Abstract
[Purpose]
This study investigated the effect of the vinegar, which is made of 4-year-old mountain-cultivated ginseng ripened into 4-year-matured persimmon vinegar, on the blood lipids level and inflammatory cytokines concentration in obese female adolescents.
[Methods]
Subjects ingested the vinegar, so-called 'mountain-cultivated ginseng persimmon vinegar (MPV)', without meals every day for 6 weeks with activities control. Subjects were grouped into control (CON), persimmon vinegar (PV), and MPV with 10 people in each group. Blood lipids, triglyceride (TG), total-cholesterol (TC), and high density lipoprotein-cholesterol (HDL-C) were analyzed. Also, glutamic oxaloacetic transaminase (GOT) and glutamate pyruvate transaminase (GPT) were analyzed for the hepatotoxicity. Blood cytokines, interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) were analyzed.
[Results]
Subjects showed a high reduction in body weight and body fat. Their blood lipid level was effectively improved, and the secretion of inflammatory cytokine was suppressed as well, except for TNF-α. However, the change ratio of the cytokines was high in PV and MPV. Such results were similar to those from research subjects who took persimmon vinegar only (PV), but the effect of the vinegar (MPV) was more remarkable. Besides, this mixture was found to have no effect on the hepatotoxicity.
[Conclusion]
The significance of this study is that all the experiments were conducted without controlling research subjects' daily lives, and it is suggested that the vinegar may be recommended as a kind of health supplement food to suppress obesity. Especially, since these two products are traditional foods of Korean people, which have been taken for ages, it is expected that the fusing of two foods may be better applied to ordinary people who are concerned about obesity.
Keywords: Mountain-cultivated ginseng, persimmon vinegar, blood lipids, inflammatory cytokines, obese adolescents
INTRODUCTION
In the past, obesity was regarded just as a phenomenon; however, it is now a contagious disease, which is controlled by WHO and Korean health authority, with disease classification code (E65-E68) corresponding to obesity within endocrine system disorder, nutrition disorder, metabolic disease, and other hyperalimentation. Especially, it is possible to know that comprehensive management on excess fat is the focus, because localized adiposity, obesity, other hyperalimentation, and sequelae of hyperalimentation are all included [1].
The adolescents and adults have more possibility of exposure to obesity, because they choose their meals [2,3] in accordance with the variables in the rapidly changing social structure [4]. Accordingly, studies on the nutrition control and the effect of exercise are being conducted continuously, to decrease excess fat in body by the following means: intake calorie limitation [5], exercise [6], and combination of diet therapy and exercise [7]. Even if one may have succeeded in weight control through diet therapy, exercise therapy, or surgery therapy, it is still difficult to maintain the decreased weight due to the yo-yo syndrome. The big expenditure [8] to resolve this issue is another reason for such studies. Therefore, it is judged that an approach with new viewpoint would be required to resolve the issue of obesity.
Obesity includes chronic inflammatory response [9], and it is related to pathogenesis mechanism of inflammatory response at hypothalamus [10]. In general, immunity response is improved by exercise training [11]; however, excess exercise can also cause disease by boosting the production of cytokine [12]. Calorie limitation improves health and extends the life of a monkey [13]; however, there is a report that dietary limitation [14,15] or starvation for a long time [16] weakens immunity response. In the case of obesity by weight-cycling, it has been reported that the immunity metabolism should be interpreted differently from the existing interpretation, and it should be investigated again [17]. Therefore, an easier solution for relieving obesity is required.
Meanwhile, persimmon vinegar, which is one of traditional foods, contains various organic acids and amino acids. If persimmon vinegar is taken before exercise, it increases free fatty acid in blood, keeps low respiration exchange ratio, and promotes oxidization [18]. Meanwhile, mountain-cultivated ginseng has high concentration of phenol compound [19] and various ginsenosides [20]. It has been reported that taking mountain-cultivated ginseng decreases blood lipids [21] and restricts inflammatory response by ginsenosides [22]. Another literature reports that taking mountain-cultivated ginseng ripened into persimmon vinegar (MPV) improves blood lipids, enhances anti-oxidized function, and increases energy metabolism [23]. However, all of these existing studies were based on rodents, and there is no study based on an actual application to human body. As interest on health has increased recently, various vinegar drinks are increasing introduced in the market. Therefore, it is judged that a study applying the results confirmed on rodents to human body would be meaningful.
Therefore, this study will use MPV, which is prepared by mixing the mountain-cultivated ginseng with persimmon vinegar, according to the method suggested in literature previously reported [24]. Obese female university students will take MPV for a long time, without any control over daily living, any encouragement on exercise training, or meal education.; and the changes in blood lipid and the concentration of proinflammatory cytokine will be investigated. This study will verify whether taking MPV, which is a mixture of Korean persimmon vinegar and the mountain-cultivated ginseng, would be an easier way for the relief of obesity.
METHODS
Subjects
Initially, 56 obese female university students were chosen with body fat percentage of 27% or more, as measured by a device utilizing bioelectrical impedance method (InBody 720, Biospace, Korea). Then, 30 obese female university students out of 56 were selected, in order to align the age, height, and weight at the similar level as control group (CON), persimmon vinegar taking group (PV), and mountain-cultivated ginseng ripened with persimmon vinegar taking group (MPV). Each group had 10 obese female university students. Explanation was given to the chosen subjects about the food to take. We promised to supply MPV to CON group and PV group, even after the study period. Blood-gathering and partial body exposures during the study period were also explained, and their consent was obtained. We did not restrict meal, alcohol drinking, snack, physical activity, or periodical exercise of the subjects during the study period. We also asked the subjects not to try any weight decrease by dietary control, including fasting or eating less. The basic ethics for study followed the 1964 Helsinki Declaration. The general physical characteristics of the subjects are shown in <Table 1>.
Table 1.
Physical characteristics of the subjects
| CON (n = 10) | PV (n = 10) | MPV (n = 10) | |
|---|---|---|---|
| Age (years) | 21.6 ± 0.5 | 21.8 ± 0.5 | 21.0 ± 0.9 |
| Height (cm) | 159.4 ± 4.0 | 158.8 ± 3.7 | 159.2 ± 3.2 |
| Body weight (kg) | 63.2 ± 1.7 | 64.4 ± 1.6 | 65.4 ± 2.5 |
| Fat (%) | 27.3 ± 2.7 | 27.9 ± 1.7 | 28.8 ± 2.1 |
| BMI (kg/m2) | 24.9 ± 1.4 | 25.6 ± 1.6 | 25.8 ± 1.1 |
Values are mean ± SE. CON: control group; PV: persimmon vinegar ingestion group; MPV: mountain ginseng ripened into persimmon vinegar ingestion group; BMI: body mass index. All items were not significant among the groups.
Preparation of MPV
We used 4-year ripened persimmon vinegar produced at Sangju of Gyeongsangbuk-do and 4-year mountain-cultivated ginseng produced at Andong of Gyeongsangbuk-do [25]. Preparation of MPV followed the method given in domestic patent [25]. The components of MPV are the same with those given in the report of Korea Forest Office [26].
Intake method
All subjects visited laboratory at 8 o’clock in the morning of every day and received the intakes. The control group was supposed to drink only water, but they also visited the laboratory at 8 a.m. so that the time of awaking in all test subjects would be kept the same as possible. Double blinded method was not applied because of its odor and taste, which was the limitation of this study. PV and MPV of 200 ml each were diluted 5 times with water, and the subjects in the groups drank it in portions throughout the daily living. Therefore, the total daily intake was 1,000 ml based on diluted PV or MPV. We gave these in two 500 ml P.E.T. bottles. It continued on Sundays in the same way as weekdays. If a test subject cannot come on a certain day, due to personal reasons, we gave two times the daily quantity so that the test subject can continue to take it on that missing day. The total period of intake was 6 weeks. For the same material ingested experiments [23,24], they were administered for 4 weeks to the rodents. Considering the duration of adaptation of the material, 6-weeks was chosen as the ingestion periods.
Measurement and analysis
The test subjects visited lab after fasting for 12 hours or more and after relieving bowel. Their physiques were measured and blood samples were taken after a short explanation on the instructions for measurement and blood gathering. Their heights, weights, and body fat ratio were measured, while BMI’s were also calculated. Then, the medical technologist took 10 cc of blood from the subjects’ forearm vein. Serums were separated from the gathered blood by a centrifuge. The serums were kept at -80℃ until analysis.
Total cholesterol (TC) and triacylglycerol (TG) concentrations were measured by a measuring kit using enzymatic method (Asan Pharmaceutical of Korea) and spectrophotometer (Optizen pop, Mecasys, Korea). Glutamic oxaloacetic transaminase (GOT), high-density lipoprotein cholesterol (HDL-C), and glutamic pyruvic transaminase (GPT) were analyzed to investigate the impact on liver function by using the reagents (Asan Pharmaceutical of Korea) and automatic biochemistry analyzer (BS-220, Mindray, China).
Interleukin-6 (IL-6) was analyzed to learn about the change in proinflammatory cytokine concentration, by using immunoassay kit (R&D Systems, USA); and tumor necrosis factor alpha (TNF-α) was analyzed by TNF-α Immunoassay kit (R&D Systems, USA). Nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) was analyzed by NFκB Active kit (Imgenex Corporation, USA). The concentration of proinflammatory cytokine was applied using the method suggested by each reagent. ELISA reader (Infinite F50, Tacan, Switzerland) was used for all absorbance measurements.
Statistical processing
All data are shown as mean and standard error. Paired t-test was used for the comparison of before intake and after intake in each group. Δ value ratio was obtained by Excel software (Excel 2013, Microsoft Office, USA) to learn about the difference between before intake and after intake among CON, PV, and MPV. One way ANOVA was done by statistical program SPSS 18.0 for the comparison of data. Post-hoc test was done by Tukey method. All statistically significant differences were set as p <0.05.
RESULTS
Body composition change
There was no significant difference in the weight, body fat ratio, and BMI of PV and MPV compared to CON <Table 2>. However, they showed the tendency of decrease after the intake, compared to before the intake. The difference between before the intake and after the intake within each group were in the order as follows: CON < PV < MPV. MPV had significant decrease in weight and body fat ratio, compared to CON (p < 0.05; Table 3).
Table 2.
Changes in body weight, fat ratio, and BMI
| CON |
PV |
MPV |
||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| Body weight (kg) | 63.2 ± 1.7 | 64.2 ± 1.2 | 64.4 ± 1.6 | 62.2 ± 2.3 | 65.4 ± 2.5 | 61.2 ± 1.8 |
| Fat (%) | 27.3 ± 2.7 | 27.7 ± 2.0 | 27.9 ± 1.7 | 26.2 ± 1.2 | 28.8 ± 2.1 | 26.3 ± 1.3 |
| BMI (kg/m2) | 24.9 ± 1.4 | 25.3 ± 1.4 | 25.6 ± 1.6 | 24.7 ± 1.4 | 25.8 ± 1.1 | 24.2 ± 1.1 |
Values are mean ± SE. CON: control group; PV: persimmon vinegar ingestion group; MPV: mountain ginseng ripened into persimmon vinegar ingestion group; BMI: body mass index. All items were not significant among the groups.
Table 3.
Delta values of the body weight, fat ratio, and BMI
| CON | PV | MPV | |
|---|---|---|---|
| Body weight change (%) | 1.6 ± 1.9a | -3.3 ± 5.8a,b | -6.3 ± 4.4b |
| Fat ratio change (%) | 1.8 ± 2.8a | -6.1 ± 4.7a,b | -8.4 ± 2.4b |
| BMI change (%) | 1.6 ± 1.9a | -3.3 ± 5.8a | -6.3 ± 4.4a |
Values are mean ± SE. CON: control group; PV: persimmon vinegar ingestion group; MPV: mountain ginseng ripened into persimmon vinegar ingestion group; BMI: body mass index. Different letter means significant within the measured item, p < 0.05.
Blood lipids change
<Fig 1A-1C> show the changes in blood lipids within each group. Triglyceride in blood and total cholesterol had statistically significant decrease in PV and MPV after the intake, compared to before the intake (p < 0.05). There was no significant difference in high-density lipoprotein cholesterol in all groups after the intake, compared to before the intake.
Fig. 1.
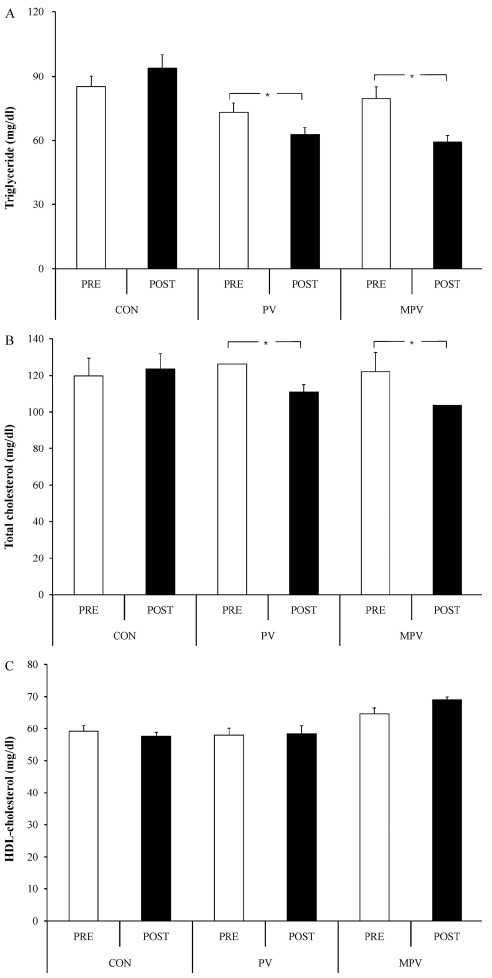
Changes of blood lipids, A: triglyceride; B: total cholesterol; C: HDL-cholesterol at pre- and post-ingestion in each group. Bars are mean and standard error. White bars are at pre-ingestion, and black bars are at the post-ingestion. CON: control group; PV: persimmon vinegar ingestion group; MPV: mountain ginseng ripened into persimmon vinegar ingestion group. Asterisks between trials means statistically significant at p < 0.05.
<Fig. 2A-2C> shows the group comparison of blood lipid change rate. Neutral fat in blood and total cholesterol had statistically significant decrease in PV and MPV, compared to CON, and after the intake compared to before the intake (p < 0.05). On the contrary, there was a significant increase in high-density lipoprotein cholesterol in PV and MPV compared to CON. Especially MPV had statistically significant difference compared to other two groups (p < 0.05).
Fig. 2.
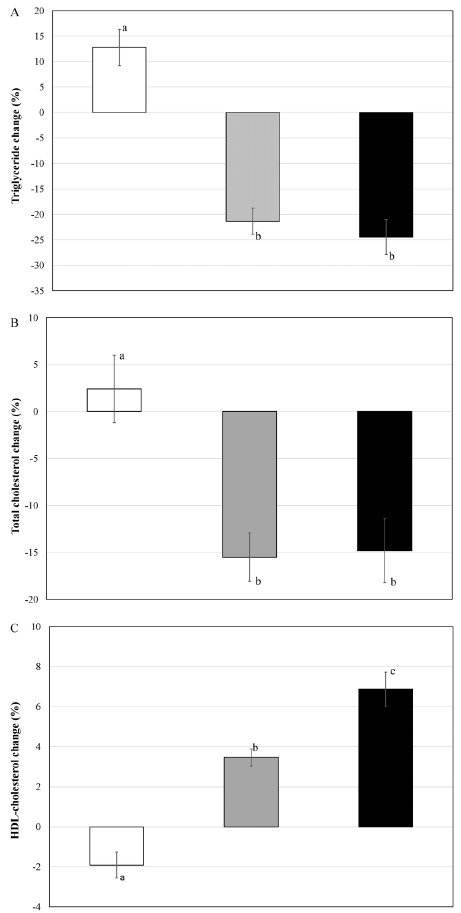
Differences in blood lipids concentration change ratio, A: triglyceride; B: total cholesterol; C: HDL-cholesterol at pre- and post-ingestion in each group. Bars are mean and standard error. White bars are CON, gray bars are PV, and black bars are MPV. Different letters among the group indicate statistical significance at p < 0.05.
GOT and GPT change
<Table 4, 5> show the concentration changes and change ratio of glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT) in blood. There was no significant change after the intake, compared to before the intake, except for the GOT in MPV. GPT also significantly decreased in MPV (p < 0.05).
Table 4.
Changes of GOT and GPT concentration
| CON |
PV |
MPV |
||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| GOT (IU/l) | 26.7 ± 2.1 | 28.4 ± 2.5 | 24.5 ± 2.4 | 25.4 ± 1.0 | 29.7 ± 2.2* | 23.5 ± 1.8* |
| GPT (IU/l) | 17.3 ± 2.4 | 21.3 ± 0.9 | 17.8 ± 2.1 | 16.9 ± 2.3 | 21.8 ± 1.0* | 19.5 ± 0.9* |
Values are mean ± SE. GOT: glutamic oxaloacetic transaminase; GPT: glutamic pyruvic transaminase. CON: control group; PV: persimmon vinegar ingestion group; MPV: mountain ginseng ripened into persimmon vinegar ingestion group; BMI: body mass index. NS: not significant. Asterisks between pre- and post- within group are statistically significant at p < 0.05.
Table 5.
Change ratio of GOT and GPT concentration
| CON | PV | MPV | |
|---|---|---|---|
| GOT change (%) | 6.46 ± 0.88a | 5.93 ± 0.78a | -19.33 ± 1.89b |
| GPT change (%) | 7.71 ± 2.57a | 6.39 ± 2.13a | -16.40 ± 5.46b |
Values are mean ± SE. GOT: glutamic oxaloacetic transaminase; GPT: glutamic pyruvic transaminase. CON: control group; PV: persimmon vinegar ingestion group; MPV: mountain ginseng ripened into persimmon vinegar ingestion group; BMI: body mass index. Different letter indicates significance within the measured item, p < 0.05.
Regarding the group comparison of GOT and GPT change rates, MPV had significant decrease in both items, compared to CON and PV (p < 0.05).
Inflammatory cytokines change
<Fig. 3A-3C> show the concentration changes of proinflammatory cytokine. Only MPV had significant decrease (p < 0.05) after the intake of Interleukin-6 (IL-6), compared to before the intake; and the other two groups did not have significant change. There was no significant difference in the tumor necrosis factor-α (TNF-α) in all groups after the intake, compared to before the intake; however, PV and MPV showed decreasing tendency. There were statistically significant differences in the nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) of all groups after the intake, compared to before the intake (p < 0.05). It increased in CON, but it significantly decreased in PV and MPV (p <0.05). <Fig. 4A-4C> show the group comparison of change rate after the intake, compared to before the intake. PV and MPV showed significant decrease in all proinflammatory cytokine analysis items compared to CON (p < 0.05).
Fig. 3.
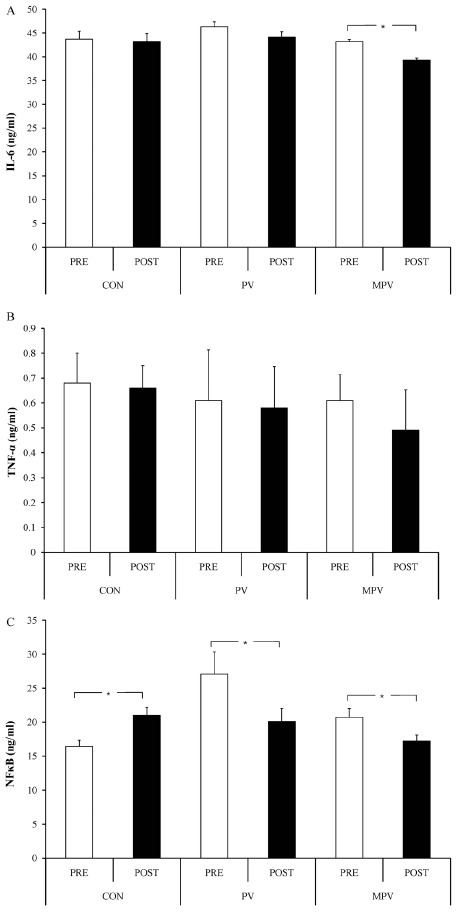
Changes of inflammatory cytokines, A: IL-6; B: TNF-α; C: NFκB, at pre- and post-ingestion in each group. Bars are mean and standard error. White bars are at pre-ingestion, and black bars are at the post-ingestion. CON: control group; PV: persimmon vinegar ingestion group; MPV: mountain ginseng ripened into persimmon vinegar ingestion group. Asterisks between trials indicate statistical significant at p < 0.05.
Fig. 4.
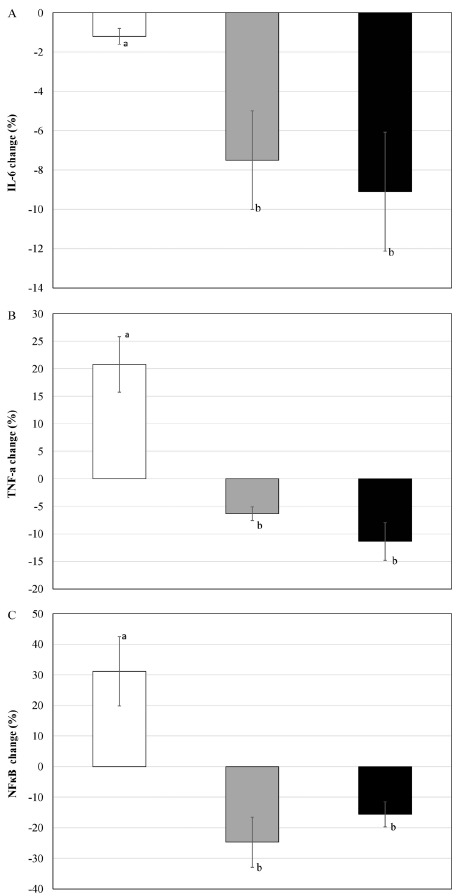
Differences of inflammatory cytokines concentration change ratio, A: IL-6; B: TNF-α; C: NFκB, at pre- and post-ingestion in each group. Bars are mean and standard error. White bars are CON, gray bars are PV, and black bars are MPV. Different letter among the group indicates statistical significance at p < 0.05.
DISCUSSION
This study investigated the change in blood lipids and proinflammatory cytokines, when obese female university students took mountain-cultivated ginseng ripened into persimmon vinegar (MPV) for a long time. The characteristic of this study is that it considered about the sense of burden for practicing and continuing various methods, such as exercise training or control of nutrition, which follow to resolve obesity. In other words, this study suggested an easier method of resolving obesity by studying the impact from a convenient intake of MPV for a long time. Everyday living was kept as usual without participating in exercise training or controlling nutrition intake, because such methods have high possibility of yo-yo syndrome, once the weight decreasing method is not continued.
There was no statistically significant change in body weight, between before and after the intake of PV or MPV. However, there was a difference in the change rates among the groups. PV group only had a trend of weight decrease; however, MPV group had significant decrease in both the weight and body fat ratio. From this, we can determine that the impact of MPV was higher. Liu et al. [27] reported that epididymis fat was significantly decreased when total saponin in ginseng was supplied for 4 weeks to a rat on high fat diet. The reason for this is that total saponin partially promotes the secretion of glucagon-like peptide-1 (GLP-1). There is a report that subcutaneous transfusion of panax notoginseng saponin for 12 days to a KK-Ay mice resulted in the restriction of weight increase, fat-storing restriction, and anti-obesity effect [28]. In addition, there is a report that ginsenoside Rb1 increases the AMP-activated protein kinase (AMPK) and restricts fat-storing in liver [29]. The weight increase restriction and fat-storing restriction by saponin and ginsenoside may be caused by the thermogenesis increasing. [30]. It is judged that the saponin in ginseng may increase energy consumption and prevent metabolic syndrome [31]. Actually, a study reported that the intake of saponin in ginseng has an effect of increasing body heat production in a cold environment [32]. Further, another study reported that the intake of MPV had an effect of weight increase restriction in laboratory animals [23,24]. From a different viewpoint, the decrease in fat quantity by using vinegar can also be considered. There have been study reports as follows: the lipolysis increase in obese rat on high fat diet by vinegar intake [33], decrease in weight and visceral fat by the intake of tomato vinegar [34], restriction on 3T3-L1 fat cell differentiation of obese fat by the intake of tomato vinegar without any change in meal quantity or intake calories [35], appetite restriction and empty stomach time restriction by the intake of vinegar [36], and decrease in fat cell size by the intake of ‘ginsam’ which is obtained during the vinegar fermentation of ginseng [37]. According to these reports, it is believed that MPV must have had similar effect in this study, because MPV is also a kind of vinegar drink. Since the PV group just had the trend of weight decrease, it is believed that there was more synergy effect with MPV than the independent effect of persimmon vinegar. It is believed that more studies and analyses would be required on this matter.
Meanwhile, vinegar is known to prevent hyperlipidemia by impacting on lipid metabolism [38]. There are many reports on the hyperlipidemia restricting effect of acids including acetic acid, which a lot of them are found in vinegar [39-41]. Therefore, this study investigated the impact of MPV, as a vinegar drink, on the blood lipid component. It was found that PV and MPV decreased triglyceride in blood and total cholesterol. In the change ratio by group, PV and MPV showed more significant decrease compared to CON. The increase ratio of high-density lipoprotein cholesterol was significantly higher in MPV than in PV or CON. This was believed to be due to vinegar drink, as reported in the literature, where the rat supplied with high fat diet and apple vinegar showed decrease in blood lipid [42]; and an obese rat showed increase in fatty acid oxidization and significant decrease in triacylglycerol in blood, by the intake of tomato vinegar [42]. It is possible to expect the effect of saponin, as reported in the literature to show significant decrease in total cholesterol and triglyceride; and the increase in high-density lipoprotein cholesterol was shown by the intake of total saponin in ginseng [27]. However, it is possible that the characteristic effect of MPV was slightly decreased, because only the high-density lipoprotein cholesterol in MPV had significant difference from other groups, considering the change ratio. However, this result can also be determined as positive, because it can be a base for the resolution of metabolic syndrome. Especially, there was a study reporting that the intake of vinegar after meal has the possibility of decreasing the risk of atherosclerosis, by improving blood lipid [43]. Regarding the fused material, there is a report that blood lipid and total cholesterol was decreased and high-density lipoprotein cholesterol concentration was increased, when a rat on high fat diet had the intake of germinated small bean (yakkong) pickled in brown rice vinegar [44]. Similarly, there is an approach from the viewpoint of converged material, in which different foods interact with each other. Therefore, the mountain-cultivated ginseng and persimmon vinegar in this study is also meaningful, since MPV is also a converged material.
Glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT), which are the indicators in hepatoxicity, are the characteristics of obesity [45]; while they are also high in a person with non-alcoholic fatty liver [46]. The GOT and GPT in this study showed significant post-decrease only in MPV group, and the change ratio between the groups was significantly low. The reason is believed to be more on the mountain-cultivated ginseng than the vinegar. It is possible to expect improvement on liver function, by various ginsenosides in mountain-cultivated ginseng, in the view of the literatures as follows: a significant restriction of GPT in a rat with damaged liver, which is caused by long term intake of alcohol and by the intake of red ginseng extract [47]; the significant decrease in GOT and GPT, by the intake of ginseng, in a rat with damaged liver caused by cadmium; [48] and the decrease in GOT and GPT by the intake of plants [49] and beans [50] which have high content of saponin. However, more diverse studies are required on this subject, because past studies on hepatoxicity and ginsenoside are not sufficient.
Obesity is related to low-grade inflammatory response in general [51], and it is the major driver of inflammatory response [52]. Actually, obesity has a deep correlation with chronic inflammatory state [53]. Especially, IL-6 and TNF-α have static correlation with the occurrence of metabolic syndrome [54]. In this study, the change in proinflammatory cytokine related to IL-6 had significant post-decrease only in MPV group. In TNF-α, there was a tendency of decrease by the intake; while NFκB had significant decrease in PV group and MPV group. The significant change before and after the intake, only in IL-6, is believed to be the effect of mountain-cultivated ginseng. In other words, the result is caused by the various kinds of ginsenosides within persimmon vinegars from mountain-cultivated ginseng such as: the decrease in proinflammatory cytokine IL-6 by the intake of ginsenoside Rg1 [55], the restriction of IL-6 concentration increase in the patients with ischemia-reperfusion of heart muscle by Rb3 [56], and the restriction of IL-6 in osteoporosis patients by the intake of Rb2 [57]. NFκB is believed to have a direct relationship with the decrease in fat quantity, because there was a significant decrease in PV and MPV. In other words, the existing studies support the result of this study, as follows: NFκB concentration of an obese rat has the tendency of increasing compared to a rat with normal weight [58]; and the NFκB of an obese rat on high fat diet was significantly higher than the NFκB of a rat with normal weight [59]. However, it is believed that there is another reason, because the CON group had increase in NFκB concentration; while there was no change in fat quantity from before the test. More studies on this matter would be required. Regarding the decreasing rate among the groups, PV and MPV had more significant decrease than CON; while there was no difference between PV and MPV. The reason is believed to be the decrease in body fat quantity and blood lipid in PV and MPV, in the view of existing studies as follows: the increase in cytokine secretion by excessive storage of body fat [60] and the decrease in proinflammatory cytokine secretion accompanied by the decrease in blood triglyceride [61].
CONCLUSION
This study investigated the impact of MPV intake on the blood lipid and proinflammatory cytokine concentration of obese female university students. For the investigation, the change occurred when MPV was taken for 6 weeks daily, without any restriction on meal and activities. The decrease rate of body weight and body fat ratio was higher when MPV was taken. MPV also effectively improved blood lipid concentration and restricted the secretion of proinflammatory cytokine. These results occurred also when the subjects took only persimmon vinegar; however, the effect was more conspicuous when MPV was taken. In addition, there was no impact on hepatoxicity. The significance of this study is that it was conducted without controlling anything in the daily life. MPV is believed to be a valid food for the restriction of obesity. Persimmon vinegar and mountain-cultivated ginseng are traditional foods, which have been taken for a long time. It is expected that the converged material of the two foods, MPV, would be an easier method for resolving obesity. After the study, simple check was done to find the physique change of the subjects, and no change was found. However, more studies and educations would be continuously required on this matter, because weight-cycling can occur when the intake of MPV is stopped for a long time.
Acknowledgments
This research was supported by Kyungpook National University Academic Cooperation Division Research Seed Fund, 2014.
REFERENCES
- 1.Statistics Korea Standard classes of diseases and cause of death in Korea. 2009 http://www.nso.go.kr/statclass/StatClassAction.do?method=disDepth3&s_scode=E65&e_scode=E68.
- 2.Velazquez CE, Pasch KE. Attention to food and beverage advertisements as measured by eye-tracking technology and the food preferences and choices of youth. J Acad Nutr Diet. 2014;114(4):578–582. doi: 10.1016/j.jand.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 3.Kruger AK, Reither EN, Peppard PE, Krueger PM, Hale L. Do sleep-deprived adolescents make less-healthy food choices? Br J Nutr. 2014;111(10):1898–1904. doi: 10.1017/S0007114514000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Penney TL, Almiron-Roig E, Shearer C, McIsaac JL, Kirk SF. Modifying the food environment for childhood obesity prevention: challenges and opportunities. Proc Nutr Soc. 2014;73(2):226–236. doi: 10.1017/S0029665113003819. [DOI] [PubMed] [Google Scholar]
- 5.Giordani I, Malandrucco I, Donno S, Picconi F, Di Giacinto P, Di Flaviani A, Chioma L, Frontoni S. Acute caloric restriction improves glomerular filtration rate in patients with morbid obesity and type 2 diabetes. Diabetes Metab. 2014;40(2):158–160. doi: 10.1016/j.diabet.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Deighton K, Batterham RL, Stensel DJ. Appetite and gut peptide responses to exercise and calorie restriction. The effect of modest energy deficits. Appetite. 2014;81C:52–59. doi: 10.1016/j.appet.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Franklin NC, Ali M, Goslawski M, Wang E, Phillips SA. Reduced vasodilator function following acute resistance exercise in obese women. Front Physiol. 2014;5:253. doi: 10.3389/fphys.2014.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McMillen TS, Minami E, Leboeuf RC. Atherosclerosis and cardiac function assessment in low-density lipoprotein receptor-deficient mice undergoing body weight cycling. Nutr Diabetes. 2013;3: doi: 10.1038/nutd.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winer DA, Winer S, Shen L, Chng MH, Engleman EG. B lymphocytes as emerging mediators of insulin resistance. Int J Obes Suppl. 2012;2(Suppl 1):S4–S7. doi: 10.1038/ijosup.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan J, Zhang H, Yin Y, Li J, Tang Y, Purkayastha S, Li L, Cai D. Obesity- and aging-induced excess of central transforming growth factor-β potentiates diabetic development via an RNA stress response. Nat Med. 2014;20(9):1001–1008. doi: 10.1038/nm.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krüger K, Mooren FC. Exercise-induced leukocyte apoptosis. Exerc Immunol Rev. 2014;20:117–134. [PubMed] [Google Scholar]
- 12.Pisetsky DS, Trace SE, Brownley KA, Hamer RM, Zucker NL, Roux-Lombard P, Dayer JM, Bulik CM. The expression of cytokines and chemokines in the blood of patients with severe weight loss from anorexia nervosa: An exploratory study. Cytokine. 2014;69(1):110–115. doi: 10.1016/j.cyto.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489(7415):318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCaskey S, Rondini EA, Langohr IM, Fenton JI. Differential effects of energy balance on experimentally-induced colitis. World J Gastroenterol. 2012;18(7):627–636. doi: 10.3748/wjg.v18.i7.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joesting JJ, Moon ML, Gainey SJ, Tisza BL, Blevins NA, Freund GG. Fasting induces IL-1 resistance and free-fatty acid-mediated up-regulation of IL-1R2 and IL-1RA. Front Immunol. 2014;5:315. doi: 10.3389/fimmu.2014.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson-Baucum EK, Major AS, Hasty AH. A possible secondary immune response in adipose tissue during weight cycling: The ups and downs of yo-yo dieting. Adipocyte. 2014;3(2):141–145. doi: 10.4161/adip.27556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seo H, Nam J-O, Jeon B-D, Kim P-K, Ryu S. Persimmon vinegar ingestion before endurance exercise on energy substrates utilization. Jour Korean For Soc. 2012;101:626–634. [Google Scholar]
- 18.Lee SM, Park SY, Jang GS, Ly SY. The protective effects of ethanol extract of wild simulated ginseng on carbon tetracholoride induced acute hepatic injury in mouse. Korean J Nutr. 2008;41:701–710. [Google Scholar]
- 19.Han Y-J, Kwon K-R, Cha B-C, Kwon O-M. Component analysis of cultivated ginseng, cultivated wild ginseng, and natural wild ginseng by structural parts using HPLC method. J Pharmacopuncture. 2007;10:37–53. [Google Scholar]
- 20.Kwon D-K, Kang J-Y, Song Y-J, Kim P-G, Seo H, Ryu S. Effects of mountain ginseng-added high fat diet on lipid peroxidation and antioxidant protein expression of skeletal muscle in rats. Jour Korean For Soc. 2012;101:69–76. [Google Scholar]
- 21.Choo MK, Sakurai H, Kim DH, Saiki I. A ginseng saponin metabolite suppresses tumor necrosis factor-alpha-promoted metastasis by suppressing nuclear factor-kappaB signaling in murine colon cancer cells. Oncol Rep. 2008;19(3):595–600. [PubMed] [Google Scholar]
- 22.Ma L, Liu H, Xie Z, Yang S, Xu W, Hou J, Yu B. Ginsenoside Rb3 protects cardiomyocytes against ischemia-reperfusion injury via the inhibition of JNK-mediated NF-κB pathway: a mouse cardiomyocyte model. PLoS One. 2014;9(8): doi: 10.1371/journal.pone.0103628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeon B-D, Kim P-G, Ryu S. Ripened persimmon vinegar with mountain-cultivated ginseng ingestion reduce blood lipids and enhance anti-oxidants capacity in rats. Jour Korean For Soc. 2013;102:182–188. [Google Scholar]
- 24.Jeon B-D, Kim P-G, Ryu S. Change of ripened persimmon vinegar with mountain-cultivated ginseng ingestion on energy metabolism in rats. Jour Korean For Soc. 2012;101:182–188. [Google Scholar]
- 25.Kim C, Hwang I-H, Kim J-H, Hyun M-Y, Kim P-G, Ryu S. Food composition for reducing cholesterol and its preparation methods. 2014 Patent No. 10-143022. Korea.
- 26.Korea Forest Service Development of anti-obesity foods and programs by using fusion ingredients of mountain-cultivated ginseng and persimmon vinegar. 2013:88–111. Report.
- 27.Liu C, Hu MY, Zhang M, Li F, Li J, Zhang J, Li Y, Guo HF, Xu P, Liu L, Liu XD. Association of GLP-1 secretion with anti-hyperlipidemic effect of ginsenosides in high-fat diet fed rats. Metabolism. 2014 doi: 10.1016/j.metabol.2014.06.015. S0026-0495(14)00182-6. [DOI] [PubMed] [Google Scholar]
- 28.Zhong ZD, Wang CM, Wang W, Shen L, Chen ZH. Major hypoglycemic ingredients of panax notoginseng saponins for treating diabetes. Sichuan Da Xue Xue Bao Yi Xue Ban. 2014;45(2):235–239. [PubMed] [Google Scholar]
- 29.Shen L, Xiong Y, Wang DQ, Howles P, Basford JE, Wang J, Xiong YQ, Hui DY, Woods SC, Liu M. Ginsenoside Rb1 reduces fatty liver by activating AMP-activated protein kinase in obese rats. J Lipid Res. 2013;54(5):1430–1438. doi: 10.1194/jlr.M035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chidrawar VR, Patel KN, Sheth NR, Shiromwar SS, Trivedi P. Antiobesity effect of Stellaria media against drug induced obesity in Swiss albino mice. Ayu. 2011;32(4):576–584. doi: 10.4103/0974-8520.96137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin J, Zhang H, Ye J. Traditional chinese medicine in treatment of metabolic syndrome. Endocr Metab Immune Disord Drug Targets. 2008;8(2):99–111. doi: 10.2174/187153008784534330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang LC, Lee TF. Effect of ginseng saponins on cold tolerance in young and elderly rats. Planta Med. 2000;66(2):144–147. doi: 10.1055/s-2000-11122. [DOI] [PubMed] [Google Scholar]
- 33.Ok E, Do GM, Lim Y, Park JE, Park YJ, Kwon O. Pomegranate vinegar attenuates adiposity in obese rats through coordinated control of AMPK signaling in the liver and adipose tissue. Lipids Health Dis. 2013;12:163. doi: 10.1186/1476-511X-12-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seo KI, Lee J, Choi RY, Lee HI, Lee JH, Jeong YK, Kim MJ, Lee MK. Anti-obesity and anti-insulin resistance effects of tomato vinegar beverage in diet-induced bese mice. Food Funct. 2014;5(7):1579–1586. doi: 10.1039/c4fo00135d. [DOI] [PubMed] [Google Scholar]
- 35.Lee JH, Cho HD, Jeong JH, Lee MK, Jeong YK, Shim KH, Seo KI. New vinegar produced by tomato suppresses adipocyte differentiation and fat accumulation in 3T3-L1 cells and obese rat model. Food Chem. 2013;141(3):3241–3249. doi: 10.1016/j.foodchem.2013.05.126. [DOI] [PubMed] [Google Scholar]
- 36.Darzi J, Frost GS, Montaser R, Yap J, Robertson MD. Influence of the tolerability of vinegar as an oral source of short-chain fatty acids on appetite control and food intake. Int J Obes (Lond) 2014;38(5):675–681. doi: 10.1038/ijo.2013.157. [DOI] [PubMed] [Google Scholar]
- 37.Yun SN, Ko SK, Lee KH, Chung SH. Vinegar-processed ginseng radix improves metabolic syndrome induced by a high fat diet in ICR mice. Arch Pharm Res. 2007;30(5):587–595. doi: 10.1007/BF02977653. [DOI] [PubMed] [Google Scholar]
- 38.Budak NH, Kumbul Doguc D, Savas CM, Seydim AC, Kok Tas T, Ciris MI, Guzel-Seydim ZB. Budak NH1, Kumbul Doguc D, Savas CM, Seydim AC, Kok Tas T, Ciris MI, Guzel-Seydim ZB. J Agric Food Chem. 2011;59(12):6638–6644. doi: 10.1021/jf104912h. [DOI] [PubMed] [Google Scholar]
- 39.Mokale SN, Nevase MC, Sakle NS, Dube PN, Shelke VR, Bhavale SA, Begum A. Synthesis and in-vivo hypolipidemic activity of some novel substituted phenyl isoxazol phenoxy acetic acid derivatives. Bioorg Med Chem Lett. 2014;24(9):2155–2158. doi: 10.1016/j.bmcl.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 40.Mokale SN, Shete MT, Shaikh SI, Shinde DB. Synthesis and hypolipidemic activity of novel 2-(4-(2-amino-6-(4-substituted phenyl) pyrimidin-4-yl)-2-substituted phenoxy) acetic acid derivatives. Chem Biol Drug Des. 2012;79(4):548–552. doi: 10.1111/j.1747-0285.2012.01319.x. [DOI] [PubMed] [Google Scholar]
- 41.Mokale SN, Sanap PT, Shinde DB. Synthesis and hypolipidemic activity of novel 2-(4-(2-substituted aminothiazole-4-yl) phenoxy) acetic acid derivatives. Eur J Med Chem. 2010;45(7):3096–3100. doi: 10.1016/j.ejmech.2010.03.043. [DOI] [PubMed] [Google Scholar]
- 42.Nazıroğlu M, Güler M, Ozgül C, Saydam G, Küçükayaz M, Sözbir E. Apple cider vinegar modulates serum lipid profile, erythrocyte, kidney, and liver membrane oxidative stress in ovariectomized mice fed high cholesterol. J Membr Biol. 2014;247(8):667–673. doi: 10.1007/s00232-014-9685-5. [DOI] [PubMed] [Google Scholar]
- 43.Setorki M, Asgary S, Eidi A, Rohani AH, Khazaei M. Acute effects of vinegar intake on some biochemical risk factors of atherosclerosis in hypercholesterolemic rabbits. Lipids Health Dis. 2010;9:10. doi: 10.1186/1476-511X-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park KM, Lee SH. Anti-hyperlipidemic activity of Rhynchosia nulubilis seeds pickled with brown rice vinegar in mice fed a high-fat diet. Nutr Res Pract. 2013;7(6):453–459. doi: 10.4162/nrp.2013.7.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsu HM, Chen WY, Hu TK, Mao FC. Supplementation of Vitis thunbergii root extract alleviated high-fat diet-induced obesity in C57BL/6J mice. Biosci Biotechnol Biochem. 2014;78(5):867–873. doi: 10.1080/09168451.2014.905181. [DOI] [PubMed] [Google Scholar]
- 46.Guijarro de Armas MG, Monereo Megías S, Navea Aguilera C, Merino Viveros M, Vega Piñero MB. Non-alcoholic fatty liver in children and adolescents with excess weight and obesity. Med Clin (Barc) 2014 doi: 10.1016/j.medcli.2014.02.018. S0025-7753(14)00195-X. [DOI] [PubMed] [Google Scholar]
- 47.Seo SJ, Cho JY, Jeong YH, Choi YS. Effect of Korean red ginseng extract on liver damage induced by short-term and long-term ethanol treatment in rats. J Ginseng Res. 2013;37(2):194–200. doi: 10.5142/jgr.2013.37.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shukla R, Kumar M. Role of Panax ginseng as an antioxidant after cadmium-induced hepatic injuries. Food Chem Toxicol. 2009;47(4):769–773. doi: 10.1016/j.fct.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Yen MH, Weng TC, Liu SY, Chai CY, Lin CC. The hepatoprotective effect of Bupleurum kaoi, an endemic plant to Taiwan, against dimethylnitrosamine-induced hepatic fibrosis in rats. Biol Pharm Bull. 2005;28(3):442–448. doi: 10.1248/bpb.28.442. [DOI] [PubMed] [Google Scholar]
- 50.Lin CY, Tsai CY, Lin SH. Effects of soy components on blood and liver lipids in rats fed high-cholesterol diets. World J Gastroenterol. 2005;11(35):5549–5552. doi: 10.3748/wjg.v11.i35.5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim Y, Park Y, Namkoong S, Lee J. Esculetin inhibits the inflammatory response by inducing heme oxygenase-1 in cocultured macrophages and adipocytes. Food Funct. 2014 doi: 10.1039/c4fo00351a. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 52.Christian LM, Porter K. Longitudinal changes in serum proinflammatory markers across pregnancy and postpartum: Effects of maternal body mass index. Cytokine. 2014 doi: 10.1016/j.cyto.2014.06.018. S1043-4666(14)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cortese S, Angriman M. Attention-deficit/hyperactivity disorder, iron deficiency, and obesity: Is there a link? Postgrad Med. 2014;126(4):155–170. doi: 10.3810/pgm.2014.07.2793. [DOI] [PubMed] [Google Scholar]
- 54.Bae YJ, Kim SH, Chung JH, Song SW, Kim KS, Kim MK, Kwon O, Choi MS, Sung MK. Evaluation of adiposity-related biomarkers as metabolic syndrome indicators. Clin Nutr Res. 2013;2(2):91–99. doi: 10.7762/cnr.2013.2.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu J, Mu X, Zeng J, Xu C, Liu J, Zhang M, Li C, Chen J, Li T, Wang Y. Ginsenoside Rg1 prevents cognitive impairment and hippocampus senescence in a rat model of D-galactose-induced aging. PLoS One. 2014;9(6): doi: 10.1371/journal.pone.0101291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma L, Liu H, Xie Z, Yang S, Xu W, Hou J, Yu B. Ginsenoside Rb3 Protects Cardiomyocytes against Ischemia-Reperfusion Injury via the Inhibition of JNK-Mediated NF-κB Pathway: A Mouse Cardiomyocyte Model. PLoS One. 2014;9(8): doi: 10.1371/journal.pone.0103628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang Q, Gao B, Jie Q, Wei BY, Fan J, Zhang HY, Zhang JK, Li XJ, Shi J, Luo ZJ, Yang L, Liu J. Ginsenoside-Rb2 displays anti-osteoporosis effects through reducing oxidative damage and bone-resorbing cytokines during osteogenesis. Bone. 2014;66:306–314. doi: 10.1016/j.bone.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 58.Mizutani K, Park K, Mima A, Katagiri S, King GL. Obesity-associated Gingival Vascular Inflammation and Insulin Resistance. J Dent Res. 2014;93(6):596–601. doi: 10.1177/0022034514532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amaral LS, Silva JA, Trindade TM, Ribas WB, Macedo CL, Coimbra TM, Belo NO, Magalhaes AC, Soares TJ. Renal changes in the early stages of diet-induced obesity in ovariectomized rats. Physiol Res. 2014;63(6):723–732. doi: 10.33549/physiolres.932619. [DOI] [PubMed] [Google Scholar]
- 60.Kiss RS, Nilsson T. Rab proteins implicated in lipid storage and mobilization. J Biomed Res. 2014;28(3):169–177. doi: 10.7555/JBR.28.20140029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Y, Gao Z, Guo Q, Wang T, Lu C, Chen Y, Sheng Q, Chen J, Nie Z, Zhang Y, Wu W, Lv Z, Shu J. Anti-diabetic effects of CTB-APSL fusion protein in type 2 diabetic mice. Mar Drugs. 2014;12(3):1512–1529. doi: 10.3390/md12031512. [DOI] [PMC free article] [PubMed] [Google Scholar]