Abstract
Although obesity is associated with osteoarthritis, it is unclear whether body weight (BW) independently affects articular cartilage catabolism (i.e., independent from physiological factors that also accompany obesity). The primary purpose of this study was to evaluate the independent effect of BW on articular cartilage catabolism associated with walking. A secondary purpose was to determine how decreased BW influenced cardiovascular response due to walking. Twelve able-bodied subjects walked for 30 minutes on a lower-body positive pressure treadmill during three sessions: control (unadjusted BW), +40%BW, and -40%BW. Serum cartilage oligomeric matrix protein (COMP) was measured immediately before (baseline) and after, and 15 and 30 minutes after the walk. Heart rate (HR) and rate of perceived exertion (RPE) were measured every three minutes during the walk. Relative to baseline, average serum COMP concentration was 13% and 5% greater immediately after and 15 minutes after the walk. Immediately after the walk, serum COMP concentration was 14% greater for the +40%BW session than for the -40%BW session. HR and RPE were greater for the +40%BW session than for the other two sessions, but did not differ between the control and -40%BW sessions. BW independently influences acute articular cartilage catabolism and cardiovascular response due to walking: as BW increases, so does acute articular cartilage catabolism and cardiovascular response. These results indicate that lower-body positive pressure walking may benefit certain individuals by reducing acute articular cartilage catabolism, due to walking, while maintaining cardiovascular response.
Key points.
Walking for 30 minutes with adjustments in body weight (normal body weight, +40% and -40% body weight) significantly influences articular cartilage catabolism, measured via serum COMP concentration.
Compared to baseline levels, walking with +40% body weight and normal body weight both elicited significant increases in articular cartilage catabolism, while walking with -40% body weight did not.
Cardiovascular response (HR and RPE) was not significantly different during walking with normal body weight and when compared to walking with -40% body weight.
Key words: Positive pressure treadmill, obesity, osteoarthritis, body mass, cartilage oligomeric matrix protein
Introduction
Thirty six percent of adult Americans are obese, and 69% are overweight (Flegal et al., 2012). Obesity is associated with knee osteoarthritis (OA) onset and progression (Felson et al., 2000; Griffin and Guilak, 2005; Hart and Spector, 1993). For each kilogram increase in body mass, knee OA genesis risk increases by 14% (Cicuttini et al., 1996), and a 5-kg body mass gain increases knee OA genesis likelihood by 35% (Hart and Spector, 1993). Relative to healthy-weighted peers, obese individuals experience greater vertical ground reaction forces (GRF) and altered GRF characteristics (Nimbarte and Li, 2011), which likely influences knee load and accelerates articular cartilage degradation (Creaby et al., 2013; Griffin and Guilak, 2005; Herzog et al., 2003; Whittle, 1999). Although obesity promotes knee OA due to mechanical and physiological factors (Felson et al., 2000), the role of each factor, independent from the other, is unclear. It is unclear how increased body mass, independent from physiological factors associated with obesity, influences articular cartilage catabolism. This knowledge is important to those who hope to solve the problem of knee OA, especially related to obesity.
Ambulation on a lower-body positive pressure treadmill is a relatively novel exercise mode that involves pressure differences between the lower- and upper-body (Hunter et al., 2014). The upward-directed pressure that is applied to the user reduces ambulatory GRF (Raffalt et al., 2013) and corresponding knee load (Patil et al., 2013). For this reason, these treadmills are being used in rehabilitative settings (Eastlack et al., 2005, Webber et al., 2014) by individuals with muscle and/or joint dysfunction (Kurz et al., 2011; Rose et al., 2013; Takacs et al., 2013). Although it has been established that low-impact aerobic exercise can help individuals decrease body mass and corresponding knee load (Hunter and Eckstein, 2009; Messier et al., 2005), low-impact exercise may elicit decreased cardiovascular response (Denning et al., 2010, Grabowski, 2010, Webber et al., 2014). Other researchers, however, have indicted no difference in cardiovascular measures during unloaded ambulation (Ruckstuhl et al., 2010; Thomas et al., 2007). The simultaneous effect of lower-body positive pressure on articular cartilage catabolism and cardiovascular response associated with walking has not yet been studied. It is important to determine whether walking with lower-body positive pressure can decrease articular cartilage catabolism, while simultaneously maintaining the cardiovascular benefit that is traditionally associated with walking.
Cartilage oligomeric matrix protein (COMP) is a noncollagenous extracellular matrix protein (Hedbom et al., 1992) that interacts with collagen and other matrix components to increase the structural integrity (Halász et al., 2007) and load bearing ability of articular cartilage (Tseng et al., 2009). In able-bodied individuals, serum COMP concentration acutely increases after walking and running (Kersting et al., 2005; Kim et al., 2009; Mündermann et al., 2005; Niehoff et al., 2010). This increase is thought to represent articular cartilage catabolism due to exercise-induced load (Erhart-Hledik et al., 2012, Niehoff et al., 2011). Also, increased resting serum COMP concentration indicates chronic articular cartilage degradation (Neidhart et al., 1997; Verma and Dalal, 2013), and is associated with OA onset and progression (Chaganti et al., 2008, Sharif et al., 1995). Resting serum COMP concentration decreases after massive weight loss (Richette et al., 2011).
The primary purpose of this study was to evaluate the acute and independent effect of body weight (BW) on articular cartilage catabolism associated with walking. A secondary purpose was to evaluate how walking on a lower-body positive pressure treadmill simultaneously affects articular cartilage catabolism and cardiovascular response. We hypothesized that walking with increased BW would acutely and independently increase articular cartilage catabolism. We also hypothesized that walking with lower-body positive pressure, in order to potentially reduce articular cartilage catabolism, would reduce cardiovascular response due to walking.
Methods
Subjects
In this cross-over designed study, a convenience sample of 12 able-bodied volunteers was used (Table 1). This sample size was chosen because previous researchers have found statistically significant differences using methods that were quite similar to the present methods, including the same dependent variables and similar sample sizes (Denning et al., 2014; Mündermann et al., 2005; Neidhart et al., 2000; Niehoff et al., 2011). Subjects were required to have no history of (1) lower-extremity injury six months prior to data collection, or (2) knee-related surgery in their lifetime. Subjects refrained from moderate to intense physical activity throughout participation in this study. Before participating, subjects read and signed an informed consent form that was approved by the Brigham Young University Institutional Review Board (IRB #X120398; Approved 1-2-2-13). All study procedures complied with the Helsinki Declaration.
Table 1.
Means (± 1 SD) for subject characteristics.
| Female (n = 6) | Male (n = 6) | |
|---|---|---|
| Age (years) | 20(2) | 21(2) |
| Height (m) | 1.66 (.09) | 1.80 (.06) |
| Mass (kg) | 61.6 (6.2) | 71.8 (7.0) |
| BMI (kg·m-2) | 22.4 (1.2) | 22.0 (1.2) |
| Speed (m·s-1) | 1.15 (.14) | 1.25 (.15) |
Procedures
Subjects completed three data collection sessions in a counterbalanced order: control (unadjusted BW), +40%BW (40% greater than unadjusted BW), and -40% BW (40% less than unadjusted BW). The sessions were completed 48 hours apart at the same time of day. For each session, subjects walked at the same self-selected speed for 30 minutes on a lower-body positive pressure treadmill (AlterG, Fremont, CA, USA). The self-selected walking speed was determined on the first day of data collection, 45 minutes before the start of the session: treadmill speed was increased, or decreased, until the subject described the speed to be the same speed that they would use to walk across a parking lot. For each session, subjects wore neoprene shorts that zipped into a lower-body positive pressure chamber that enclosed the subject from the waist down (Figure 1). For the control session, subjects walked with no BW manipulation. For the +40%BW session, subjects wore a weighted exercise vest (ZFO Sports, San Jose, CA, USA) that weighed 40% of their BW. For the -40%BW session, subjects walked while being unloaded 40% of BW, via the lower-body positive pressure chamber. For all sessions, subjects wore a HR monitor directly below the xiphoid process. HR and rate of perceived exertion (RPE; Borg 6-20 scale) were measured every three minutes, throughout each 30-minute walk, for each session, to quantify cardiovascular response.
Figure 1.
Subject using the lower-body positive pressure treadmill during the +40%BW session (note the use of the weighted vest).
Before each walk, subjects rested on a chair for 30 minutes to minimize potential influence of preceding activity on serum COMP concentration (Mündermann et al., 2005). Next, the baseline blood sample was collected. Subjects then completed one of the three 30-minute walks. Blood samples were then collected immediately, 15 minutes, and 30 minutes after the completion of the 30-minute walk. These specific time points were selected because serum COMP concentration is known to return to baseline levels within 30 minutes following a 30-minute walking exercise (Mündermann et al., 2005). All blood samples were collected with the subject in the same body position, sitting in a chair, to minimize potential influence of plasma volume shift on serum COMP concentration.
All blood samples (3 ml) were collected from an antecubital vein using a 20 gage shielded I.V. catheter (Becton Dickinson & Company, Franklin Lakes, NJ, USA) that was flushed every 15 minutes with 1-ml of isotonic saline (0.9%NaCl) to prevent clotting. A 1-ml waste sample was drawn prior to each blood sample. Each collected blood sample was placed in an EDTA vacutainer (Becton Dickinson & Company, Franklin Lakes, NJ, USA), centrifuged using an Eppendorf 5403 refrigerated centrifuge (Hamburg, Germany) for 15 minutes at 3000 × gravity, and then stored at -20°C. Serum COMP concentration was determined using a commercially available enzyme-linked immunosorbent assay (ELISA; R&D Systems, Inc., Minneapolis, MN, USA). Each sample was analyzed in triplicate and average concentration was calculated. The inter- and intra-assay coefficients of variation were 13.5% and 2.8%, respectively, for a 146.8 ±19.8 ng·ml-1 sample. Potential differences, due to inter-plate variation, were eliminated by comparing serum COMP concentration within subjects and testing all samples from each subject on the same plate.
Statistical analysis
Statistical computations were performed using JMP Pro 10 software (SAS Institute Inc., Cary, NC, USA). The independent variables were session (control, +40%BW, and -40%BW), draw (baseline, and immediately, 15, and 30 minutes after the walk for serum COMP) and time (every 3 minutes during the walk for HR and RPE).The dependent variables were serum COMP concentration, HR, and RPE. A mixed model repeated measures analysis of covariance (p < 0.05) was used to evaluate the potential effects of session and draw on serum COMP concentration. Because baseline COMP concentration differed between subjects and walking speed influences GRF and knee joint load (Nilsson and Thorstensson, 1989, Verma and Dalal, 2013), baseline COMP concentration and walking speed were included as covariates. A mixed-model repeated measures analysis of variance (p < 0.05) was used to evaluate the potential effects of session and time on HR and RPE. If a session × draw or session × time interaction existed, Tukey’s post hoc tests were performed to further evaluate for significant differences (p < 0.05).
Results
The 30-minute walk caused a significant increase in serum COMP concentration for the control and +40%BW sessions: relative to baseline, serum COMP concentration was 10% (p = 0.03) and 22% (p < 0.01) greater immediately after the 30-minute walk for the control and +40%BW sessions, respectively (Table 2). When values for each session were averaged, serum COMP concentration was 13% (p < 0.001) and 5% (p = 0.04) greater immediately after the walk and 15 minutes after the walk, respectively, relative to baseline (Table 2). Serum COMP concentration returned to baseline levels 30 minutes after the completion of the walk for all sessions (Table 2). When comparing between sessions, immediately after the walk, serum COMP concentration was 14% (p = 0.01) greater for the +40%BW session than for the -40%BW session. Lastly, when values for each draw were averaged, serum COMP concentration was 8% (p = 0.03) greater for the +40%BW session, relative to the -40%BW session (Table 2).
Table 2.
Mean (95% CI) serum COMP concentration (ng·ml-1) for all sessions and draws.
| SESSION | Baseline | DRAWS | |||
|---|---|---|---|---|---|
| Immediately After 30-minute Walk |
15 Minutes After 30-minute Walk |
30 Minutes After 30-minute Walk |
Session Mean | ||
| -40%BW | 106.5 (100.3 – 112.7) |
114.5† (108.3 – 120.7) |
108.0 (101.8 – 114.2) |
105.6 (99.3 – 111.8) |
108.7† (103.6 – 113.7) |
| Control | 107.1 (100.9 – 113.4) |
117.9* (111.7 – 124.2) |
109.4 (103.2 – 115.6) |
106.8 (100.5 – 113.0) |
110.3 (105.2 – 115.4) |
| +40%BW | 106.5 (100.3 – 112.7) |
130.4*, † (124.2 – 136.6) |
119.1* (112.8 – 125.3) |
112.4 (106.2 – 118.6) |
117.1† (112.0 – 122.1) |
| Draw Mean | 106.7 (102.3 – 111.1) |
121.0* (116.5 – 125.4) |
112.2* (107.8 – 116.6) |
108.2 (103.8 – 112.6) |
|
* indicate significant increase relative to baseline (p < 0.05).
† indicate significant between-session differences: COMP was greater for the +40%BW session, relative to the -40%BW session immediately after the 30-minute walk and when data were pooled across draws (p < 0.05).
Session and time both influenced HR (p < 0.01; Figure 2A) and RPE (p < 0.01; Figure 2B). For most of the walk, HR was significantly greater for the +40%BW session than for the -40%BW session (p < 0.05; Figure 2A). Similarly, for most of the walk, RPE was greater for the +40%BW session than for the control and -40%BW sessions (p < 0.05; Figure 2B). Mean HR for the +40%BW session (109 beats/min; 95% confidence intervals (CI): 103, 115) was 8.9% and 14.8% greater than for the control (99 beats/min; 95% CI: 93, 105; p < 0.01) and -40%BW sessions (93 beats/min; 95% CI: 87, 99; p < 0.01). Similarly, mean RPE for the +40%BW session (13.6; 95% CI: 12.6, 14.5) was 38% and 44% greater than for the control (8.5; 95% CI: 7.6, 9.5; p < 0.01) and -40% BW sessions (7.6; 95% CI: 6.7, 8.6; p < 0.01). There was no difference between HR nor RPE values at any point in time between the control and -40%BW sessions (p > 0.05).
Figure 2.
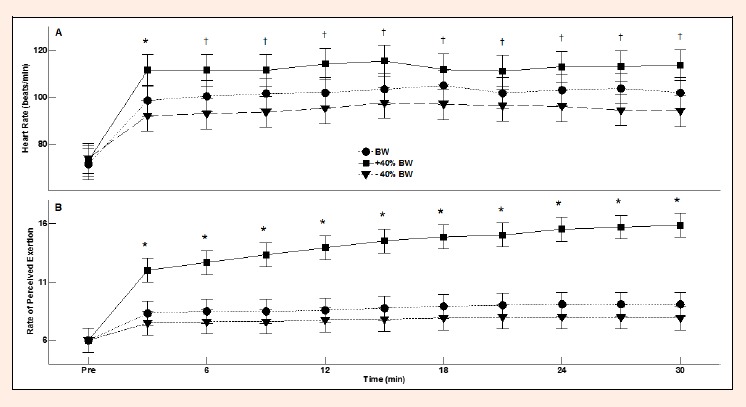
Mean and 95% CI for heart rate (2A) and rate of perceived exertion (2B) for the 30-minute walk for each session. The asterisks indicate that values for the +40%BW session were significantly greater than for the BW and -40%BW sessions (p < 0.05). The cross symbols indicate significant differences between +40%BW and -40%BW sessions (p < 0.05).
Discussion
The primary purpose of this study was to evaluate the independent effect of BW on acute articular cartilage catabolism. We hypothesized that walking with increased BW would acutely increase articular cartilage catabolism. The findings supported this hypothesis. Acute articular cartilage catabolism was greatest after subjects walked with increased BW (+40%BW session) and least after subjects walked with decreased BW (-40%BW session; Table 2). The results, however, partially contradicted our second hypothesis and showed that walking with reduced BW (from unadjusted BW to -40%BW) does not significantly decrease HR and RPE (Figure 2). Reducing BW from +40%BW to unadjusted BW did, however, decrease HR and RPE (Figure 2). As we initially hypothesized, BW, articular cartilage catabolism due to walking, and cardiovascular response due to walking appear to be related: as BW increases, acute articular cartilage catabolism and cardiovascular response associated with walking also increase.
This is the first study that has used BW, independently, to manipulate articular cartilage catabolism due to physical activity. Presently, serum COMP concentration increased nearly 14% due to a 30-minute walk and returned to baseline levels within 30 minutes after the completion of the walk (Table 2). This fits with previous reports that showed that physical activity, for subjects with unadjusted BW, resulted in articular cartilage catabolism (Kim et al., 2009; Mündermann et al., 2005; Neidhart et al., 2000; Niehoff et al., 2010; 2011). The present results add to the scientific literature by showing that walking for 30 minutes with more or less BW can independently affect acute articular cartilage catabolism associated with physical activity (Table 2). After increasing load to the musculoskeletal system, via additional mass (+40%BW), articular cartilage catabolism acutely increased. Walking with decreased BW (-40%BW), however, resulted in no significant increase for articular cartilage catabolism (Table 2). We speculate that the observed independent effect of BW on articular cartilage catabolism is linked to increased joint contact forces that accompany increased BW. This idea is supported by researchers who showed that lower-extremity joint contact forces are increased up to eight times BW while walking with an additional 20 kg of mass (Simonsen et al., 1995). A unique characteristic of the present study is that healthy-weighted subjects were used to provide evidence that excessive BW alone, independent from physiological factors that are related to obesity (e.g., inflammation), increases acute articular cartilage catabolism.
BW independently increased cardiovascular response due to walking: reducing BW from +40%BW to unadjusted BW resulted in a decrease for HR and RPE (Figure 2). HR and RPE, however, did not differ between the control and -40%BW sessions, showing that cardiovascular response during walking might be able to be maintained by limiting the decrease of BW (i.e., not decreasing BW too much). The present results regarding HR and RPE corroborate researchers who reported that neither HR nor RPE decreases as a result of unloaded (near -40% of BW) walking at a comfortable speed, relative to walking with unadjusted BW (Colby et al., 1999; Ruckstuhl et al., 2010; Thomas et al., 2007). Differences in HR and RPE have been reported for unloaded walking involving greater than preferred walking speeds and unloaded walking involving percentages greater than -40% BW (Ruckstuhl et al., 2010; Thomas et al., 2007). Contradicting all results mentioned thus far in this paragraph, however, Grabowski reported decreased cardiovascular response during unloaded walking of -25% BW (Grabowski, 2010). The reason for this discrepancy is unclear, however, Grabowski indicated that cardiovascular response at this unloading percentage could be increased by increasing walking speed (Grabowski, 2010). Collectively, present and previous data generally indicate that cardiovascular response to unloaded walking depends on the unloaded percentage and walking speed.
The present results might have implications regarding articular cartilage response, to walking, for obese patients who are simultaneously concerned with cartilage loss and cardiovascular response. Low-impact aerobic exercise that can reduce GRF (Barela and Duarte, 2008) and maintain cardiovascular response (Denning et al., 2010) likely benefits obese OA patients (Hunter and Eckstein, 2009). Using lower-body positive pressure to unload 50% of BW during walking reduces peak axial knee forces by more than 40% (Patil et al., 2013); this fact fits with the lack of change, between draws, for serum COMP concentration during the -40%BW walk (Table 2). Further, our data showed that cardiovascular response did not differ between the BW and -40%BW sessions. Our serum COMP and cardiovascular results collectively indicate that the use of a lower-body positive pressure treadmill may reduce articular cartilage catabolism that results from walking, while maintaining cardiovascular response. Importantly, however, generalizations of the present data to an obese and/or OA sample should be made cautiously: the present design only allows for inferences toward young able-bodied male and females of healthy weight.
This study has limitations. Although COMP is predominantly expressed in articular cartilage, it is also found in ligaments, tendons, menisci, and dermal and synovial fibroblasts (Dodge et al., 1998; Muller et al., 1998). Consequently, it is unclear which anatomical structure(s) contributed to the observed increase of serum COMP concentration. Researchers have hypothesized that increased serum COMP concentration, following physical activity, is at least partially due to knee load (Kersting et al., 2005; Niehoff et al., 2011). Our serum COMP results should be interpreted as general articular cartilage catabolism, not articular cartilage catabolism of a particular joint. Lastly, a larger sample might have elicited statistical differences where statistically insignificant trends now exist. For example, a post-hoc power analysis, using 80% power, revealed that 24 subjects would have been needed to find a significant 7% difference in serum COMP between the control and +40% BW sessions.
Conclusion
There are two primary findings from this study. First, and most importantly, BW appears to independently and acutely affect articular cartilage catabolism that occurs due to walking. Walking with unadjusted BW and increased BW resulted in measureable articular cartilage catabolism (via serum COMP); however, walking with decreased BW did not. Second, walking with acutely increased BW increased cardiovascular response (HR and RPE), compared to walking with unadjusted BW; however, there was no difference between walking with unadjusted BW and decreased BW for HR or RPE. In combination, the presently observed markers of articular cartilage catabolism (serum COMP) and cardiovascular response (HR and RPE) indicated that the use of lower-body positive pressure during walking may potentially benefit individuals who wish to simultaneously minimize knee joint load and maintain cardiovascular response.
Acknowledgments
We thank Dr. Allen Parcell for his direction regarding the analysis of serum COMP.
Biographies

W. Matt DENNING
Employment
Department of Health Promotion and Human Performance, Weber State University, Ogden, UT, USA
Degree
PhD
Research interest
Biomechanics of human gait, mechanical effects of knee pain, exercise treatment for osteoarthritis
E-mail: mattdenning@weber.edu

Jason G. WINWARD
Employment
Department of Exercise Sciences, Brigham Young University, Provo, UT, USA
Degree
BSc
Research interest
Biomechanics of human gait
E-mail: jasonwinward@gmail.com

Michael Becker PARDO
Employment
Department of Exercise Sciences, Brigham Young University, Provo, UT, USA
Degree
BSc
Research interest
Biomechanics of human gait
E-mail: malel_b@hotmail.com

J. Ty HOPKINS
Employment
Department of Exercise Sciences, Brigham Young University, Provo, UT, USA
Degree
PhD
Research interest
Neruomechanics of joint injury and rehabilitation.
E-mail: tyhopkins@byu.edu

Matthew K. SEELEY
Employment
Department of Exercise Sciences, Brigham Young University, Provo, UT, USA
Degree
PhD
Research interest
Biomechanics of human gait, neuromechanical effects of knee pain during human movement
E-mail: matthewkseeley@gmail.com
References
- Barela A. M., Duarte M. (2008) Biomechanical characteristics of elderly individuals walking on land and in water. Journal of Electromyography and Kinesiology 18, 446-454. [DOI] [PubMed] [Google Scholar]
- Chaganti R.K., Kelman A., Lui L., Yao W., Javaid M.K., Bauer D., Nevitt M., Lane N.E. (2008) Change in serum measurements of cartilage oligomeric matrix protein and association with the development and worsening of radiographic hip osteoarthritis. Osteoarthritis Cartilage 16, 566-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicuttini F.M., Baker J.R., Spector T.D. (1996) The association of obesity with osteoarthritis of the hand and knee in women: a twin study. Journal of Rheumatology 23, 1221-1226. [PubMed] [Google Scholar]
- Colby S.M., Kirkendall D.T., Bruzga R.F. (1999) Electromyographic analysis and energy expenditure of harness supported treadmill walking: implications for knee rehabilitation. Gait Posture 10, 200-205. [DOI] [PubMed] [Google Scholar]
- Creaby M.W., Hunt M.A., Hinman R.S., Bennell K.L. (2013) Sagittal plane joint loading is related to knee flexion in osteoarthritic gait. Clinical Biomechanics 28, 916-920. [DOI] [PubMed] [Google Scholar]
- Denning W.M., Bressel E., Dolny D.G. (2010) Underwater treadmill exercise as a potential treatment for adults with osteoarthritis. International Journal of Aquatic Research & Education 4, 70-80. [Google Scholar]
- Denning W.M., Woodland S., Winward J.G., Leavitt M.G., Parcell A.C., Hopkins J.T., Francom D., Seeley M.K. (2014) The influence of experimental anterior knee pain during running on electromyography and articular cartilage metabolism. Osteoarthritis and Cartilage 22, 1111-1119. [DOI] [PubMed] [Google Scholar]
- Dodge G.R., Hawkins D., Boesler E., Sakai L., Jimenez S.A. (1998) Production of cartilage oligomeric matrix protein (COMP) by cultured human dermal and synovial fibroblasts. Osteoarthritis and Cartilage 6, 435-440. [DOI] [PubMed] [Google Scholar]
- Eastlack R.K., Hargens A.R., Groppo E.R., Steinbach G.C., White K.K., Pedowitz R. A. (2005) Lower body positive-pressure exercise after knee surgery. Clinical Orthopaedics and Related Research 213-219. [DOI] [PubMed] [Google Scholar]
- Erhart-Hledik J.C., Favre J., Asay J.L., Smith R.L., Giori N.J., Mundermann A., Andriacchi T.P. (2012) A relationship between mechanically-induced changes in serum cartilage oligomeric matrix protein (COMP) and changes in cartilage thickness after 5 years. Osteoarthritis and Cartilage 20, 1309-1315. [DOI] [PubMed] [Google Scholar]
- Felson D.T., Lawrence R.C., Dieppe P.A., Hirsch R., Helmick C.G., Jordan J.M., Kington R.S., Lane N.E., Nevitt M.C., Zhang Y., Sowers M., McAlindon T., Spector T.D., Poole A.R., Yanovski S.Z., Ateshian G., Sharma L., Buckwalter J.A., Brandt K.D., Fries J.F. (2000) Osteoarthritis: new insights. Part 1: the disease and its risk factors. Annals of Internal Medicine 133, 635-646. [DOI] [PubMed] [Google Scholar]
- Flegal K.M., Carroll M.D., Kit B.K., Ogden C.L. (2012) Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. Journal of the American Medical Association 307, 491-497. [DOI] [PubMed] [Google Scholar]
- Grabowski A.M. (2010) Metabolic and biomechanical effects of velocity and weight support using a lower-body positive pressure device during walking. Archives of Physical Medicine and Rehabilitation 91, 951-7. [DOI] [PubMed] [Google Scholar]
- Griffin T.M., Guilak F. (2005) The role of mechanical loading in the onset and progression of osteoarthritis. Exercise and Sport Sciences Reviews 33, 195-200. [DOI] [PubMed] [Google Scholar]
- Halász K., Kassner A., Mörgelin M., Heinegård D. (2007) COMP acts as a catalyst in collagen fibrillogenesis. Journal of Biological Chemistry 282, 31166-31173. [DOI] [PubMed] [Google Scholar]
- Hart D.J., Spector T.D. (1993) The relationship of obesity, fat distribution and osteoarthritis in women in the general population: the Chingford Study. Journal of Rheumatology 20, 331-335. [PubMed] [Google Scholar]
- Hedbom E., Antonsson P., Hjerpe A., Aeschlimann D., Paulsson M., Rosapimentel E., Sommarin Y., Wendel M., Oldberg A., Heinegard D. (1992) Cartilage matrix proteins-an acidic oligomeric protein (COMP) detected only in cartialge Journal of Biological Chemistry 267, 6132-6136. [PubMed] [Google Scholar]
- Herzog W., Longino D., Clark A. (2003) The role of muscles in joint adaptation and degeneration. Langenbeck’s Archives of Surgery 388, 305-315. [DOI] [PubMed] [Google Scholar]
- Hunter D.J., Eckstein F. (2009) Exercise and osteoarthritis. Journal of Anatomy 214, 197-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter I., Seeley M.K., Hopkins J. T., Carr C., Franson J.J. (2014) EMG activity during positive-pressure treadmill running. Journal of Electromyography and Kinesiology 24, 348-352. [DOI] [PubMed] [Google Scholar]
- Kersting U.G., Stubendorff J.J., Schmidt M.C., Bruggemann G.P. (2005) Changes in knee cartilage volume and serum COMP concentration after running exercise. Osteoarthritis and Cartilage 13, 925-934. [DOI] [PubMed] [Google Scholar]
- Kim H.J., Lee Y.H., Kim C.K. (2009) Changes in serum cartilage oligomeric matrix protein (COMP), plasma CPK and plasma hs-CRP in relation to running distance in a marathon (42.195 km) and an ultra-marathon (200 km) race. European Journal of Applied Physiology 105, 765-770. [DOI] [PubMed] [Google Scholar]
- Kurz M.J., Corr B., Stuberg W., Volkman K.G., Smith N. (2011) Evaluation of lower body positive pressure supported treadmill training for children with cerebral palsy. Pediatric Physical Therapy 23, 232-239. [DOI] [PubMed] [Google Scholar]
- Messier S.P., DeVita P., Cowan R.E., Seay J., Young H.C., Marsh A.P. (2005) Do older adults with knee osteoarthritis place greater loads on the knee during gait? A preliminary study. Archives of Physical Medicine and Rehabilitation 86, 703-709. [DOI] [PubMed] [Google Scholar]
- Muller G., Michel A., Altenburg E. (1998) COMP (cartilage oligomeric matrix protein) is synthesized in ligament, tendon, meniscus, and articular cartilage. Connective Tissue Research 39, 233-244. [DOI] [PubMed] [Google Scholar]
- Mündermann A., Dyrby C.O., Andriacchi T.P., King K.B. (2005) Serum concentration of cartilage oligomeric matrix protein (COMP) is sensitive to physiological cyclic loading in healthy adults. Osteoarthritis and Cartilage 13, 34-38. [DOI] [PubMed] [Google Scholar]
- Neidhart M., Hauser N., Paulsson M., DiCesare P.E., Michel B.A., Häuselmann H.J. (1997) Small fragments of cartilage oligomeric matrix protein in synovial fluid and serum as markers for cartilage degradation. British Journal of Rheumatology 36, 1151-1160. [DOI] [PubMed] [Google Scholar]
- Neidhart M., Müller-Ladner U., Frey W., Bosserhoff A.K., Colombani P.C., Frey-Rindova P., Hummel K. M., Gay R. E., Häuselmann H., Gay S. (2000) Increased serum levels of non-collagenous matrix proteins (cartilage oligomeric matrix protein and melanoma inhibitory activity) in marathon runners. Osteoarthritis and Cartilage 8, 222-229. [DOI] [PubMed] [Google Scholar]
- Niehoff A., Kersting U.G., Helling S., Dargel J., Maurer J., Thevis M., Bruggemann G.P. (2010) Different mechanical loading protocols influence serum cartilage oligomeric matrix protein levels in young healthy humans. European Journal of Applied Physiology 110, 651-657. [DOI] [PubMed] [Google Scholar]
- Niehoff A., Muller M., Bruggemann L., Savage T., Zaucke F., Eckstein F., Muller-Lung U., Bruggemann G.P. (2011) Deformational behaviour of knee cartilage and changes in serum cartilage oligomeric matrix protein (COMP) after running and drop landing. Osteoarthritis and Cartilage 19, 1003-1010. [DOI] [PubMed] [Google Scholar]
- Nilsson J., Thorstensson A. (1989) Ground reaction forces at different speeds of human walking and running. Acta Physiologica Scandinavica 136, 217-227. [DOI] [PubMed] [Google Scholar]
- Nimbarte A.D., Li L. (2011) Effect of added weights on the characteristics of vertical ground reaction force during walk-to-run gait transition. Human Movement 12, 81-87. [Google Scholar]
- Patil S., Steklov N., Bugbee W.D., Goldberg T., Colwell C.W., Jr., D’Lima D.D. (2013) Anti-gravity treadmills are effective in reducing knee forces. Journal of Orthopaedic Research 31, 672-679. [DOI] [PubMed] [Google Scholar]
- Raffalt P.C., Hovgaard-Hansen L., Jensen B.R. (2013) Running on a lower-body positive pressure treadmill: VO2max, respiratory response, and vertical ground reaction force. Research Quarterly for Exercise and Sport 84, 213-222. [DOI] [PubMed] [Google Scholar]
- Richette P., Poitou C., Garnero P., Vicaut E., Bouillot J.L., Lacorte J.M., Basdevant A., Clement K., Bardin T., Chevalier X. (2011) Benefits of massive weight loss on symptoms, systemic inflammation and cartilage turnover in obese patients with knee osteoarthritis. Annals of Rheumatic Diseases 70, 139-144. [DOI] [PubMed] [Google Scholar]
- Rose M.H., Lokkegaard A., Sonne-Holm S., Jensen B.R. (2013) Effects of training and weight support on muscle activation in Parkinson’s disease. Journal of Electromyography and Kinesiology 23, 1499-1504. [DOI] [PubMed] [Google Scholar]
- Ruckstuhl H., Schlabs T., Rosales-Velderrain A., Hargens A.R. (2010) Oxygen consumption during walking and running under fractional weight bearing conditions. Aviation, Space, and Environmental Medicine 81, 550-554. [DOI] [PubMed] [Google Scholar]
- Sharif M., Saxne T., Shepstone L., Kirwan J.R., Elson C.J., Heinegård D., Dieppe P.A. (1995) Relationship between serum cartilage oligomeric matrix protein levels and disease progression in osteoarthritis of the knee joint. British Journal Of Rheumatology 34, 306-310. [DOI] [PubMed] [Google Scholar]
- Simonsen E.B., Dyhre-Poulsen P., Voigt M., Aagaard P., Sjogaard G., Bojsen-Moller F. (1995) Bone-on-bone forces during loaded and unloaded walking. Acta Anatomica (Basel) 152, 133-142. [DOI] [PubMed] [Google Scholar]
- Takacs J., Anderson J.E., Leiter J.R., MacDonald P.B., Peeler J.D. (2013) Lower body positive pressure: an emerging technology in the battle against knee osteoarthritis? Clinical Interventions in Aging 8, 983-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E.E., De Vito G., Macaluso A. (2007) Physiological costs and temporo-spatial parameters of walking on a treadmill vary with body weight unloading and speed in both healthy young and older women. European Journal of Applied Physiology 100, 293-299. [DOI] [PubMed] [Google Scholar]
- Tseng S., Reddi A.H., Di Cesare P.E. (2009) Cartilage oligomeric matrix protein (COMP): a biomarker of arthritis. Biomarker Insights 4, 33-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma P., Dalal K. (2013) Serum cartilage oligomeric matrix protein (COMP) in knee osteoarthritis: a novel diagnostic and prognostic biomarker. Journal of Orthopaedic Research 31, 999-1006. [DOI] [PubMed] [Google Scholar]
- Webber S.C., Horvey K.J., Pikaluk M.T.Y., Butcher S.J. (2014) Cardiovascular responses in older adults with total knee arthroplasty at rest and with exercise on a positive pressure treadmill. European Journal of Applied Physiology 114, 653-662. [DOI] [PubMed] [Google Scholar]
- Whittle M.W. (1999) Generation and attenuation of transient impulsive forces beneath the foot: a review. Gait and Posture 10, 264-275. [DOI] [PubMed] [Google Scholar]


