Abstract
[Purpose]
We investigated the effects of 8 weeks of treadmill exercise on nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and synapsin I protein expression and on the number of 5-bromo-2'-deoxyuridine-5'-mono-phosphate (BrdU)-positive cells in the dentate gyrus of the hippocampus in socially isolated rats. Additionally, we examined the effects of exercise on the number of serotonin (5-HT)- and tryptophan hydroxylase (TPH)-positive cells in the raphe nuclei and on depression behaviors induced by social isolation.
[Methods]
Forty male Sprague-Dawley rats were divided into four groups: (1) group housing and control group (GCG, n = 10); (2) group housing and exercise group (GEG, n = 10); (3) isolated housing and control group (ICG, n = 10); and (4) isolated housing and exercise group (IEG, n = 10). After 1 week of housing under the normal condition of 3 animals per cage, rats were socially isolated via transfer to individual cages for 8 weeks. Rats were then subjected to treadmill exercise for 5 days per week for 8 weeks during which time the speed of the treadmill was gradually increased.
[Results]
Compared to the GCG, levels of NGF, BDNF, and synapsin I were significantly decreased in the ICG and significantly increased in the IEG (p < 0.001 respectively). Significantly more BrdU-positive cells in the GEG were present as compared to the GCG and ICG, and more BrdU-positive cells were found in the IEG as compared to the ICG (p < 0.001). 5-HT-positive cells in the GEG were significantly increased compared to the GCG and ICG, and more of these cells were found in the IEG as compared to the ICG (p < 0.01). TPH-positive cells in the GEG were significantly increased compared to those in the GCG and ICG (p < 0.05). In the forced swim test, immobility time was significantly increased in the ICG and significantly decreased in the IEG as compared to the ICG (p < 0.01).
[Conclusion]
These results showed that regular treadmill exercise following social isolation not only increased the levels of NGF, BDNF, and synapsin I to induce survival of neurons in the hippocampus but also improved depression by increasing the number of serotonergic cells in the raphe nuclei.
Keywords: Nerve growth factor, brain-derived neurotrophic factor, synapsin I, serotonin, tryptophan hydroxylase, treadmill exercise
INTRODUCTION
Social isolation is recognized as an important factor contributing to the development of various social problems in contemporary society and has been found to be closely connected to many brain disorders such as anxiety, restlessness, depression, and memory loss [1]. Social isolation not only increases the levels of stress hormones by stimulating the hypothalamic-pituitary-adrenal (HPA) axis [2,3] but also increases the sensitivity to other stressors [1]. According to the study of Wosiski-Kuhn et al. [4], long-term increases in stress hormones due to social isolation decreases the expression of brain-derived neurotrophic factor (BDNF) in the dentate gyrus of the hippocampus and adversely affects brain function by decreasing the formation of neurons and neuronal plasticity. Lu et al. [5] and Mitra et al. [6] have confirmed the decrease in neurogenesis and cognitive function following social isolation, thus seeing a significant decrease in the number of 5-bromo-2'-deoxyuridine-5'-mono-phosphate (BrdU)-positive cells in the hippocampus and increased latencies in the Morris water maze test. Additionally, decreased brain function induced by social isolation is connected to various behavioral problems and psychological disorders such as anxiety and depression [7,8]. In the case of depression, reduced serotonin (5-HT), tryptophan hydroxylase (TPH), and secretion of neurotransmitter in the raphe nuclei lead to decreased function of various other brain regions such as the spinal cord, hypothalamus, and hippocampus. Moreover, such phenomena are worse during brain development in children (~6-12 years of age). Silva-Gómez et al. [9] reported that social isolation in early childhood induces neuronal dysfunction in the limbic system by suppressing dendritogenesis in the hippocampus. Quan et al. [10] reported that social isolation disrupts long-term potential (LTP) in the hippocampus, thereby affecting plasticity. In particular, social isolation in early childhood induces neurobehavioral disorders such as performance anxiety and depression [11] and possibly leads to long-term brain dysfunction and depression in old age [12]. Therefore, social isolation, frequently occurring throughout the entire lifespan of an individual, along with the development of contemporary society is assumed to cause decreased brain function or depression; yet precise ways to ameliorate these effects are not known.
Regular exercise improves brain function including cognitive ability [13,14] and increases brain plasticity and the expression of neurotrophins [15]. Additionally, Daley [16] reports that exercise improves signal transduction for neurotransmitters such as 5-HT, dopamine, and noradrenaline, thereby positively contributing to the treatment of depression. Therefore, we hypothesize that regular exercise prevents the decline in brain function or depression-related behaviors caused by social isolation. To test this, we investigated the effects of treadmill exercise for 8 weeks on the levels of nerve growth factor (NGF), BDNF, and synapsin I proteins and on survival of neurons through BrdU immunohistochemistry in the dentate gyrus. We also examined the effects of exercise on the numbers of 5-HT- and TPH-positive cells in the raphe nuclei and on depression-like behaviors in socially isolated rats.
METHODS
Animals
Male Sprague-Dawley rats (n = 40; age, 4 weeks; weight, 90.0 ± 4.2 g) were adapted to the laboratory environment (temperature, 22℃ ± 1℃; relative humidity, 55% ± 3%; 12-h light/dark cycle) for 2 weeks. All rats were housed in pairs, given free access to water, and fed a standard chow diet (protein, 21%; fat, 5%; nitrogen-free extract, 55%; fiber, 4%; adequate mineral and vitamin content; Purina Mills Inc, Korea). Studies were approved by the Ethical Committee of Korea National Sport University.
Rats were allocated to the following groups: (1) group housing and control group (GCG, n = 10); (2) group housing and exercise group (GEG, n = 10); (3) isolated housing and control group (ICG, n = 10); (4) isolated housing and exercise group (IEG, n = 10). In the study of Stranahan et al. [17], rats were socially isolated by being moved from group breeding of 3 per cage for 1 week to individual cages for 8 weeks. The rats in the GEG and IEG after social isolation were subjected to moderate-intensity treadmill exercise for 5 days per week for 8 weeks. The speed and duration of the treadmill exercise were gradually increased from 10 to 12 m/min for 10 min (grade 0%) in the first week, 10-12 m/min for 20 min (grade 0%) in the second week, 18-20 m/min for 20 min (grade 0%) in the third week, 18-20 m/min for 30 min (grade 0%) in the fourth week, and 18-20 m/min for 50 min (grade 0%) from the fifth week onwards. At the same time, BrdU (50 mg/kg bodyweight), a thymidine analog, was injected into 5 rats of each group for 5 days during the last week of exercise to observe cell survival in the dentate gyrus. Additionally, they were tested using the forced swim paradigm during the 7 days prior to the end of exercise.
Tissue collection
Upon completion of the 8-week exercise program, the rats were anesthetized 48 h after the final exercise session by an intraperitoneal (i.p.) injection of xylazine (8 mg/kg) and ketamine (40 mg/kg). For the detection of cell survival in the hippocampal dentate gyrus and expression of 5-HT and TPH cells in the raphe nuclei, 5 rats of each group were selected, transcardially perfused with 50 mM phosphate-buffered saline (pBS), and fixed with a freshly prepared solution of 4% paraformaldehyde in 100 mM phosphate buffer (pB, pH 7.4). The brains were dissected and postfixed in the same fixative overnight before being transferred into a 30% sucrose solution for cryoprotection. Coronal sections of 40-µm thickness were made using a freezing microtome (Leica, Nussloch, Germany). For the analysis of protein levels, brains were quickly excised, and the hippocampus was dissected and stored at -70℃.
NGF, BDNF, and synapsin I protein levels
To prepare proteins for western blotting, the hippocampus was crushed in a solution containing 150 mM NaCl, 5 mM ethylenediamine tetraacetic acid (EDTA), 50 mM Tri-HCl (pH 8.0), 1% NP 40, 1 mM aprotinin, 0.1 mM leupeptin, and 1 mM pepstatin and then centrifuged at 12,000 g for 15 min at 4℃. The extracted proteins (30 µg) were separated with 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane (Nitrobind; 0.45 µm; Geneworks, SA, Australia). The membrane was blocked by incubation in a Tris-buffered saline solution containing Tween (TBST) and 5% nonfat milk at 4℃. After washing, the membrane was incubated with anti-NGF, BDNF, and synapsin I antibodies (dilution, 1:1,000) (Cell Signaling, Beverly, MA, USA), washed with TBST (3 × 10 min), and incubated with a goat anti-rabbit IgG secondary antibody conjugated with alkaline phosphatase (AP) (dilution, 1:2,000) (Santa Cruz Biotech, Santa Cruz, CA, USA) for 1 h. The membrane was washed with TBST (3 × 10 min). The protein bands were imaged using a Kodak Image Station (440CF; PerkinElmer Life Sciences, Boston, MA, USA), and the proteins were quantified using densitometry software (Kodak ID 3.5).
Immunohistochemistry
BrdU-positive cells
For the detection of newly generated neuronal cells in the dentate gyrus (-3.30 to -3.80 mm relative to bregma), BrdU-specific immunohistochemistry was performed as previously described [18]. Brain sections were permeabilized by incubation in 0.5% Triton X-100 in PBS for 20 min, pretreated in 50% formamide-2 × standard saline citrate (SSC) at 65℃ for 2 h, denatured in 2N HCl at 37℃ for 30 min, and rinsed twice in 100 mM sodium borate (pH 8.5). The sections were incubated overnight at 4℃ with a BrdU-specific mouse monoclonal antibody (1:600; Roche, Mannheim, Germany). The sections were then washed 3 times with PBS and incubated for 1 h with the biotinylated mouse secondary antibody (1:200; Vector Laboratories). The sections were incubated for another 1 h with an avidin-peroxidase complex (1:100; Vector Laboratories). For visualization, the sections were incubated for 5 min in 50 mM Tris-HCl (pH 7.6) containing 0.02% diaminobenzidine (DAB), 40 mg/mL nickel chloride, and 0.03% hydrogen peroxide. Subsequently, the slides were air dried overnight at room temperature, and coverslips were mounted using Permount®. The number of BrdU-positive cells in the subgranular layer of the hippocampal dentate gyrus was counted hemilaterally in every eighth section throughout the entire extent of the dentate gyrus at 400 × magnification. The area of the granular layer of the dentate gyrus was traced using the Image Pro®Plus image analyzer (Media Cybernetics Inc, Silver Springs, MD, USA) at 40 × magnification. The number of BrdU-positive cells was expressed as the mean number of cells per millimeter square of the cross sectional area of the granular layer of the dentate gyrus.
5-HT- and TPH-positive cells
For the detection of 5-HT- and TPH-positive cells in the dorsal raphe nuclei (-7.04 to - 7.30 mm relative to bregma), immunohistochemistry was performed using a previously described method [19]. Free-floating tissue sections were washed twice in 50 mM PBS and then permeablized by incubating in 0.5% Triton X-100 in PBS for 20 min. Sections were next incubated in 1% hydrogen peroxide (H2O2) for 30 min. Following this, sections were incubated overnight in rabbit anti-5-HT antibody (1:1000; Millipore Co, MA, USA) for visualization of 5-HT expression or in mouse anti-TPH antibody (1:1000; Millipore Co, MA, USA) for visualization of TPH expression. Next, sections were incubated for 1 h in anti-rabbit secondary antibody (1:1000; Vector Laboratories, Burlingame, CA, USA) for 5-HT immunohistochemistry or in anti-mouse secondary antibody (1:200; Vector Laboratories) for TPH immunohistochemistry. Sections were subsequently incubated in avidin-biotin-peroxidase complex (1:100; Vector Laboratories) for 1 h at room temperature. The immunoreactivity was visualized by incubating sections in a solution consisting of 0.02% 3,3’-diaminobenzidine tetrahydrochloride (DAB) and 0.03% H2O2 in 50 mM Tris-buffer (pH 7.6) for approximately 5 min. Sections were then washed with PBS, mounted onto gelatin-coated slides, air-dried, and coverslipped using Permount®. 5-HT- and TPH-positive cells in the dorsal raphe nuclei were counted in 4 to 5 coronal sections per animal. Immunopositive cells were counted using a BX-51 microscope (Olympus, Tokyo, Japan) with an Image Pro®Plus image analysis system (Media Cybernetics Inc, Silver Spring, MD, USA), and the image was displayed on a computer monitor. Data were expressed as the average number of 5-HT- and TPH-positive cells per section.
Forced swim test
The forced swim test was carried out during the final week of exercise as previously described [20]. For pre-swim trials, each rat was allowed to swim in a glass cylinder (54 cm in height and 24 cm in diameter) filled with water to a depth of 40 cm (23-25℃) for 15 min. They were then removed from the cylinder, dried with a paper towel, and returned to their home cage. On the following day, rats were subjected to 5 min of the forced swim test. All test sessions were recorded by a video camera positioned at the side of the cylinder. Duration of immobility in the water was scored from videotapes by a trained observer who was blinded to the experimental conditions. Immobility was defined as the state in which rats were judged to be making only those movements necessary to keep their head above water.
Statistical analysis
All data were analyzed using the SAS software package (SAS Institute, Cary, NC, USA) and tested against the normal distribution. We performed one-way analysis of variance (ANOVA) followed by least significant difference (LSD) test to compare the data among the experimental groups. All values were expressed as means ± standard deviation (SD), and p < 0.05 was accepted as statistically significant.
RESULTS
The effect of treadmill exercise after social isolation on NGF, BDNF, and synapsin I protein levels
The levels of NGF, BDNF, and synapsin I in the hippocampus of rats in each group were significantly different (p < 0.001, Fig. 1), respectively. NGF, BDNF, and synapsin I protein levels significantly decreased in the ICG as compared to the GCG, and these levels significantly improved in the IEG.
Fig. 1.
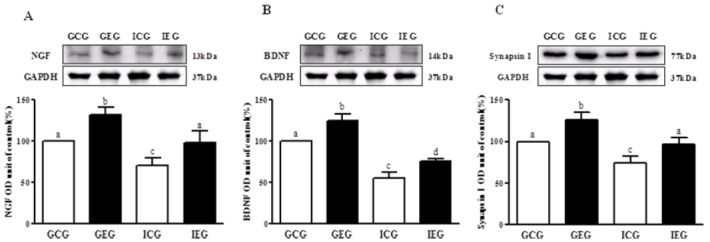
NGF, BDNF, and synapsin I protein levels in hippocampal dentate gyrus following treadmill exercise after social isolation. (A) NGF, (B) BDNF, and (C) synapsin I. NGF, BDNF, and synapsin I protein level were significantly decreased in the ICG as compared to the GCG, and these levels were significantly improved in the IEG. GCG: group housing and control group, n = 10; GEG: group housing and exercise group, n = 10; ICG: isolated housing and control group, n = 10; IEG: isolated housing and exercise group, n = 10 (respectively 5 rats for immunohistochemistry, 5 rats for western blotting). Significant difference among groups at p < 0.001. Different letters represent significant variations calculated by 1-way analysis of variance (ANOVA) and the LSD test.
The effect of treadmill exercise after social isolation on the number of BrdU-positive cells
A significant difference between groups occurred with regard to the number of BrdU-positive cells in the dentate gyrus (p < 0.001, Fig. 2). The number of BrdU-positive cells in the GEG was significantly greater as compared to the GCG and ICG, and the number of cells were significantly greater in the IEG as compared to the ICG.
Fig. 2.
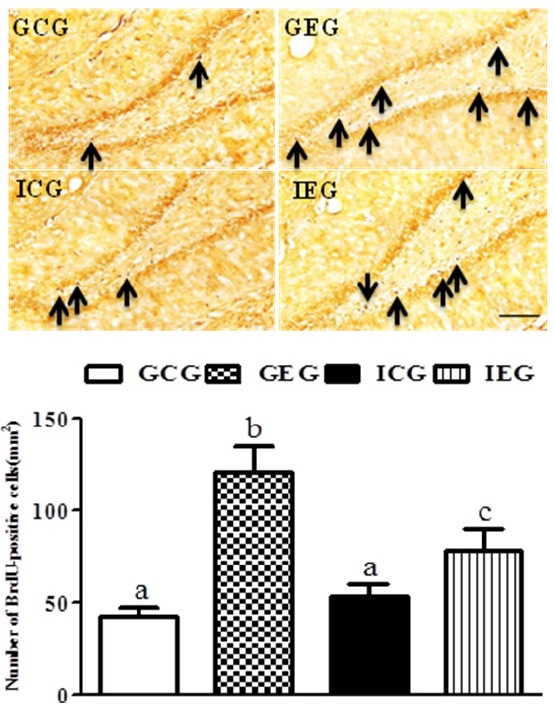
BrdU-labeled cells in the hippocampal dentate gyrus following treadmill exercise after social isolation. The number of BrdU-positive cells in the GEG was significantly greater as compared to the GCG and ICG, and they were significantly greater in the IEG as compared to the ICG. GCG: group housing and control group, n = 10; GEG: group housing and exercise group, n = 10; ICG: isolated housing and control group, n = 10; IEG: isolated housing and exercise group, n = 10 (respectively 5 rats for immunohistochemistry, 5 rats for western blotting). Significant difference among groups at p < 0.001. Different letters represent significant variations calculated by 1-way analysis of variance (ANOVA) and the LSD test. The scale bar represents 50 μm.
The effect of treadmill exercise after social isolation on the number of 5-HT- and TPH-positive cells
A significant difference between groups occurred with regard to the number of 5-HT- (p < 0.01, Fig. 3) and TPH (p < 0.05, Fig. 4)-positive cells in the raphe nuclei. The number of 5-HT-positive cells in the GEG was significantly greater than in the GCG and ICG, and they were significantly greater in the IEG as compared to the ICG. The number of TPH-positive cells in the GEG was significantly greater than in the GCG and ICG. Despite no statistically significant difference, a trend of an increased number of TPH-positive cells occurred in the IEG as compared to the ICG.
Fig. 3.
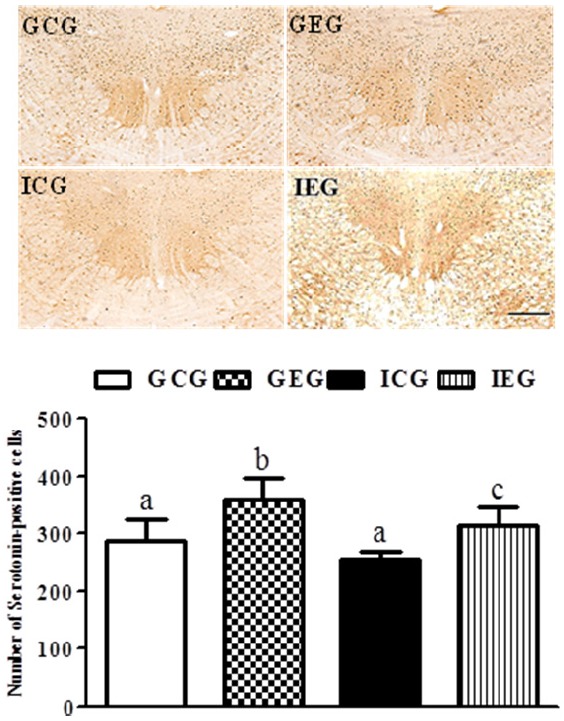
5-HT-positive cells in the raphe nuclei following treadmill exercise after social isolation. The number of 5-HT-positive cells in the GEG was significantly greater than in the GCG and ICG, and they were significantly greater in the IEG as compared to the ICG. GCG: group housing and control group, n = 10; GEG: group housing and exercise group, n = 10; ICG: isolated housing and control group, n = 10; IEG: isolated housing and exercise group, n = 10 (respectively 5 rats for immunohistochemistry, 5 rats for western blotting). Significant difference among groups at p < 0.01. Different letters represent significant variations calculated by 1-way analysis of variance (ANOVA) and the LSD test. The scale bar represents 100 μm.
Fig. 4.
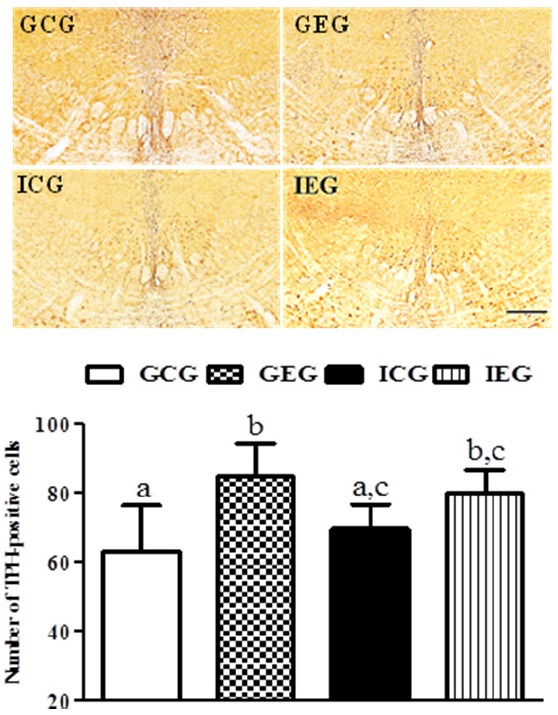
TPH-positive cells in the raphe nuclei following treadmill exercise after social isolation. The number of TPH-positive cells in the GEG was significantly greater than in the GCG and ICG. GCG: group housing and control group, n = 10; GEG: group housing and exercise group, n = 10; ICG: isolated housing and control group, n = 10; IEG: isolated housing and exercise group, n = 10 (respectively 5 rats for immunohistochemistry, 5 rats for western blotting). Significant difference among groups at p < 0.05. Different letters represent significant variations calculated by 1-way analysis of variance (ANOVA) and the LSD test. The scale bar represents 100 μm.
The effect of treadmill exercise after social isolation on the forced swim test
The results of the forced swim test showed that the groups differed significantly from each other with regard to depression (p < 0.01, Fig. 5). Immobility time in the ICG significantly increased, and it significantly decreased in the IEG as compared to the ICG.
Fig. 5.
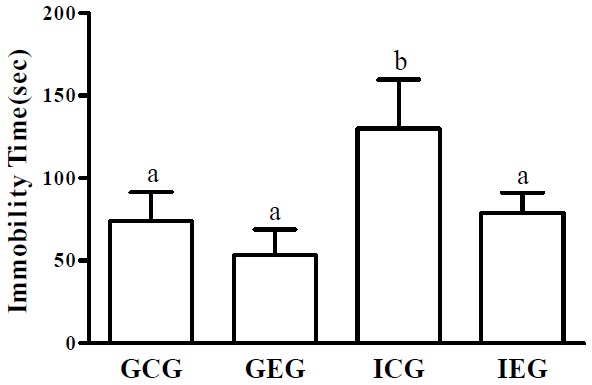
Forced swim test following treadmill exercise after social isolation. Immobility time in the ICG significantly increased and significantly decreased in the IEG as compared to the ICG. GCG: group housing and control group, n = 10; GEG: group housing and exercise group, n = 10; ICG: isolated housing and control group, n = 10; IEG: isolated housing and exercise group; n = 10. Significant difference among groups at p < 0.01. Different letters represent significant variations calculated by 1-way analysis of variance (ANOVA) and the LSD test.
DISCUSSION
This study has confirmed the potential use of exercise for attenuating brain dysfunction and depression caused by social isolation. We subjected socially isolated rats to regular treadmill exercise and measured the levels of NGF, BDNF, and synapsin I proteins as well as the number of BrdU-positive cells in the hippocampus and 5-HT- and TPH-positive cells in the raphe nucleus. We also conducted a forced swim test to assess depression. We discovered that regular treadmill exercise following social isolation not only increased the levels of NGF, BDNF, and synapsin I to induce survival of neurons in the hippocampus but also improved depression by increasing the number of serotonergic cells in the raphe nucleus.
Social isolation as a kind of environmental stress negatively influenced various factors related to brain function. It reduced the expression of BDNF in the dentate gyrus, thereby decreasing neuronal plasticity and brain function [4-6]. Our study also showed that the levels of NGF and BDNF in the ICG significantly decreased. This result supported previous studies demonstrating that social isolation increased stress hormones and decreased the expression of neurotrophic factors [2-4]. We found that regular treadmill exercise following social isolation increased the expression of NGF and BDNF in the hippocampus. As neurotrophic factors that are retrogradely transported, NGF and BDNF promoted neuronal growth, differentiation, and survival [21-23]; they also acted as factors controlling cholinergically mediated plasticity in the hippocampus through turnover of neurotransmitters such as acetylcholine and dopamine [24]. However, the levels of NGF and BDNF dramatically decreased during aging, disease, or environmental stress, thereby contributing to a decline in brain function. We found that the decrease in NGF and BDNF expression in the hippocampus after social isolation could be attenuated by treadmill exercise. Therefore, regular exercise might help prevent hippocampal dysfunction following social isolation. Our findings were similar to previous reports showing that 8-week regular treadmill exercise increased the level of NGF in the hippocampus [25] and that exercise preconditioning increased the level of NGF in the hippocampus of rats in social isolation [26]. Moreover, an increase in NGF and BDNF in the hippocampus was assumed to contribute to improved neuronal plasticity and survival of neurons. Our results also proved that treadmill exercise following social isolation increased the level of synapsin I and significantly increased the number of BrdU-positive cells. Synapsin I was involved in controlling synaptic density in the hippocampus, in spatial and emotional memory, in behavioral disorders, and in cholinergically mediated neuronal plasticity [27]. Synapsin I was important for hippocampal function, and synapsin I deficiency is known to cause epilepsy, schizophrenia, decreased cognitive function, and learning disability [28]. Hermes et al. [29] and Lim et al. [30] indirectly suggested that decreased hippocampal function due to social isolation was likely, but that regular treadmill exercise increased the level of synapsin I.
Social isolation is associated with various behavioral and psychological disorders such as anxiety and depression. In particular, depression results in damage to various regions of the brain via decreased levels of neurotransmitters including 5-HT, which is important for brain development during early childhood. We also found a decreased number of 5-HT- and TPH-positive cells in the raphe nuclei in the ICG, which could be attenuated by treadmill exercise following social isolation. Furthermore, this phenomenon was more apparent in 5-HT- positive cells. 5-HT was synthesized by TPH when tryptophan entered cells and was isolated from the presynaptic membrane, physiologically working by combing with the 5-HT receptor in the postsynaptic membrane. However, in depression, 5-HT was re-absorbed into the presynaptic terminal by the serotonin transporter (5-HTT) and then converted to the inactive 5-hydroxyindoleacetic acid (5-HIAA) by monoamine oxidase (MAO) [31]. In an animal model of depression, neurotransmitters such as 5-HT decreased but could be increased with anti-depressant therapy [32]. As such, 5-HT is considered to be as an important neurotransmitter involved in the etiology of depression [33], and most anti-depressants are designed to increase the amount of 5-HT in the synapse [34]. Because we found that social isolation resulted in a decreased number of 5-HT-positive neurons, it is likely that social isolation contributed to depression. However, this reduction in 5-HT-positive neurons could be ameliorated by treadmill exercise, thereby supporting the hypothesis that regular exercise can contribute to improving depression. This finding was also proven by the results of the forced swim test, where immobility time was significantly increased in the ICG but decreased by treadmill exercise following social isolation. This result showed that exercise could be an effective strategy for improving depression-related behaviors. This conclusion was also reached by Greenwood et al. [35], who showed that the number of 5-HT-positive cells and the amount of 5-HT1A receptor mRNA in the dorsal and ventral raphe nuclei significantly increased and the shuttle box latency was improved by 6 weeks of voluntary wheel running in depression-induced rats with uncontrollable stress. Furthermore, Huang et al. [36] demonstrated that 6 weeks of treadmill exercise significantly improved immobility time in the forced swim test following maternal deprivation. This finding also suggests that regular exercise contributed to relieving stress caused by various environmental factors [26,37], influenced neurotransmitter secretion [38], and increased the expression of neurotrophic factors such as BDNF. Interestingly, 5-HT is known to be related to BDNF; according to Sakata et al. [39], 5-HT receptor levels significantly decreased in the frontal cortex and hippocampus of knock-out mice with suppressed BDNF expression. Additionally, the expression of 5-HT and 5-HT receptors in the basolateral amygdala of BDNF-mutated rats significantly decreased [40].
Neuronal growth and BDNF levels in the hippocampus significantly increased following direct 5-HT injection [41]; therefore, BDNF was assumed to play an important role in 5-HT expression and transmission. From this point of view, the increased number of 5-HT-positive cells in the raphe nuclei following treadmill exercise after social isolation could possibly be explained by the accompanying increase in BDNF. Although it was not clear whether the increased number of 5-HT-positive cells was due to increased BDNF or to exercise itself, the results indicated that treadmill exercise can potentially prevent the decrease in 5-HT- and TPH-positive cells in the raphe nuclei caused by social isolation.
CONCLUSION
We demonstrated that regular treadmill exercise following social isolation increased the levels of NGF, BDNF, and synapsin I to induce the survival of neurons in the hippocampus and was considered to have improved depression by increasing the number of serotonergic cells in the raphe nuclei.
Acknowledgments
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2012S1A5A2A01015855).
REFERENCES
- 1.Serra M, Sanna E, Mostallino MC, Biggio G. Social isolation stress and neuroactive steroids. Eur Neuropsychopharmacol. 2007;17:1–11. doi: 10.1016/j.euroneuro.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Serra M, Pisu MG, Floris I, Biggio G. Social isolation-induced changes in the hypothalamic-pituitary-adrenal axis in the rat. Stress. 2005;4:259–264. doi: 10.1080/10253890500495244. [DOI] [PubMed] [Google Scholar]
- 3.Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, Carter CS. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007;32:966–980. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wosiski-Kuhn M, Stranahan AM. Opposing effects of positive and negative stress on hippocampal plasticity over the lafespan. Agening Res Rev. 2012;11:399–403. doi: 10.1016/j.arr.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Lu L, Bao G, Chen H, Xia P, Fan X, Zhang J, Pei G, Ma L. Modification of hippocampal neurogenesis and neuroplasticity by social environments. Exp Neurol. 2003;183:600–609. doi: 10.1016/s0014-4886(03)00248-6. [DOI] [PubMed] [Google Scholar]
- 6.Mitra R, Sundlass K, Parker KJ, Schatzberg AF, Lyons DM. Social stress-related behavior affects hippocampal cell proliferation in mice. Physiol Behav. 2006;89:123–127. doi: 10.1016/j.physbeh.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 7.Heidbreder CA, Weiss IC, Domeney AM, Pryce C, Homberg J, Hedou G, Feldon J, Moran MC, Nelson P. Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience. 2000;100:749–768. doi: 10.1016/s0306-4522(00)00336-5. [DOI] [PubMed] [Google Scholar]
- 8.Fone KC, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents: Relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev. 2008;32:1087–1102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Silva-Gómez AB, Rojas D, Juárez I, Flores G. Decreased dendritic spine density on prefrontal cortical and hippocampal pyramidal neurons in postweaning social isolation rats. Brain Res. 2003;983:128–136. doi: 10.1016/s0006-8993(03)03042-7. [DOI] [PubMed] [Google Scholar]
- 10.Quan MN, Tian YT, Xu KH, Zhang T, Yang Z. Post weaning social isolation influences spatial cognition, prefrontal cortical synaptic plasticity and hippocampal potassium ion channels in wistar rats. Neuroscience. 2010;169:214–222. doi: 10.1016/j.neuroscience.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 11.Ku HY, Huang YF, Chao PH, Huang CC, Hsu KS. Neonatal isolation delays the developmental decline of long-term depression in the CA1 region of rat hippocampus. Neuropsychopharmacology. 2008;33:2847–2859. doi: 10.1038/npp.2008.36. [DOI] [PubMed] [Google Scholar]
- 12.Hicks LE, Wood N. Depression and suicide risks in older adults: a case study. Home Healthc Nurse. 2009;27:482–487. doi: 10.1097/01.NHH.0000360922.56851.04. [DOI] [PubMed] [Google Scholar]
- 13.Radak Z, Kaneko T, Tahara S, Nakamoto H, Pucsok J, Sasvari M, Nyakas C, Goto S. Regular exercise improves cognitive function and decreases oxidative damage in rat brain. Neurochem Int. 2001;38:17–23. doi: 10.1016/s0197-0186(00)00063-2. [DOI] [PubMed] [Google Scholar]
- 14.Uysal N, Tugyan K, Kayatekin BM, Acikgoz O, Bagriyanik HA, Gonenc S, Ozdemir D, Aksu I, Topcu A, Semin I. The effects of regular aerobic exercise in adolescent period on hippocampal neuron density, apoptosis and spatial memory. Neurosci Lett. 2005;383:241–245. doi: 10.1016/j.neulet.2005.04.054. [DOI] [PubMed] [Google Scholar]
- 15.Radak Z, Toldy A, Szabo Z, Siamilis S, Nyakas C, Silye G, Jakus J, Goto S. The effects of training and detraining on memory, neurotrophins and oxidative stress markers in rat brain. Neurochem Int. 2006;49:387–392. doi: 10.1016/j.neuint.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Daley A. Exercise and depression: A review of review. J Clin Psychol Med Settings. 2008;15:140–147. doi: 10.1007/s10880-008-9105-z. [DOI] [PubMed] [Google Scholar]
- 17.Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat Neurosci. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim EK, Lee MH, Kim H, Sim YJ, Shin MS, Lee SJ, Yang HY, Chang HK, Lee TH, Jang MH, Shin MC, Lee HH, Kim CJ. Maternal ethanol administration inhibits 5-hydroxytryptamine synthesis and tryptophan hydroxylase expression in the dorsal raphe of rat offspring. Brain Dev. 2005;27:472–476. doi: 10.1016/j.braindev.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 21.Mobley WC, Rutkowski JL, Tennekoon GI, Buchanan K, Johnston MV. Choline acetyltransferase activity in stratum of neonatal rats increased by nerve growth factor. Science. 1985;229:284–287. doi: 10.1126/science.2861660. [DOI] [PubMed] [Google Scholar]
- 22.Chan JP, Cordeira J, Calderon GA, Iyer LK, Rios M. Depletion of central BDNF in mice impedes terminal differentiation of new granule neurons in the adult hippocampus. Mol Cell Neurosci. 2008;39:372–383. doi: 10.1016/j.mcn.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frielingsdorf H, Simpson DR, Thal LJ, Pizzo DP. Nerve growth factor promotes survival of new neurons in the adult hippocampus. Neurobiol Dis. 2007;26:47–55. doi: 10.1016/j.nbd.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Neeper SA, Gómez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- 25.Chae CH, Kim HT. Forced, moderate-intensity treadmill exercise suppresses apoptosis by increasing the level of NGF and stimulating phosphatidylinositol 3-kinase signaling in the hippocampus of induced aging rats. Neurochem Int. 2009;55:208–213. doi: 10.1016/j.neuint.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 26.Hong YP, Kim HT. Effects of exercise preconditioning on the expression of NGF, Synapsin I, and ChAT in the hippocampus of socially isolated rats. J Life Sci. 2012;22:1180–1186. [Google Scholar]
- 27.Corradi A, Zanardi A, Giacomini C, Onofri F, Valtorta F, Zoli M, Benfenati F. Synapsin-I- and synapsin-II-null mice display an increased age-dependent cognitive impairment. J Cell Sci. 2008;121:3042–3051. doi: 10.1242/jcs.035063. [DOI] [PubMed] [Google Scholar]
- 28.Bogen IL, Haug KH, Roberg B, Fonnum F, Walaas S. The importance of synapsin I and II for neurotransmitter levels and vesicular storage in cholergic, glutamatergic and GABAergic nerve terminals. Neurochem Int. 2009;55:13–21. doi: 10.1016/j.neuint.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Hermes G, Li N, Duman C, Duman R. Post-weaning chronic social isolation produces profound behavioral dysregulation with decreases in prefrontal cortex synaptic-associated protein expression in female rats. Physiol Behav. 2010;104:354–359. doi: 10.1016/j.physbeh.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim AL, Taylor DA, Malone DT. Isolation rearing in rats: Effect on expression of synaptic, myelin and GABA-related immunoreactivity and its utility for drug screening via the subchronic parenteral route. Brain Res. 2011;1381:52–65. doi: 10.1016/j.brainres.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 31.Knapp DJ, Sin-Selley LJ, Breese GR, Overstreet DH. Selective breeding of 5-HT(1A) receptor-mediated responses: application to emotion and receptor action. Pharmacol Biochem Behav. 2000;67:701–708. doi: 10.1016/s0091-3057(00)00415-9. [DOI] [PubMed] [Google Scholar]
- 32.Hamon M, Blier P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog Neuropsychopharmacol Biol Psychiatry. 2013;40:54–63. doi: 10.1016/j.pnpbp.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 33.Ohno Y. New insight into the therapeutic role of 5-HT1A receptors in central nervous system disorders. Cent Nerv Syst Agents Med Chem. 2010;10:148–157. doi: 10.2174/187152410791196341. [DOI] [PubMed] [Google Scholar]
- 34.Kennedy SH, Rizvi SJ. Emerging drugs for major depressive disorder. Expert Opin Emerg Drugs. 2009;14:439–453. doi: 10.1517/14728210903107751. [DOI] [PubMed] [Google Scholar]
- 35.Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, Maier SF, Fleshner M. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci. 2003;23:2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang TY, Lin CH. A comparison between chronic exercise training and desipramine as treatments for the depression-like behavior of early-life maternal deprivation rats. Neurosci Lett. 2010;480:201–205. doi: 10.1016/j.neulet.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 37.Huang AM, Jen CJ, Chen HF, Yu L, Kuo YM, Chen HI. Compulsive exercise acutely upregulates rat hippocampal brain-derived neurotrophic factor. J Neural Transm. 2006;113:803–811. doi: 10.1007/s00702-005-0359-4. [DOI] [PubMed] [Google Scholar]
- 38.Banasr M, Hery M, Brezun JM, Daszuta A. Serotonin mediates oestrogen stimulation of cell proliferation in the adult dentate gyrus. Eur J Neurosci. 2001;14:1417–1424. doi: 10.1046/j.0953-816x.2001.01763.x. [DOI] [PubMed] [Google Scholar]
- 39.Sakata K, Duke SM. Lack of BDNF expression through promoter IV disturbs expression of monoamine genes in the frontal cortex and hippocampus. Neuroscience. 2013;260:265–275. doi: 10.1016/j.neuroscience.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 40.Shabrine SD, German C, Maribel R. Essential role of brain-derived neurotrophic factor in the regulation of serotonin transmission in the basolateral amygdala. Neuroscience. 2012;224:125–134. doi: 10.1016/j.neuroscience.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang D, Chen M, Russo-Neustadt A. Antidepressants are neuroprotective against nutrient deprivation stress in rat hippocampal neurons. Eur J Neuroscience. 2012;36:2573–2587. doi: 10.1111/j.1460-9568.2012.08187.x. [DOI] [PubMed] [Google Scholar]