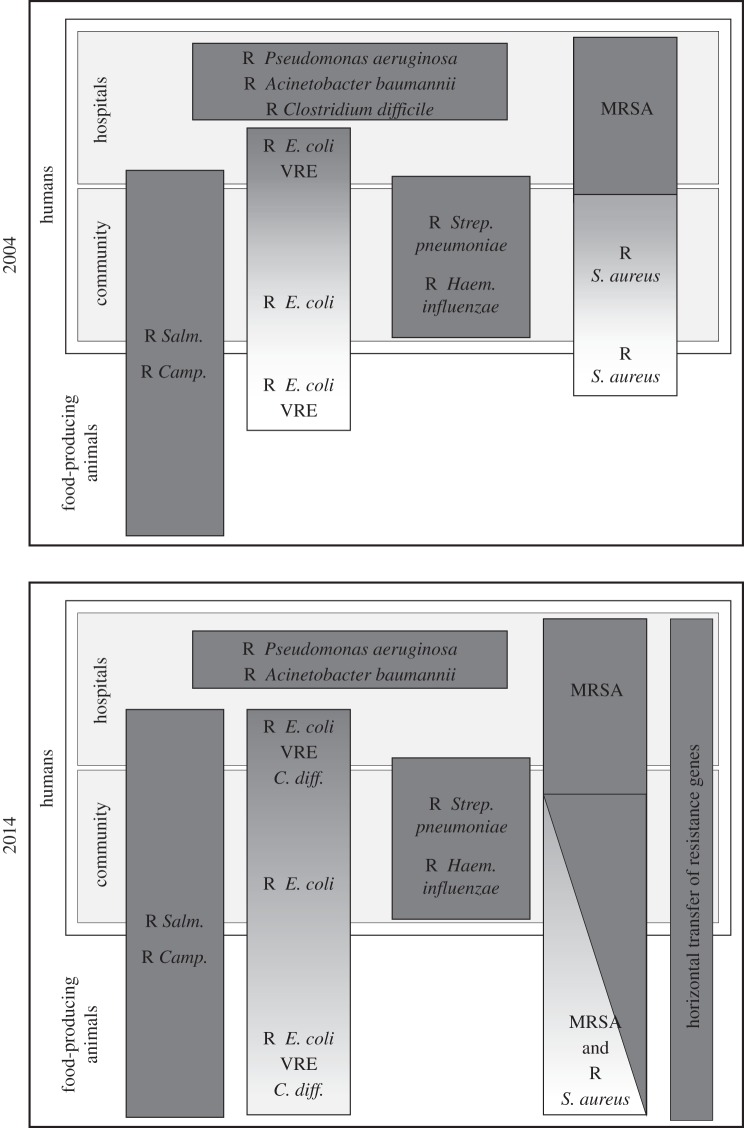
The actual importance of the animal reservoir of antimicrobial resistance for human health is an area of great controversy and unfortunately no precise estimates exist. Estimates have ranged from almost zero to a major contribution to the human health burden [38]. Data for precise risk assessments are also scare, because there is no systematic registration of the burden associated with infections in humans caused by resistant bacteria and thus no impact factor to include in risk assessments. Today, most experts agree that the contribution from food animals is a concern and that the development of resistance in this reservoir should be also limited as much as possible. In addition, our increased knowledge of the complex biology of microorganisms is constantly changing, and regarding antimicrobial resistance, the accumulating evidence seems to indicate that the importance of the food-animal reservoir is larger than we estimated a decade ago (figure 1).
Figure 1.
Overview of some of the most important antimicrobial-resistant pathogens and the overlap between the different reservoirs (VRE, vancomycin-resistant enterococci). The human reservoir (community and hospital) and food animal reservoir is indicated. Boxes and colouring indicate the degree of overlap of specific resistant bacterial species between these reservoirs. As indicated, some pathogens such as Pseudomonas aeruginosa, are strictly confined within the human reservoir, whereas others have a mainly or partly animal reservoir, such as Salmonella and Campylobacter. The two pictures show the changes in the author's personal perception over the past 10 years (2004–2014).
A decade ago it was generally agreed that the human health problems associated with antimicrobial resistance in the food-animal reservoir were mainly related to selection of resistant Salmonella and Campylobacter and to a more limited extent resistant variants of enterococci and E. coli. However, studies during the last decade have shown that some variants of methicillin-resistant S. aureus [36,39,40] and Clostridium difficile [41] also may have an animal reservoir, even though the precise burden on human health has not been determined. In addition, the importance of foodborne E. coli [42] as well as horizontally transferred resistance genes and potentially also the emergence of novel resistance genes is probably larger than previously expected. Thus, reducing the risk of antimicrobial resistance in the animal reservoir is today more important than ever.
4. Reducing the risk
The risk of antimicrobial resistance can basically be reduced by either limiting the selection of resistance or limiting the spread of resistance genes and/or resistant bacterial clones [30].
Several options for reducing the risk exist, but are not all equally likely to be successful and some might be hindered by national or international legislation. Furthermore, some interventions might work only if they are introduced in parallel. Thus, reducing antibiotic use for therapy might, in some cases, only be feasible when at the same time introducing improved biosecurity and/or use of vaccines. From the international point of view one of the main problems is as mentioned, that resistant bacteria might be selected for by antimicrobial usage in one country, but may be causing problems in several other countries, as exemplified by the recent spread of cephalosporin-resistant E. coli from England, though Sweden to Denmark [35].
A large number of national and international rules and regulations as well as marketing and consumer factors regulate and change the international trade of food products and live animals. Internationally, the most important standard-setting organization is the World Trade Organization (WTO). As the WTO has no scientific competence, the Office International des Epizooties is the agency responsible for setting appropriate global standards for animal heath, whereas Codex Alimentarius Commission sets standards for food safety. Other important organizations providing guidelines but not binding standards are the World Health Organization (WHO), the Food and Agriculture Organization (FAO) and OIE (the World Organisation for Animal Health). These organizations have developed common guidelines and strategies for containment of antimicrobial resistance [43–45].
In 2011, Codex set guidelines for risk analysis of antimicrobial resistance. Even though this might be a time-consuming procedure and not possible for all countries, it does provide countries with the option to limit the import of food products with antimicrobial-resistant bacteria. In addition to the legal regulations from WTO, several communities (e.g. the EU) and countries require higher standards especially with regard to food safety. Examples are Sweden, which has a Salmonella-free status and does not accept the importation of meat products with Salmonella. Denmark has a special case-by-case control programme in place for resistant Salmonella and Campylobacter [46].
(a). Knowledge on the magnitude and nature of the problem and documenting changes
Continuous and updated knowledge on the size of the problem is essential to guide risk management and to determine the effect of possible interventions. Thus, continuous monitoring of the occurrence of food-borne pathogens, antimicrobial resistance and drug use, as well as research studies determining the associations between different reservoirs, the spread of bacterial clones and genes, risk factors for the development and spread of resistance, are all essential for efficient risk management. The essential first step is the establishment of coordinated and standardized monitoring systems for determining the occurrence of antimicrobial resistance in all countries and all reservoirs. This has been emphasized in several reports, and recommendations for how to establish such monitoring have been published [47–49]. Most detailed is the common protocol for antimicrobial resistance monitoring for Europe [50]. Unfortunately, only a few countries have so far established continuous monitoring programmes.
The first country to establish a continuous monitoring of antimicrobial resistance among food animals was Denmark in 1995 [51]. This made it possible to document that major reductions in the use of antimicrobial agents for growth promotion, implemented during 1995–1999, were followed by major reductions in the occurrences of resistance to those specific antimicrobial agents [52], except in cases where antimicrobial resistance genes were genetically linked [53]. However, in Denmark, no monitoring was established for antimicrobial resistance in humans prior to the major reductions in use of AGPs and so an opportunity was missed to document the effects in the human reservoir.
All current monitoring is based on isolation of bacterial isolates and phenotypic susceptibility testing. It should be noted that the rapid development in whole genome and whole community sequencing today makes it feasible to routinely sequence both single isolates and entire bacterial communities [54,55]. The first evaluations of using whole genome and whole community sequencing for diagnostics and surveillance have already been published [56–59] and it may be expected that this will replace the current phenotypic approaches in the near future.
No matter what, the collection of data prior to and following interventions is an absolute must if we wish to learn from both good and bad experiences. This has unfortunately more been the exception than the rule so far, but is an absolute must for the future.
(b). Reducing the selective pressure
The main factor leading to reduced selection of resistance is a more limited use of antimicrobial agents. A number of different options, including drug approval, prescriber behaviour, limiting profit, taxation and complete withdrawal or bans have previously been reviewed in more detail [30].
It should in theory be easy to remove unnecessary use—overuse—of antimicrobial agents. Defining unnecessary use might not be easy though, because perspectives might differ (table 1). However, it should also be borne in mind that in some cases reduction in antimicrobial usage is not possible without the introduction of improved management, biosecurity and in some cases use of vaccines. In many cases, it is expected that good management may replace the easy use of antimicrobials.
In general, the selective pressure may be reduced by reducing the total amounts of antimicrobial used, the way they are used or by selectively reducing the use of specific ‘critically important’ antimicrobial agents. Good evidence for the long-term effects and sustainability of interventions are not available, except for major interventions such as complete bans.
(i). General reduction
Incentives for general reduction in the use of antimicrobial agents for food animals are constantly imposed on prescribers and farmers. These range from peer-pressure, general acceptance in the community to more scientific guidelines from professional societies. In organic farming, more specific rules apply, where only a very restrictive use or no use all are allowed. More systematic approaches have, to the best of my knowledge, only been attempted with the withdrawals and bans on use of antimicrobial agents for growth promotion in Denmark from 1995 to 1999, the reductions in therapeutic usage in the Netherlands from 2008 and in Denmark with the yellow card scheme in 2010.
The antimicrobial growth promoter interventions in Denmark
In the period May 1995 to December 1999, major changes in the consumption of antimicrobial agents for growth promotion occurred in Denmark [17]. Until May 1995, a total of 11 different antimicrobial agents were approved for use as growth promoters in Denmark and this constituted approximately two-thirds of all usage of antimicrobials for food animals in the country. In May 1995, the use of the glycopeptide avoparcin was banned owing to concern over selection for glycopeptide-resistant enterococci in the animal reservoir. This was followed by a ban in Germany in January 1996 and in all EU countries in April 1997. In January 1998, virginiamycin was banned in Denmark because of cross-resistance to Synercid, a streptogramin of potential value for human treatment. Simultaneously, the Danish poultry producers decided to implement a complete voluntary withdrawal of all use of AGPs. In December 1998, the European Commission decided to ban the use of bacitracin, spiramycin, tylosin and virginiamycin for growth promotion from July 1999. In Denmark, the Danish pig producers decided to follow the Danish poultry producers and voluntarily stop all use of AGPs from the end of 1999. In 2003, the European Union decided to stop all use of AGPs by the beginning of 2006.
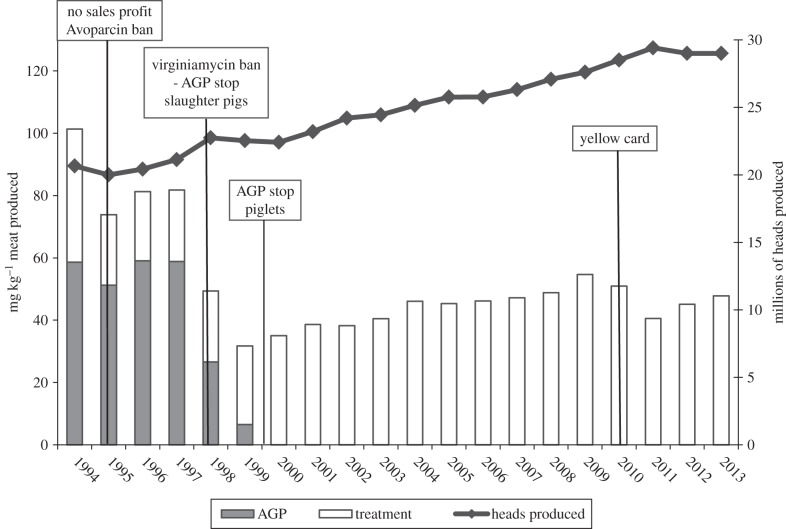
These different precautionary steps were taken to prevent future increase in the problems with antimicrobial resistance in the health sector. In Denmark, data on the consumption of antimicrobial agents for therapy prior to the different interventions on AGPs are only available for swine. However, consumption in Danish swine production accounts for more than 80% of all antimicrobial use for animals in Denmark. Figure 2 illustrates the changes in total use of antimicrobial agents for swine in Denmark from 1992 to 2013 adjusted for the total production of swine, which has increased considerably over time.
Figure 2.
Antimicrobial consumption and millions of heads of pigs produced in Denmark from 1994 to 2013. Black line indicates number of heads. Bars indicate total antimicrobial consumption adjusted to mg kg−1 of pork produced (grey indicates use as antimicrobial growth promoter (AGP), white used for treatment). Also depicted are important events over time, including: no sales profit in 1995 reducing the use of antimicrobials for treatment, ban of the AGP avoparcin in 1995, ban of the AGP virginiamycin and voluntary stop for all AGP use in 1998, complete stop for all AGP use at the end of 1999 and implementation of the yellow card scheme in 2010.
Other interventions in Denmark
Following the phasing out of AGPs, there was a minor increase in therapeutic usage in 2000 and 2001. Consumption for therapy continued to increase in the following years also, with minor fluctuations. In 2010 this led to a public debate about the use of antimicrobials in Danish pigs. As a consequence, two further reductive actions were implemented in July 2010. The first was a voluntary ban on the use of cephalosporins in Danish swine herds for a 2-year period, adopted by the Danish pig industry. The second was the so-called yellow card antimicrobial scheme implemented by the Danish government, where restrictions are imposed on farmers who have more than twice the national average consumption of antimicrobials for any of the three swine age groups (sow/piglets, weaners, finishers). In July 2010, farmers with an antimicrobial use close to these limits received a warning letter from the Danish Veterinary and Food Administration stating that unless actions were taken to reduce antimicrobial use, the producer would receive a yellow card by December 2010. A yellow card would result in restrictions on oral medication usage and supervision by the authorities. This intervention immediately led to a major reduction in total consumption and the consumption in 2001 was 25% lower than 2010 (figure 2). However, during the past 2 years, the total consumption has again started to increase and it seems questionable whether this yellow card scheme is effective in the long term. There is currently no hard evidence as to why consumption again is increasing, but it may be due to considerable creativity by some farmers, drug companies and veterinarians to set consumption levels very close to the accepted thresholds. However, this experience also shows that it is necessary to keep a constant pressure on restrictions. More details are available in the DANMAP reports [60].
The Dutch experience
Ever since the surveillance of antimicrobial consumption in human medicine was initiated in Europe in 1997, The Netherlands has had the lowest consumption for humans [61,62], which has been in sharp contrast to the situation in livestock. Following decades of being the European country with the highest antimicrobial usage for food animals compared with the size of the production system, a memorandum of understanding was signed by the animal sectors and the Dutch Association for Veterinarians in 2008 [63]. Mandatory targets for total reduction in consumption compared with 2009 were set by the government at 20% in 2011, 50% in 2013 and 70% in 2015. An independent Dutch Veterinary Medicines Authority was established and a fully transparent monitoring of drug use at farm level enabling bench-marking and identification of high users. A mandatory one-to-one relationship between farmer and veterinarian was established. In addition, no use of new antibiotics in animals will be allowed, fluoroquinolones and cephalosporins are only allowed if no other antibiotics work, and colistin, beta-lactams and aminoglycosides are second choice antibiotics only. It is still too early to evaluate the long-term effects of these interventions, but the first results have been extremely promising [64]: the reduction has been larger than the targets, with an almost 60% reduction achieved in 2013 [64]. The first results on antimicrobial resistance suggest that resistance in E. coli from healthy animals is also being reduced in the animal reservoir [64]. However, the data for Salmonella and Campylobacter so far do not allow for any preliminary conclusions regarding changes in resistance.
(ii). Targeted reduction
Not all antimicrobial agents are equally important for human health. Even the first restrictions in Denmark targeted specific AGPs and not all substances. Realizing this, the World Health Organization in 2005 provided the first guidelines for ranking antimicrobial agents which should be considered most critically important for human health and where interventions should first take place [65,66]. The most critically important antimicrobial agents were considered third-generation cephalosporins, fluoroquinolones and macrolides. Antimicrobials not approved for animal use were not considered, but it is implicit that any use of, for example, carbapenems should be avoided.
Governmental restrictions or voluntary withdrawals of specific classes have already been implemented in a number of countries. In Australia, it was decided in the mid-1990s not to approve the use of the fluoroquinolone enrofloxacin for pigs [67]. In Denmark, this has included restrictions on use of fluoroquinolones and, as mentioned, the recent voluntary withdrawal of all uses of third-generation cephalosporins for swine from July 2010. In the USA, the approval of fluoroquinolone for use in poultry was withdrawn in September 2005. Also in 2005, a temporary voluntary withdrawal of extra-label use of ceftiofur was implemented by the poultry producers in Quebec, Canada and, as mentioned, specific limitations were imposed on the use of fluoroquinolones and cephalosporins in The Netherlands in 2008. In most cases where a monitoring programme has been in place, these restrictions have resulted in a reduced occurrence of resistance [52,68–71].
(iii). Modes of use
Escalating or reducing the dose or changing the duration of treatments have often been suggested as ways to reduce the selection of resistance. Experimental studies with both quinolone resistant Salmonella and E. coli, as well as beta-lactam resistance, have provided promising results indicating that the selection for resistance might be drastically reduced while using the same or even larger amounts of antimicrobial agents [72–76]. These studies have so far not been systematically attempted under field conditions and thus there is no data available on the efficacy of the changed treatment regime. The feasibility of drastically changing the current modes of use is also questionable because it might require an entirely novel approval of the different drugs, which would be a very expensive procedure. Furthermore, systematically optimizing the way we use drugs to limit selection would most likely also require very strict adherence to a specific treatment regime. This could prove very difficult in food-animal production where most drugs are given by farmers or animal handlers.
(c). Controlling the spread of resistant bacteria
Improved hygiene and microbiological control has for millenniums been an essential part of controlling infectious diseases associated with food production. Thresholds for acceptable risk today exist for a number of pathogenic bacteria, but specific thresholds for resistance are rare. In Denmark, a zero tolerance for multiple resistant Salmonella DT104 was implemented for both domestic and imported food products in 1998. The aim was to offer the Danish consumers maximum protection against this type of Salmonella, because it was feared that it had a larger potential for spread in food animals than other Salmonella types, and also that it had increased virulence for humans. The impact of these restrictions has not been quantified and these restrictions have since been lifted.
The risk of transmission of macrolide-resistant Campylobacter from Danish pork has been estimated to be very low [77]. In Denmark, the slaughterhouses use blast chilling after slaughter in combination with a high level of hygiene. Blast chilling is a process in which ice-cold air is blown across the carcass at a high velocity, which kills most Campylobacter present on the carcasses. This, however, has no effect on the occurrence in imported meat.
To reduce the risk of human salmonellosis, Denmark, in 2006, started testing Danish and imported meat for Campylobacter and Salmonella [46]. The testing determines whether the meat imposes a risk for the consumer, and if so, the meat would be deemed to be unsafe according to article 14 of regulation (EC) no. 178/2002. In that case, the meat would be withdrawn from the market. As parameters to determine whether the meat will impose a risk for the consumers, numbers of infected samples, type of Salmonella and antimicrobial resistance profile are included. The evaluation is done for each case individually and not as a general threshold that a food product has to be below. The effect of this case-by-case procedure has not been finally evaluated for Salmonella, but an analysis of Campylobacter data has shown this procedure significantly reducing the occurrence of Campylobacter in fresh meat available on the Danish retail market [78].
To the best of my knowledge, no other restrictions specifically targeting antimicrobial resistance in food animals or food-animal products have been attempted even though the Codex guidelines do give such possibilities.
Considering the widespread occurrences of similar resistance genes and resistant bacterial clones globally, there can be little doubt that resistance has multiple routes of efficient transmission [29,32], even though the importance of the multiple routes, including environmental spread, trade with live animals, human vectors, etc., have not been quantified. Modern livestock production is, however, highly susceptible to the introduction of novel threats, including resistance genes, because an increasing number of animals are confined at each farm and we very often have a highly efficient pyramid-like breeding system where a very limited number of farms deliver the genetic material to a much larger number of production farms. This is exemplified by the recent spread of cephalosporin resistant E. coli from the UK, though Sweden to Denmark [35], as mentioned above. This makes intensive surveillance of all risks, especially at the top of the breeding pyramids, essential.
5. Effect of interventions on livestock health, welfare and productivity
Numerous studies have been performed on the economic benefit of the use of AGPs, and in the approval of therapeutic antimicrobial agents, it is often mandatory to document a beneficial effect on treatment of diseases. It has, thus, been argued that limiting the use of antimicrobial agents for therapy, prophylaxis, metaphylaxis or even growth promotion would have disastrous negative consequences for animal production, health and welfare.
There is, however, very little hard evidence documenting the negative or positive effects of reducing the usage, especially under real life conditions. Berge et al. [79] performed an intervention study on a single dairy cattle farm where all pre-weaned calves routinely received prophylactic antimicrobial treatment for diarrhoea. They compared this normal routine with a changed strategy where only calves showing clinical symptoms were treated. Surprisingly, they found less diarrhoea using this targeted strategy, and besides a reduced use of antimicrobials, a total of around 10 US$ per calf could be saved by changing the routine.
In Denmark, it was expected that problems in relation to loss of productivity and increased disease would follow as a consequence of removing AGPs from food-animal production. However, data from the Danish Poultry Council suggest that the removal of antimicrobial agents for growth promotion from poultry production has not had any negative effect, such as decreased productivity or increased mortality [20,80]. On the contrary, the mean kilogram broilers produced per square metre immediately increased and the trend in mortality immediately changed from continuously increasing to decreasing. A minor effect was observed on the feed conversion rate, where there was an increase in the amount of feed needed to produce 1 kg of broiler, but this has since decreased again.
Similar observations have also been reported from the USA. Thus, an analysis of production data from one of the largest poultry producers (Perdue farms), covering 7 million broilers, estimated that the net effect of using AGPs was a loss of around one cent per chicken [21].
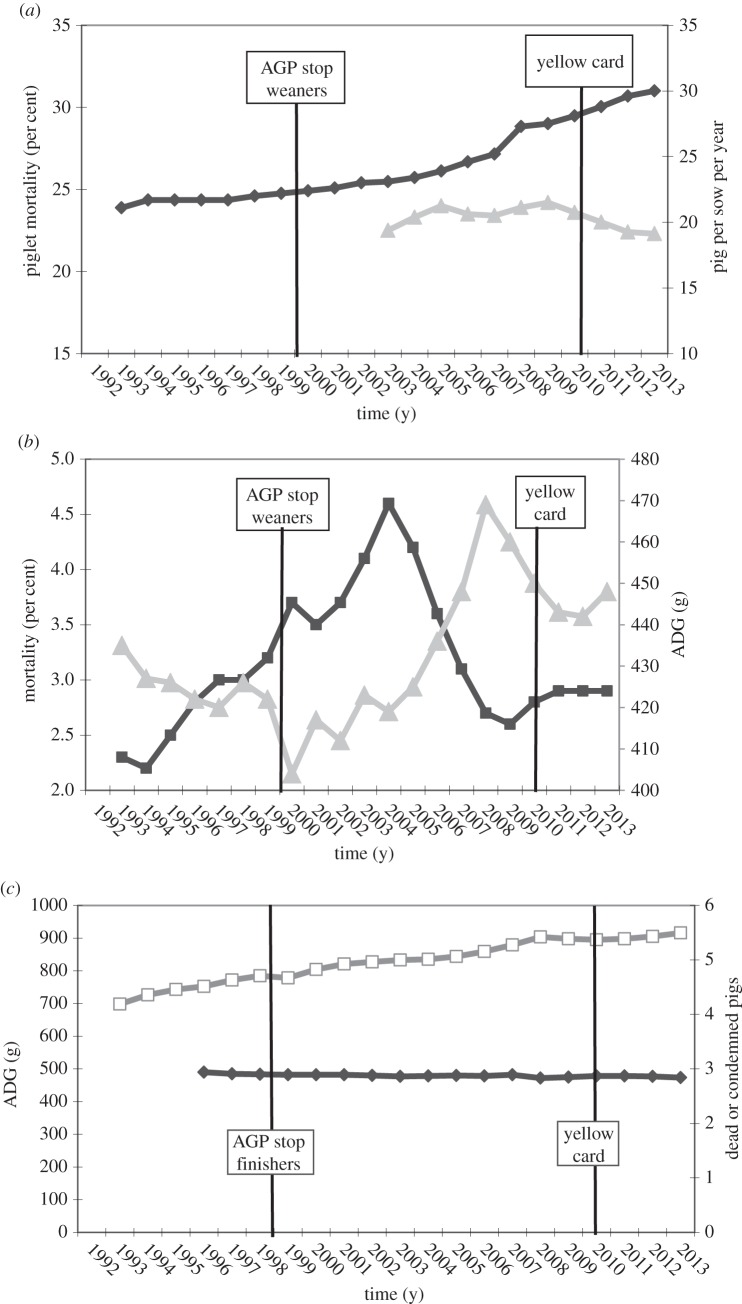
In Danish swine production, the effects of the different interventions over time have been an area of more controversy. Initial analysis only looking at production parameters the year before and the year after the ban of AGPs suggested that this was without any effect in older pigs, but associated with increased mortality and reduced average daily gain in weaners. In 2010, a study was published analysing the trends of a number of available production parameters from 1992 to 2008 [18]. Figure 3 gives an update of these data including an additional 5 years of data from 2009 to 2013, as well as an additional inclusion of piglet mortality in the period 2003–2013. These data over time, contrary to initial observations, suggest that there was either none or a very limited effect of the growth promoter ban and perhaps even suggest that average daily gain decreased before but increased after the ban, and thus that this intervention had a positive effect.
Figure 3.
Data on production characteristics for piglet mortality (per cent; grey triangles) and mean number of pigs produced per sow per year (black diamonds) (a), average daily gain (ADG, grey triangles) and mortality rate (black squares) in weaning pigs (b), and average daily gain (white squares) and the percentage of dead or condemned finishing pigs (black diamonds; (c)) raised in the Danish swine production system from 1992 to 2013. The ban on antimicrobial growth promoters (vertical line) was instituted on 1 April 1998, and 1 January 2000, for finishing and weaning pigs, respectively. Weaning and finishing pigs weighed <35 and >35 kg, respectively. The yellow card scheme was introduced from 1 July 2010. Based on references [18,81].
Another major intervention in Denmark was the yellow card scheme in 2010. A recent study analysed data on vaccine sale around the implementation of the yellow card scheme and compared data on different disease lesions recorded by the Danish meat inspection of finisher pigs in winter 2010 with winter 2011 [82]. They found an increased sale of vaccines following the intervention. Statistical differences were found for several diseases between winter 2010 and 2011 periods, but some diseases increased and others decreased. Whether this is due to a natural year-to-year fluctuation or the intervention is difficult to conclude. However, it was concluded that the intervention did not adversely affect animal health or welfare. In this study, no analyses of swine production parameters were included. In figure 3, a number of selected swine production parameters and data for mortality over several years are included. However, it is not possible to observe any major impact of the yellow card scheme on any of these parameters. Thus, it may be concluded that the yellow card intervention has had very limited if any negative impact on swine productivity in Denmark.
6. The way forward
Even though the precise burden of selection for antimicrobial resistance in the food animal reservoir has not been determined, in the author's opinion there is sufficient evidence to conclude that it is an important burden on human health. Current experience suggests that reducing the routine use of antimicrobial agents for food animals is associated with either very limited negative effects or possibly even positive effects for animal health and welfare as well as economy. It, thus, might seem strange that most countries and farmers continue with a procedure that could be seen as both unnecessary or even having negative consequences. However, it should also be realized that the drug industry, for example, make sales and profits from this usage and has very strong and influential lobbying, marketing and advertisement activities. In addition, it might also be the case that some farmers see the routine use of antimicrobial agents as an easier and safer option than relying on proper clinical diagnosis. Thus, antimicrobial usage might be seen as something that has an average negative effect, but is an insurance against, for example, disastrous outbreaks.
In the coming years, there is a need to strengthen international and cross-sectional collaboration, not only in research but especially between governmental institutions, and to support the work of international organizations such as WHO, OIE and Codex. There is a major need to try different forms of large- and small-scale interventions and particularly to document the negative and positive effects. Thus, the establishment of surveillance programmes collecting data on antimicrobial usage and resistance as well as different animal health and production parameters is greatly needed.
There is a major need for continued research into novel procedures for limiting the use of antimicrobial agents and transmission of resistance. However, we do already know sufficient to begin interventions and there is no need to get paralysed by analysis.
References
- 1.Levy SB. 1982. Microbial resistance to antibiotics. An evolving and persistent problem. Lancet 2, 83–88. ( 10.1016/S0140-6736(82)91701-9) [DOI] [PubMed] [Google Scholar]
- 2.Norrby SR, Nord CE, Finch R, European Society of Clinical Microbiology and Infectious Diseases. 2005. Lack of development of new antimicrobial drugs: a potential serious threat to public health. Lancet Infect. Dis. 5, 115–119. ( 10.1016/S1473-3099(05)01283-1) [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. 2001. WHO global strategy for containment of antimicrobial resistance. Geneva: WHO; http://www.who.int/drugresistance/WHO_Global_Strategy_English.pdf?ua=1. [Google Scholar]
- 4.World Health Organization. 2011. The evolving threat of antimicrobial resistance: options for action. Geneva: WHO; http://whqlibdoc.who.int/publications/2012/9789241503181_eng.pdf?ua=1. [Google Scholar]
- 5.Allen HK, Donato J, Wang HH, Cloud-Hansen KA, Davies J, Handelsman J. 2010. Call of the wild: antibiotic resistance genes in natural environments. Nat. Rev. Microbiol. 8, 251–259. ( 10.1038/nrmicro2312) [DOI] [PubMed] [Google Scholar]
- 6.Wright GD. 2010. Antibiotic resistance in the environment: a link to the clinic? Curr. Opin. Microbiol. 13, 589–594. ( 10.1016/j.mib.2010.08.005) [DOI] [PubMed] [Google Scholar]
- 7.Fraser-Moodie W. 1971. Struggle against infection. Proc. R. Soc. Med. 64, 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacFarlane G. 1984. Alexander Fleming: the man and the myth. Cambridge, MA: Harvard University Press. [Google Scholar]
- 9.Fleming A. 1929. On the antibacterial action of cultures of a penicillum, with special reference to their use in the isolation of B. influenza. Br. J. Exp. Path. 10, 226–236. [PubMed] [Google Scholar]
- 10.Abraham EP, Chain E, Fletcher CM, Gardner AD, Heatley NG, Jennings MA, Florey HW. 1941. Further observations on penicillin. Lancet Aug. 16, 178–189. [PubMed] [Google Scholar]
- 11.Prescott JF. 2006. History of antimicrobial usage in agriculture: an overview. In Antimicrobial resistance in bacteria of animal origin (ed. Aarestrup FM.), pp. 19–27. Washington, DC: ASM Press. [Google Scholar]
- 12.Moore PR, Evenson A, Luckey TD, McCoy E, Elvehjem CA, Hart EB. 1946. Use of sulfasuxidine, streptothricin, and streptomycin in nutritional studies with the chick. J. Biol. Chem. 165, 437–441. [PubMed] [Google Scholar]
- 13.Catron D. 1949. Recent development in swine nutrition. Vet. Med. 44, 215–220. [PubMed] [Google Scholar]
- 14.Whitehill AR, Oleson JJ, Hutchings BL. 1950. Stimulatory effect of aureomycin on the growth of chicks. Proc. Soc. Exp. Biol. Med. 74, 11–13. ( 10.3181/00379727-74-17793) [DOI] [PubMed] [Google Scholar]
- 15.Speer VC, Maddock HM, Cuff PWW, Catron DV. 1951. Growth response of swine fed penicillin. Antibiotics Chemother. 1, 41–46. [PubMed] [Google Scholar]
- 16.Swann MM. 1969. Joint committee on the use of antibiotics in animal husbandry and veterinary medicine. London, UK: HMSO. [Google Scholar]
- 17.Aarestrup FM. 2000. Occurrence, selection and spread of resistance to antimicrobial agents used for growth promotion for food animals in Denmark. APMIS 101, 1–48. ( 10.1111/j.1600-0463.2000.tb05380.x) [DOI] [PubMed] [Google Scholar]
- 18.Aarestrup FM, Jensen VF, Emborg HD, Jacobsen E, Wegener HC. 2010. Changes in the use of antimicrobials and the effects on productivity of swine farms in Denmark. Am. J. Vet. Res. 71, 726–733. ( 10.2460/ajvr.71.7.726) [DOI] [PubMed] [Google Scholar]
- 19.Collignon P, Wegener HC, Braam P, Butler CD. 2005. The routine use of antibiotics to promote animal growth does little to benefit protein undernutrition in the developing world. Clin. Infect. Dis. 41, 1007–1013. ( 10.1086/433191) [DOI] [PubMed] [Google Scholar]
- 20.Emborg H, Ersbøll AK, Heuer OE, Wegener HC. 2001. The effect of discontinuing the use of antimicrobial growth promoters on the productivity in the Danish broiler production. Prev. Vet. Med. 50, 53–70. ( 10.1016/S0167-5877(01)00218-5) [DOI] [PubMed] [Google Scholar]
- 21.Graham JP, Boland JJ, Silbergeld E. 2007. Growth promoting antibiotics in food animal production: an economic analysis. Public Health Rep. 122, 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leibovici L, Paul M, Ezra O. 2012. Ethical dilemmas in antibiotic treatment. J. Antimicrob. Chemother. 67, 12–16. ( 10.1093/jac/dkr425) [DOI] [PubMed] [Google Scholar]
- 23.Black WD. 1984. The use of antimicrobial drugs in agriculture. Can. J. Physiol. Pharmacol. 62, 1044–1048. ( 10.1139/y84-175) [DOI] [PubMed] [Google Scholar]
- 24.Food and Drug Administration. 2014. 2011 Summery report on antimicrobials sold or distributed for use in food-producing animals. http://www.fda.gov/downloads/forindustry/userfees/animaldruguserfeeactadufa/ucm338170.pdf (accessed 13 November 2014).
- 25.FAOSTAT. 2014. http://faostat.fao.org/site/569/DesktopDefault.aspx?PageID=569#ancor (accessed 13 November 2014).
- 26.European Medicines Agency. 2013. Sales of veterinary antimicrobial agents in 25 EU/EEA countries in 2011. Third ESVAC report. http://www.ema.europa.eu/docs/en_GB/document_library/Report/2013/10/WC500152311.pdf.
- 27.Department of Health and Human Services Public Health Service Food and Drug Administration Center for Drug Evaluation and Research Office of Surveillance and Epidemiology. 2012. Drug use review. http://www.fda.gov/downloads/Drugs/DrugSafety/InformationbyDrugClass/UCM319435.pdf (accessed 13 November 2014).
- 28.JETACAR. 1999. The use of antibiotics in food-producing animals: antibiotic-resistant bacteria in animals and humans. Report of the Joint Expert Advisory Committee on Antibiotic Resistance (JETACAR). http://www.health.gov.au/internet/main/publishing.nsf/Content/health-pubs-jetacar-cnt.htm/$FILE/jetacar.pdf (accessed 13 November 2014).
- 29.Aarestrup FM. 2006. The origin, evolution and local and global dissemination of antimicrobial resistance. In Antimicrobial resistance in bacteria of animal origin (ed. Aarestrup FM.), pp. 339–360. Washington, DC: ASM Press. [Google Scholar]
- 30.Boerlin P, Reid-Smith RJ. 2008. Antimicrobial resistance: its emergence and transmission. Anim. Health Res. Rev. 9, 115–126. ( 10.1017/S146625230800159X) [DOI] [PubMed] [Google Scholar]
- 31.Aarestrup FM, Wegener HC, Collignon P. 2008. Resistance in bacteria of the food chain: epidemiology and control strategies. Expert Rev. Anti-Infect. Ther. 6, 733–750. ( 10.1586/14787210.6.5.733) [DOI] [PubMed] [Google Scholar]
- 32.Osborn AM, Böltner D. 2002. When phage, plasmids, and transposons collide: genomic islands, and conjugative- and mobilizable-transposons as a mosaic continuum. Plasmid 48, 202–212. ( 10.1016/S0147-619X(02)00117-8) [DOI] [PubMed] [Google Scholar]
- 33.Threlfall EJ. 2000. Epidemic Salmonella typhimurium DT 104: a truly international multiresistant clone. J. Antimicrob. Chemother. 46, 7–10. ( 10.1093/jac/46.1.7) [DOI] [PubMed] [Google Scholar]
- 34.Price LB, et al. 2012. Staphylococcus aureus CC398: host adaptation and emergence of methicillin resistance in livestock. MBio 3, e00305–11. ( 10.1128/mBio.00305-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agersø Y, Jensen JD, Hasman H, Pedersen K. 2014. Spread of extended spectrum cephalosporinase-producing Escherichia coli clones and plasmids from parent animals to broilers and to broiler meat in a production without use of cephalosporins. Foodborne Pathog. Dis. 11, 740–746. ( 10.1089/fpd.2014.1742) [DOI] [PubMed] [Google Scholar]
- 36.Marshall BM, Levy SB. 2011. Food animals and antimicrobials: impacts on human health. Clin. Microbiol. Rev. 24, 718–733. ( 10.1128/CMR.00002-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis HC, Mølbak K, Reese C, Aarestrup FM, Selchau M, Sørum M, Skov RL. 2008. Pigs as source of methicillin-resistant Staphylococcus aureus CC398 infections in humans, Denmark. Emerg. Infect. Dis. 14, 1383–1389. ( 10.3201/eid1409.071576) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collignon P, Aarestrup FM, Irwin R, McEwen S. 2013. Human deaths and third-generation cephalosporin use in poultry, Europe. Emerg. Infect. Dis. 19, 1339–1340. ( 10.3201/eid.1908.120681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agersø Y, Hasman H, Cavaco LM, Pedersen K, Aarestrup FM. 2012. Study of methicillin resistant Staphylococcus aureus (MRSA) in Danish pigs at slaughter and in imported retail meat reveals a novel MRSA type in slaughter pigs. Vet. Microbiol. 157, 246–250. ( 10.1016/j.vetmic.2011.12.023) [DOI] [PubMed] [Google Scholar]
- 40.García-Álvarez L, et al. 2011. Meticillin-resistant Staphylococcus aureus with a novel mecA homologue in human and bovine populations in the UK and Denmark: a descriptive study. Lancet Infect. Dis. 11, 595–603. ( 10.1016/S1473-3099(11)70126-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hensgens MP, Keessen EC, Squire MM, Riley TV, Koene MG, de Boer E, Lipman LJ, Kuijper EJ, European Society of Clinical Microbiology and Infectious Diseases Study Group for Clostridium difficile (ESGCD). 2012. Clostridium difficile infection in the community: a zoonotic disease? Clin. Microbiol. Infect. 18, 635–645. ( 10.1111/j.1469-0691.2012.03853.x) [DOI] [PubMed] [Google Scholar]
- 42.Vieira AR, Collignon P, Aarestrup FM, McEwen SA, Hendriksen RS, Hald T, Wegener HC. 2011. Association between antimicrobial resistance in Escherichia coli isolates from food animals and blood stream isolates from humans in Europe: an ecological study. Foodborne Pathog. Dis. 8, 1295–1301. ( 10.1089/fpd.2011.0950) [DOI] [PubMed] [Google Scholar]
- 43.Anonymous. 2003. Joint FAO/OIE/WHO expert workshop on non-human antimicrobial usage and antimicrobial resistance: scientific assessment, Geneva, 1–5 December 2003 http://www.who.int/zoonoses/resources/amresistance/en/. [Google Scholar]
- 44.Anonymous. 2004. Second Joint FAO/ OIE/WHO expert workshop on non-human antimicrobial usage and antimicrobial resistance: management options, Oslo, 15–18 March 2004 http://www.who.int/zoonoses/resources/amresistance/en/. [Google Scholar]
- 45.Anonymous. 2007. Joint FAO/WHO/OIE expert meeting on critically important antimicrobials. In Report of the FAO/WHO/OIE Expert meeting FAO, Rome, Italy 26–30 November 2007 http://www.who.int/foodborne_disease/resources/Report%20joint%20CIA%20Meeting.pdf?ua=1. [Google Scholar]
- 46.Wegener HC. 2010. Danish initiatives to improve the safety of meat products. Meat Sci. 84, 276–283. ( 10.1016/j.meatsci.2009.06.025) [DOI] [PubMed] [Google Scholar]
- 47.Franklin A, et al. 2001. Antimicrobial resistance: harmonisation of national antimicrobial resistance monitoring and surveillance programmes in animals and in animal-derived food. Rev. Sci. Tech. 20, 859–870. [DOI] [PubMed] [Google Scholar]
- 48.White DG, et al. 2001. Antimicrobial resistance: standardisation and harmonisation of laboratory methodologies for the detection and quantification of antimicrobial resistance. Rev. Sci. Tech. 20, 849–858. [DOI] [PubMed] [Google Scholar]
- 49.Aarestrup FM. 2004. Monitoring of antimicrobial resistance among food animals: principles and limitations. J. Vet. Med. B Infect. Dis. Vet. Public Health 51, 380–388. ( 10.1111/j.1439-0450.2004.00775.x) [DOI] [PubMed] [Google Scholar]
- 50.European Food Safety Authority: Working Group on Developing Harmonised Schemes for Monitoring Antimicrobial Resistance in Zoonotic Agents. 2008. Harmonised monitoring of antimicrobial resistance in Salmonella and Campylobacter isolates from food animals in the European Union. Clin. Microbiol. Infect. 14, 522–533. ( 10.1111/j.1469-0691.2008.02000.x) [DOI] [PubMed] [Google Scholar]
- 51.Aarestrup FM, Bager F, Jensen NE, Madsen M, Meyling A, Wegener HC. 1998. Resistance to antimicrobial agents used for animal therapy in pathogenic-, zoonotic- and indicator bacteria isolated from different food animals in Denmark: a baseline study for the Danish integrated antimicrobial resistance monitoring programme (DANMAP). APMIS 106, 745–770. ( 10.1111/j.1699-0463.1998.tb00222.x) [DOI] [PubMed] [Google Scholar]
- 52.Aarestrup FM, Seyfarth AM, Emborg HD, Pedersen K, Hendriksen RS, Bager F. 2001. Effect of abolishment of the use of antimicrobial agents for growth promotion on occurrence of antimicrobial resistance in fecal enterococci from food animals in Denmark. Antimicrob. Agents Chemother. 45, 2054–2059. ( 10.1128/AAC.45.7.2054-2059.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aarestrup FM. 2000. Characterization of glycopeptide-resistant Enterococcus faecium (GRE) from broilers and pigs in Denmark: genetic evidence that persistence of GRE in pig herds is associated with coselection by resistance to macrolides. J. Clin. Microbiol. 38, 2774–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aarestrup FM, et al. 2012. Integrating genome-based informatics to modernize global disease monitoring, information sharing, and response. Emerg. Infect. Dis. 18, e1 ( 10.3201/eid/1811.120453) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Didelot X, Bowden R, Wilson DJ, Peto TE, Crook DW. 2012. Transforming clinical microbiology with bacterial genome sequencing. Nat. Rev. Genet. 13, 601–612. ( 10.1038/nrg3226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hasman H, Saputra D, Sicheritz-Ponten T, Lund O, Svendsen CA, Frimodt-Møller N, Aarestrup FM. 2014. Rapid whole-genome sequencing for detection and characterization of microorganisms directly from clinical samples. J. Clin. Microbiol. 52, 139–146. ( 10.1128/JCM.02452-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Köser CU, et al. 2012. Rapid whole-genome sequencing for investigation of a neonatal MRSA outbreak. N. Engl. J. Med. 366, 2267–2275. ( 10.1056/NEJMoa1109910) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Loman NJ, et al. 2013. A culture-independent sequence-based metagenomics approach to the investigation of an outbreak of Shiga-toxigenic Escherichia coli O104:H4. JAMA 309, 1502–1510. ( 10.1001/jama.2013.3231) [DOI] [PubMed] [Google Scholar]
- 59.Zankari E, Hasman H, Kaas RS, Seyfarth AM, Agersø Y, Lund O, Larsen MV, Aarestrup FM. 2013. Genotyping using whole-genome sequencing is a realistic alternative to surveillance based on phenotypic antimicrobial susceptibility testing. J. Antimicrob. Chemother. 68, 771–777. ( 10.1093/jac/dks496) [DOI] [PubMed] [Google Scholar]
- 60.DANMAP. http://danmap.org/.
- 61.Adriaenssens N, et al. 2011. European surveillance of antimicrobial consumption (ESAC): outpatient antibiotic use in Europe (1997–2009). J. Antimicrob. Chemother. 66(Suppl. 6), 3–12. ( 10.1093/jac/dkr453) [DOI] [PubMed] [Google Scholar]
- 62.European Centre for Disease Prevention and Control. 2014. Surveillance of antimicrobial consumption in Europe 2012. Stockholm, Sweden: ECDC; http://www.ecdc.europa.eu/en/publications/Publications/antimicrobial-consumption-europe-esac-net-2012.pdf. [Google Scholar]
- 63.Mevius D, Heederik D. 2014. Reduction of antibiotic use in animals ‘‘let's go Dutch’’. J. Verbr. Lebensm. 9, 177–181. ( 10.1007/s00003-014-0874-z) [DOI] [Google Scholar]
- 64.NETHMAP. 2014. Monitoring of antimicrobial resistance and antibiotic usage in animals in the Netherlands in 2013. http://www.wageningenur.nl/upload_mm/1/a/1/0704c512-5b42-4cef-8c1b-60e9e3fb2a62_NethMap-MARAN2014.pdf.
- 65.Collignon P, Powers JH, Chiller TM, Aidara-Kane A, Aarestrup FM. 2009. World Health Organization ranking of antimicrobials according to their importance in human medicine: a critical step for developing risk management strategies for the use of antimicrobials in food production animals. Clin. Infect. Dis. 49, 132–141. ( 10.1086/599374) [DOI] [PubMed] [Google Scholar]
- 66.World Health Organization. 2012. Critically important antimicrobials for human medicine, 3rd revision. http://apps.who.int/iris/bitstream/10665/77376/1/9789241504485_eng.pdf?ua=1.
- 67.Cheng AC, Turnidge J, Collignon P, Looke D, Barton M, Gottlieb T. 2012. Control of fluoroquinolone resistance through successful regulation, Australia. Emerg. Infect. Dis. 18, 1453–1460. ( 10.3201/eid1809.111515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Agersø Y, Aarestrup FM. 2013. Voluntary ban on cephalosporin use in Danish pig production has effectively reduced extended-spectrum cephalosporinase-producing Escherichia coli in slaughter pigs. J. Antimicrob. Chemother. 68, 569–572. ( 10.1093/jac/dks427) [DOI] [PubMed] [Google Scholar]
- 69.Dutil L, et al. 2010. Ceftiofur resistance in Salmonella enterica serovar Heidelberg from chicken meat and humans, Canada. Emerg. Infect. Dis. 16, 48–54. ( 10.3201/eid1601.090729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Leeuwen WJ, van Embden J, Guinée P, Kampelmacher EH, Manten A, van Schothorst M, Voogd CE. 1979. Decrease of drug resistance in Salmonella in the Netherlands. Antimicrob. Agents Chemother. 16, 237–239. ( 10.1128/AAC.16.2.237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boerlin P, Wissing A, Aarestrup FM, Frey J, Nicolet J. 2001. Antimicrobial growth promoter ban and resistance to macrolides and vancomycin in enterococci from pigs. J. Clin. Microbiol. 39, 4193–4195. ( 10.1128/JCM.39.11.4193-4195.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wiuff C, Lykkesfeldt J, Svendsen O, Aarestrup FM. 2003. The effects of oral and intramuscular administration and dose escalation of enrofloxacin on the selection of quinolone resistance among Salmonella and coliforms in pigs. Res. Vet. Sci. 75, 185–193. ( 10.1016/S0034-5288(03)00112-7) [DOI] [PubMed] [Google Scholar]
- 73.Nguyen TT, Chachaty E, Huy C, Cambier C, de Gunzburg J, Mentré F, Andremont A. 2012. Correlation between fecal concentrations of ciprofloxacin and fecal counts of resistant Enterobacteriaceae in piglets treated with ciprofloxacin: toward new means to control the spread of resistance? Antimicrob. Agents Chemother. 56, 4973–4975. ( 10.1128/AAC.06402-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Béraud R, Huneault L, Bernier D, Beaudry F, Letellier A, del Castillo JR. 2008. Comparison of the selection of antimicrobial resistance in fecal Escherichia coli during enrofloxacin administration with a local drug delivery system or with intramuscular injections in a swine model. Can. J. Vet. Res. 72, 311–319. [PMC free article] [PubMed] [Google Scholar]
- 75.Bibbal D, Dupouy V, Ferré JP, Toutain PL, Fayet O, Prère MF, Bousquet-Mélou A. 2007. Impact of three ampicillin dosage regimens on selection of ampicillin resistance in Enterobacteriaceae and excretion of blaTEM genes in swine feces. Appl. Environ. Microbiol. 73, 4785–4790. ( 10.1128/AEM.00252-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Randall LP, Cooles SW, Coldham NC, Stapleton KS, Piddock LJ, Woodward MJ. 2006. Modification of enrofloxacin treatment regimens for poultry experimentally infected with Salmonella enterica serovar Typhimurium DT104 to minimize selection of resistance. Antimicrob. Agents Chemother. 50, 4030–4037. ( 10.1128/AAC.00525-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alban L, Nielsen EO, Dahl J. 2008. A human health risk assessment for macrolide-resistant Campylobacter associated with the use of macrolides in Danish pig production. Prev. Vet. Med. 83, 115–129. ( 10.1016/j.prevetmed.2007.06.006) [DOI] [PubMed] [Google Scholar]
- 78.Christensen BB, Nauta M, Korsgaard H, Sørensen AIV, Rosenquist H, Boysen L, Perge A, Nørrung B. 2013. Case-by-case risk assessment of broiler meat batches: an effective control strategy for Campylobacter. Food Control 31, 485–490. ( 10.1016/j.foodcont.2012.10.025) [DOI] [Google Scholar]
- 79.Berge AC, Moore DA, Besser TE, Sischo WM. 2009. Targeting therapy to minimize antimicrobial use in preweaned calves: effects on health, growth, and treatment costs. J. Dairy Sci. 92, 4707–4714. ( 10.3168/jds.2009-2199) [DOI] [PubMed] [Google Scholar]
- 80.Anonymous 2002. Impacts of antimicrobial growth promoter termination in Denmark, Foulum, Denmark, 6–9 November 2002 http://apps.who.int/iris/bitstream/10665/68357/1/WHO_CDS_CPE_ZFK_2003.1.pdf?ua=1. [Google Scholar]
- 81.Vinther J. 2014. Landsgennemsnit for produktivitet in svineproduktionen. http://www.vsp.lf.dk/Publikationer/Kilder/Notater/2014/1422.aspx.
- 82.Alban L, Dahl J, Andreasen M, Petersen JV, Sandberg M. 2013. Possible impact of the ‘yellow card’ antimicrobial scheme on meat inspection lesions in Danish finisher pigs. Prev. Vet. Med. 108, 334–341. ( 10.1016/j.prevetmed.2012.11.010) [DOI] [PubMed] [Google Scholar]