Abstract
The skeletal muscle in aged rats adapts rapidly following a period of exercise. This adaptation includes structural remodeling and biochemical changes such as an up-regulation of antioxidant enzymes, content of stress and heat shock proteins (HSPs). However, the associated molecular mechanisms mediating different types of exercise training-induced adaptations are not yet completely understood. Therefore, the purpose of this study was to investigate the effects of duration and frequency exercise on the expression of HSPs, antioxidant enzymes, and mitogen-activated protein kinase (MAPKs) in the skeletal muscles of aged rats. Young (3-month-old) and aged (20-month-old) male Sprague-Dawley rats were randomly assigned to 6 groups and extensor digitorum longus (EDL; fast twitch muscle fiber) and soleus (SOL; slow twitch muscle fiber) skeletal muscles were collected immediately. The expression pattern of HSPs in skeletal muscles was decreased in old groups compared with young groups. Especially, HSPs showed lower expression in SOL than EDL muscle. Interestingly, HSPs in aged rats was increased significantly after S1 (single long-duration; 1×30 min, 5 days/week for 6 weeks) and M1 types (multiple short-duration; 3×10 min·day−1, 5 days·week−1 for 6 weeks) than S2 (single long-duration; 1×30 min, 3 days/week for 6 weeks) and M2 (multiple short-duration; 3×10 min·day−1, 3 days·week−1 for 6 weeks) types of exercise training. Also, superoxide dismutase (SODs) showed similar expression as HSP did. On the contrary, the p-ERK and p-JNK were down regulated. In addition, p-p38 level in the SOL muscle was activated markedly in all exercise groups. These results demonstrate that increasing of HSP expression through duration and frequency exercise can lead to protection and training-induced adaptation against aging-induced structural weakness in skeletal muscles.
Key points.
The expression of heat shock proteins (HSPs) in aged rats was increased significantly after single long-duration (S1) and multiple short-duration (M1) types than S2 and M2 types of exercise training in soleus (SOL) skeletal muscles.
Superoxide dismutase (SODs) showed similar expression as HSPs did. On the contrary, the p-ERK and p-JNK were down regulated. In addition, p-p38 level in the SOL muscle was activated markedly in all exercise groups.
Induction of HSPs and SODs by high duration and frequency of exercise training such as S1 and M1 types with concomitant MAPKs pathway depending on the type of muscles.
The frequency and duration of exercise training could affect the functional adaptation and protection against aging-induced structural weakness of skeletal muscles through changing expression of related molecules.
Key words: Aging, multiple short-duration exercise training, single long-duration exercise training, heat shock protein, superoxide dismutase, mitogen-activated protein kinase
Introduction
Aged skeletal muscles become smaller and weaker, which are also more susceptible to injury and take considerably longer to repair (Menshikova et al., 2006; Pasini et al., 2012). Previous studies have shown that the regular exercise leads to training-induced adaptation of aged muscles through contractile protein synthesis (Menshikova et al., 2006; Ogura et al., 2011). This adaptation includes structural remodeling and biochemical changes such as an up-regulation of antioxidant enzymes, content of stress and heat shock proteins (HSPs) (McArdle and Jackson, 2000; Vasilaki et al., 2003).
HSPs play an important role in cellular processes, such as cell survival, proliferation and prevention of apoptosis against oxidative stress and aging (Koh and Escobedo, 2004; Vasilaki et al., 2003; Weber et al., 2012). HSPs are classified into hsp 27, 60, 70 and 90 according to the molecular weight. The expression of HSPs in skeletal muscles increases with exercise training, and it causes inhibition of apoptosis and cytoskeletal protection, which are needed for maintaining homeostasis and preventing myotube degeneration (Gabai and Sherman, 2002). Furthermore, HSPs correlates with high expression of SODs, which acts as a protective mechanism against oxidative stress in skeletal muscles after exercise (Murlasits et al., 2006; Zembron-Lacny et al., 2008). Among signaling pathway, MAPKs are general mediators of the stress response during exercise training (Nader and Esser, 2001). ERK1/2, p38 and JNK of MAPKs families are activated by various environmental stresses and related to activation of HSPs (Cargnello and Roux, 2011). Study about the exercise-induced production of HSPs in the skeletal muscle of aged rats is important as it may provide a valuable insight into the molecular mechanisms which can increase muscle mass against age-related stressors by regular exercise (Morton et al., 2009). Previous studies have demonstrated that HSP may differ in aged animal skeletal muscles following a period of duration and frequency exercise as well as numerous muscle structural and biochemical changes including increased antioxidant enzymes activity (McArdle and Jackson, 2000; Nader and Esser, 2001; Noble et al., 2008). Nevertheless, the effects of duration and frequency of exercise on the expression of HSPs in the skeletal muscle of aged animals are unknown. Therefore, the purpose of this study was to investigate whether different types of exercise training affect the expression of HSPs, SODs, and MAPKs in skeletal muscles of aged rats.
Methods
Animals
Male Sprague-Dawley rats [young (4 months old) and aged (21 months old) rats] were kept in a room that had an inverted 12h light/dark cycle under standard conditions of temperature and humidity. The animals were divided into the following 8 groups: young control (YC, n = 6) and old control (OC, n = 6); single long-duration exercise-trained old groups (OS1, n = 7): 1×30 min, 5 days·week−1 for 6 weeks; second type of single long-duration exercise-trained old groups (OS2, n = 8): 1×30 min, 3 days·week−1 for 6 weeks; multiple short-duration exercise-trained old groups (OM1, n = 8): 3×10 min·day−1, 5 days·week−1 for 6 weeks; second type of multiple short-duration exercise-trained old groups (OM2, n = 8): 3×10 min, 3 days/week for 6 weeks. The rats in the exercise groups were subjected to a treadmill physical training program. A standard laboratory animal chow (56.8% carbohydrates, 22.5% proteins, 3.5% lipids and 17.2% other nutrients; Korean animals) and water were given ad libitum. The experiment was approved by the Ethical Committee of the Chonbuk National Universityv (CBU: 2012-0046) for care and experimentation of laboratory animals.
Physical training protocol
The training protocol has been described previously (Ogura et al., 2011). In the first week of the preliminary experiment, the rats were adapted to the treadmill (Omnipacer model LC-4, Omnitech, Columbus, OH), and Exercise consisted of 5~10 min at a speed of 5~10 m·min−1 with an incline of 0°. This program comprised of running on a treadmill for 30 (30×1) min, 5 days or 3 days per week for 6 weeks, and for 30 (10×3) min, 5 days or 3 days per week for 6 weeks at a maximal intensity or speed by aging rats (10 m·min−1, 5° incline). Electric shocks were used sparingly to motivate the animals to run. Electrical shocks were applied to the metal grid behind the lane to stimulate the rats that failed to run spontaneously. The rats in the non-exercise group remained in their cages. Rats were sacrificed 48 h after their last exercise bout to minimize the influence of the last bout of exercise. Samples of blood and extensor digitorum longus (EDL; fast type) and soleus (SOL; slow type) skeletal muscles were collected. Separated serum and skeletal muscles were stored at –80°Cbefore determining cytokine levels and protein activity.
Western blot analysis
The total proteins were extracted from soleus and EDL muscle tissues using a lysis buffer containing 150 mmol·L−1 NaCl, 5 mmol·L−1 EDTA, 50 mmol·L−1 Tri-HCl (pH 8.0), 1%-NP 40, 1 mmol·L−1 aprotinin, 0.1 mmol leupeptin, and 1 mmol·L−1 pepstatin and quantified according to the Bradford dye-binding procedure (Bio-Rad, Hercules, CA, USA). A total protein of 20 μg was electrophoresed on 8% SDS-polyacrylamide gel under denaturing conditions, and then transferred to a Hybond-P membrane (Amersham, Arlington, IL, USA) with a Mini-protean II system (Bio-Rad, Hercules, CA, USA). After blocking with 5% skimmed milk in phosphate buffered saline (PBS), the membranes were incubated with the appropriate antibody [i.e. HSP27 (ADI-SPA-803, Enzo Life Sciences Inc, NY, USA); HSP60 (SC-13966, Santa Cruz Biotech, Santa Cruz, CA, USA); HSP70 (ADI-SPA-810, Enzo Life Sciences Inc, NY, USA); HSP90 (ADI-SPA-836, Enzo Life Sciences Inc, NY, USA); p-ERK (#9102, Cell signaling Beverly, MA, USA); p-p38 (SC-7975-R, Santa Cruz Biotech, Santa Cruz, CA, USA); p-JNK (#9251, Cell signaling Beverly, MA, USA); Mn-SOD (SC-30080, Santa Cruz Biotech, Santa Cruz, CA, USA); Cu/Zn SOD (ADI-SOD-100, Enzo Life Sciences Inc, NY, USA); ES-SOD (SC-32220, Santa Cruz Biotech, Santa Cruz, CA, USA ); HO-1 (SC-136960, Santa Cruz Biotech, Santa Cruz, CA, USA) and tubulin (Sigma-Aldrich, St. Louis, MO, USA)] at a 1:1,000 dilution in either 1% skim milk or 3% BSA for 4 or 24 h at 4°C or room temperature. After washing with PBS containing 0.1% Tween-20, once for 15 min and twice for 5 min, the membranes were incubated with anti-mouse or anti-rabbit IgG conjugated to horseradish peroxidase at a 1:3,000 dilution in PBS for 1 h at room temperature. After the final wash, the immune-reactive bands were visualized with chemiluminescent detection using LAS-4000 CCD Imaging system (Fujifilm Corp. Tokyo, Japan). The protein expression levels were analyzed with immageQuanT TL 1D gel analysis programme (GE Healthcare Bio-Science AB, Sweden).
Statistical analyses
The group comparisons were assessed by one-way analysis of variance (ANOVA) and Student-Newman-Keuls post hoc test. Null hypotheses of no difference were rejected if p-values were p < 0.05. The values are presented as mean ± SD.
Results
Morphological characteristics
Age-related skeletal muscle mass is characterized as the loss of muscle fibers including type II fiber of EDL muscle (Pasini et al., 2012). In this study, EDL muscle mass was decreased in OC group compared to YC group. 6 weeks of exercise training types significantly increased the weight of the EDL and SOL muscle fibers in aged rats (Figure 1B. C). In particular, OS1 and OM1 groups were increased significantly compared with OS2 and OM2 groups (p < 0.05). However, Body weight didn’t show any difference in old groups despite exercise training (Figure 1A).
Figure 1.
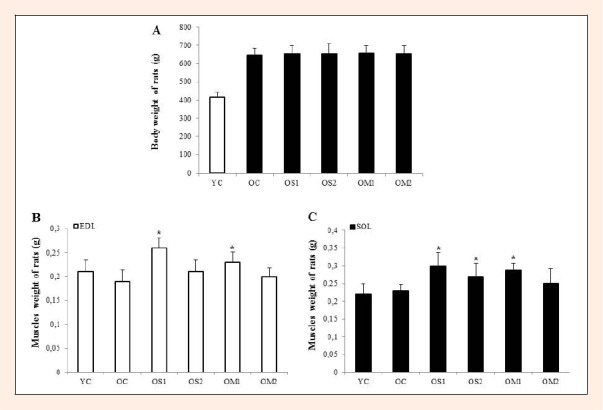
The body (A) and EDL (B), SOL (C) muscle weights were determined in young and old rats after 6 weeks of exercise training. The values are presented as mean ± standard deviation of animals per group (p < 0.05). The groups are young control (YC), old control (OC), single long-duration exercise-trained (OS1; 5 days·weeks−1, 1×30 min/day, OS2; 3 days·weeks−1, 1×30 min·day−1), and multiple short-duration exercise-trained (OM1; 5 days·weeks−1, 3×10 min·day−1, OM2; 3 days/weeks, 3×10 min·day−1). EDL muscle; extensor digitorum longus, SOL muscle; soleus. The symbols * indicates = significantly different from the OC group (p < 0.05).
The expression of HSPs after different types of exercise training in aged rats
The HSP27, 60, 70 and 90 were decreased significantly in OC group compared to the YC group (p < 0.05). HSP27 was increased markedly by almost all exercise groups in EDL and SOL muscles. In particular, OS1 and OM1 groups showed significantly increased than OS2 and OM2 groups in expression of HSP27 (p < 0.05) (Figure 2A). Similarly, the HSP60, 70 and 90 levels were increased markedly in OS1 and OM1 groups of old groups (p < 0.05) (Figure 2B, C, D). Above all, the HSPs levels were mostly increased in OS1 group than OM1 group (p < 0.05).
Figure 2.
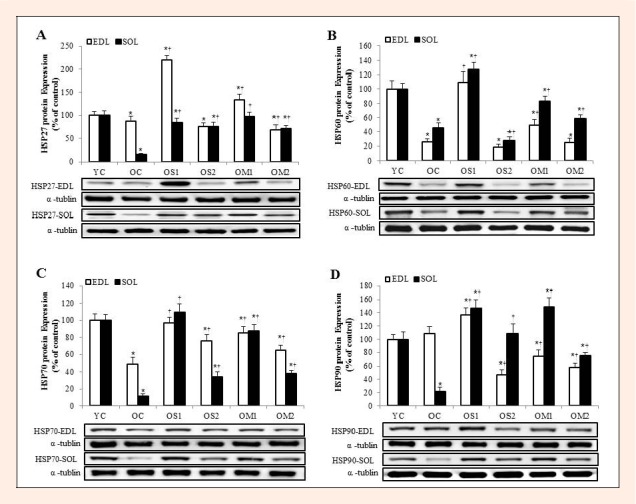
The expression levels of HSP27 (A), HSP60 (B) HSP70 (C), and HSP90 (D) proteins in the extensor digitorumlongus (EDL) and soleus (SOL) muscles. Data are presented as percentage of mean YC values. Representative blots are shown. The symbols * indicates = significantly different from the YC group (p < 0.05). The symbol † indicates = significantly different from the OC group (p < 0.05).
The expression of antioxidants after different types of exercise training in aged rats
The Mn-SOD showed different pattern of expression with exercise training in the EDL and SOL muscles. Mn-SOD in SOL muscle was increased significantly in all exercise groups. In particular, OS1 group showed significantly increased expression than OS2 group. However, in EDL muscle, all exercise groups showed reduction pattern (p < 0.05) (Figure 3A). The Cu/Zn-SOD was increased significantly after exercise training in both EDL and SOL muscles (p < 0.05) (Figure 3B). Also, EC-SOD in EDL muscle was increased significantly in OS1 group. In contrast, EC-SOD in the SOL muscle was increased significantly in OM1 group (p < 0.05) (Figure 3C). In addition, heme oxygenase-1 (HO-1) in both EDL and SOL muscles were increased significantly in OS1 exercise training group (p < 0.05) (Figure 3D).
Figure 3.
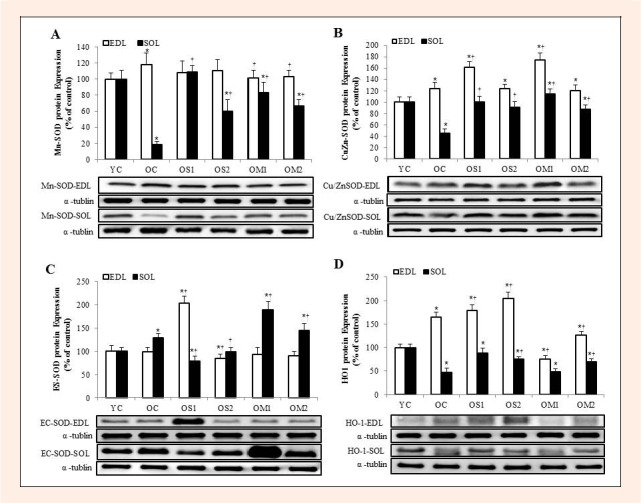
The expression levels of Mn-SOD (A), Cu/Zn-SOD (B) ES-SOD (C), and HO-1 (D) proteins in the extensor digitorum longus (EDL) and soleus (SOL) muscles. Data are presented as percentage of mean YC values. Representative blots are shown. The symbol * indicates = significantly different from the YC group (p < 0.05). The symbol † indicates = significantly different from the OC group (p < 0.05).
The expression of MAPKs after different types of exercise training in aged rats
The MAPKs showed different pattern of expression according to the muscle types, age, and exercise (Figure 4A, B, C). p-ERK in SOL muscle was inhibited significantly in OS1 and OM1 groups. In particular, OM groups were decreased markedly in both EDL and SOL muscles (p < 0.05) (Figure 4A). Also, p-p38 in EDL muscle was decreased significantly in the OS2 and OM groups. Conversely, p-p38 level in SOL muscle was increased markedly in all exercise groups (p < 0.05) (Figure 4B). Moreover, p-JNK level was decreased significantly in OS1 and OS2 groups of the EDL and SOL muscles (p < 0.05) (Figure 4C).
Figure 4.
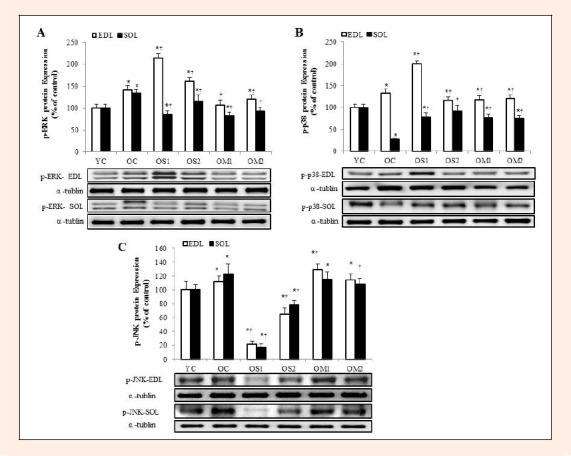
The expression levels of p-ERK (A), p-p-38 (B), p-JNK (C) proteins in the extensor digitorum longus (EDL) and soleus (SOL) muscles. Data are presented as percentage of mean YC values. Representative blots are shown. The symbol * indicates = significantly different from the YC group (p < 0.05).The symbol † indicates = significantly different from the OC group (p < 0.05).
Discussion
In this study, HSPs, SODs and MAPKs showed different pattern of expression on the types of exercise training and muscle types. The HSPs are usually named according to their molecular weight (McArdle and Jackson, 2000) and prevent muscle damage caused by exercise training and aging (McArdle et al., 2002; Murlasits et al., 2006). Recent studies showed that HSPs have demonstrated different expression patterns according to the duration of exercise, intensity, and muscle types (Noble et al., 2008; Paulsen, 2007). Especially, they show high expression in slow-twitch oxidative skeletal muscle such as the soleus (Noble et al., 2006; 2008). Therefore, the expression of HSPs may differ according to the characteristics of the skeletal muscle- and exercise-specific, which is highly sensitive to aging-induced oxidative stress (Lollo et al., 2013; Noble et al., 2008; Paulsen, 2007). In addition, hemo oxygenase-1 (HO-1) is recognized as a major heat shock and stress response protein in cellular defense against oxidative stress (Elbirt and Bonkovsky, 1999). In this study, the expressions of HSPs and HO-1 were lower in aged rats compared to young rats. However, HSPs showed a remarkable increase in skeletal muscles by exercise training. In particular, they were elevated markedly by means of OS1 and OM1 training in SOL muscle. Above all, the HSP levels were mostly increased in the OS1 group rather than the OM1 group. These results suggest that the S1 and M1 types of exercise methods (5days/week) have a similar recovery capacity against aging- and exercise-induced oxidative stress in highly oxidative muscles such as the soleus muscle of old rats. This study resulted in important and beneficial finding that HSP expression appears to be proportional to the duration and frequency of exercise training.
Skeletal muscles of aged rats are significantly susceptible to age-induced oxidative damage (McArdle and Jackson, 2000). This suggests that for improvement in the muscles, antioxidant capacity might be important following exercise (Nader and Esser, 2001). This study is consistent with the previous study which showed that the level of SODs is lower in old group compared with young group without exercise training. However, the level of SODs showed increased expression in aged rats by exercise training. In particular, the level of SODs elevated higher in OS1 and OM1 groups than OS2 and OM2 groups in both EDL and SOL muscle. It is clear that activation of SODs has associated with duration and frequency of exercise as well as the type of skeletal muscle. These findings suggest that antioxidant enzyme activity in aging may be differently activated following various types, intensity, and duration of exercise training. It is possible that the function of HSPs might have relevance to activation of SODs following exercise training in aging muscle. However, OS and OM groups in EDL muscle showed abbreviated expressions of Mn-SOD protein due to the exercise training. Because, it may be possible that the maladjustment state by the exercise caused high levels of oxidative stress in EDL muscle of aged rats.
MAPKs are known to be related to activation of HSPs. This pathway is activated by various environmental stresses and growth factors (Carlson et al., 2001). Also, it is involved in exercise-induced adaptations in skeletal muscle (van Ginneken et al., 2006). Kramer and Goodyear (2007) demonstrated that the role of MAPK activation by exercise is the transcriptional regulation of redox status in the skeletal muscle. Furthermore, exercise caused an increase in the phosphorylation of the ERK1/2, p38 and JNK proteins in rats (Cargnello and Roux, 2011). Widegren et al. (2000) reported that phosphorylation of ERK1/2 is rapidly decreased during recovery after exercise. In addition, p-38 is a stress-activated kinase that responds to cellular stress, such as metabolic stress and inflammation response (Carlson et al., 2001; Nader and Esser, 2001). Previous reports suggested that JNK level in the skeletal muscle of aged rats reduced following exercise training with activation of SOD and phosphorylation of p38 in cell survival (Kostenko and Moens, 2009; Launay et al., 2006; Lee et al., 2005). Consistent with previous report, expression of p-ERK was decreased significantly after exercise training. In particular, expression of p-ERK (its molecules was markedly reduced in the OS1 and OM1 groups compared with OC group in the SOL muscle. Also, the expression patterns of p-p38 appeared similar to HSPs expression. In addition, p-p38 was increased markedly in the SOL muscle compared to the EDL muscle after exercise training. Simultaneously, the p-JNK was decreased markedly in the OS1 group of the EDL and SOL muscles.
Taken together, HSPs are known to be activated via MAPK pathway such as phosphorylation of p38 and ERK1/2 due to exercise stimulus (Widegren et al., 2000). Thus, these results suggest that the expression of HSPs and SODs was profoundly associated with regulation of MAPKs for adaptation of skeletal muscle after regular exercise training. Perhaps these results indicate that the expression of HSPs and MAPKs in skeletal muscles elicited functional adaptations by exercise training which is evident effect of high duration and frequency of exercise in aged rat.
Conclusion
This study resulted in induction of HSPs and SODs expression by high duration and frequency of exercise training such as single long-duration exercise training (S1) and multiple short-duration (M1) types with concomitant MAPKs pathway depending on the type of muscles. Collectively, findings from the present study suggest that the frequency and duration of exercise training could affect the functional adaptation and protection against aging-induced weakness of skeletal muscles through changing expression of related molecules. Also, benefits elicited by physical exercise appear proportional to the training frequency and duration. This valuable information may have important clinical efficacy. Further studies will be required to elucidate how the recovery period after exercise affects the expression of HSPs and MAPKs in skeletal muscles.
Biographies
Jeong-Seok KIM
Employment
Lecturer of Department of Physical Education, College of Education, and Researcher at Department of Oral Biochemistry, Institute of Oral Bioscience, School of Dentistry, Chonbuk National University, Jeonju, Korea.
Degree
PhD
Research interest
Exercise physiology and sport nutrition, study of treatment of metabolic syndrome using a rat model
E-mail: kjs2002dol@hanmail.net
Young-Hee LEE
Employment
Researcher at Department of Oral Biochemistry, Institute of Oral Bioscience, School of Dentistry, Chonbuk National University, Jeonju, Korea.
Degree
PhD
Research interest
Bioscience and Biochemistry, Exercise therapy
E-mail: leeyh@jbnu.ac.kr
Do-Yourl CHOI
PhD student Department of Physical Education, College of Education, Chonbuk National University, Jeonju, Korea.
Degree
PhD
Research interest
Exercise therapy for chronic diseases in older people
E-mail: cdy5447@naver.com
Ho-Keun YI
Director of Department of Oral Biochemistry, Institute of Oral Bioscience, BK21 program, School of Dentistry, Chonbuk National University, Jeonju, Korea.
Degree
PhD
Research interest
Bioscience and Biochemistry, Exercise therapy
E-mail: yihokn@chonbuk.ac.kr
References
- Cargnello M., Roux P.P. (2011) Activation and Function of the MAPKs and Their Substrates, the MAPK-Activated Protein Kinases. Microbiology and Molecular Biology Reviews 75(1), 50-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson C.J., Fan Z., Gordon S.E., Booth F.W. (2001) Time course of the MAPK and PI3-kinase response within 24 hours of skeletal muscle overload. Journal of Applied Physiology 91, 2079-2087. [DOI] [PubMed] [Google Scholar]
- Elbirt K.K., Bonkovsky H.L. (1999) Hemeoxygenase: recent advances in understanding its regulation and role. Proceedings of the Association of American Physicians 111(5), 438-447. [PubMed] [Google Scholar]
- Gabai V.L., Sherman M.Y. (2002) Invited review: Interplay between molecular chaperon and signaling pathway in survival of heat shock. Journal of Applied Physiology 92, 1743-1748. [DOI] [PubMed] [Google Scholar]
- Koh T.J., Escobedo J. (2004) Cytoskeletal disruption and small heat shock protein translocation immediately after lengthening contractions. American Journal of Physiology. Cell Physiology 286, 713-722. [DOI] [PubMed] [Google Scholar]
- Kostenko S., Moens U. (2009) Heat shock protein 27 phosphorylation : kinases, phosphatases, functions and pathology. Cellular and Molecular Life Sciences 66, 3289-3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer H.F., Goodyear L.J. (2007) Exercise, MAPK, and NF-kB signaling in skeletal muscle. Journal of Applied Physiology 103, 388-395. [DOI] [PubMed] [Google Scholar]
- Launay N., Goudeau B., Kato K., Vicart P., Lilienbaum A. (2006) Cell signaling pathway to aB-crystallin following stresses of the cytoskeleton. Experimental Cell Research 312, 3570-3584. [DOI] [PubMed] [Google Scholar]
- Lee K.H., Lee C.T., Kim Y.W., Kim Y.W., Han S.K., Shim Y.S., Yoo C.G. (2005) Preheating Accelerates Mitogen-activated Protein (MAP) Kinase Inactivation Post-heat Shock via a Heat Shock Protein 70-mediated Increase in Phosphorylated MAP Kinase Phosphatase-1. The Journal of Biological Chemistry 13, 13179-13186. [DOI] [PubMed] [Google Scholar]
- Lollo P.C., Moura C.S., Morato P.N., Amaya-Farfan J. (2013) Differential response of heat shock proteins to uphill and downhill exercise in heart, skeletal muscle, lung and kidney tissues. Journal of Sports Science & Medicine 12(3), 461-466. [PMC free article] [PubMed] [Google Scholar]
- McArdle A., Jackson M.J. (2000) Exercise, oxidative stress and ageing. Journal of Anatomy 197, 539-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle A., Vasilaka A., Jackson M.J. (2002) Exercise and skeletal muscle ageing: cellular and molecular mechanism. Ageing Research Reviews 1(1), 79-93. [DOI] [PubMed] [Google Scholar]
- Menshikova E.V., Ritov V.B., Fairfull L., Ferrell R.E., Kelley D.E., Goodpaster B.H. (2006) Effects of exercise on mitochondrial content and function in aging human skeletal muscle. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 61, 534-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton J.P., Kayani A.C., McArdle A., Drust B. (2009) The exercise-induced stress response of skeletal muscle, with specific emphasis on humans. Sports Medicine 39(8), 643-662. [DOI] [PubMed] [Google Scholar]
- Murlasits Z., Cutlip R.G., Geronilla K.B., Rao K.M.K., Wonderlin W.F., Always S.E. (2006) Resistance training increases heat shock protein levels in skeletal muscle of young and old rats. Experimental Gerontology 41, 398-406. [DOI] [PubMed] [Google Scholar]
- Nader G.A., Esser K.A. (2001) Intracellular signalling specificity in skeletal muscle in response to different modes of exercise. Journal of Applied Physiology 90, 1936-1942. [DOI] [PubMed] [Google Scholar]
- Noble E.G., Ho R., Dzialoszynski T. (2006) Exercise is the primary factor associated with HSP70 induction in muscle of treadmill running rats. Acta Physiologica 187, 495-501. [DOI] [PubMed] [Google Scholar]
- Noble E.G., Milne K.J., Melling J. (2008) Heat shock proteins and exercise: a primer. Applied Physiology, Nutrition, and Metabolism 33, 1050-1065. [DOI] [PubMed] [Google Scholar]
- Ogura Y., Naito H., Kakigi R., Ichinoseki-Sekine N., Kurosaka M., Yoshihara T., Akema T. (2011) Effects of ageing and endurance exercise training on alpha-actin in isoforms in rat plantaris muscle. Acta Physiologica 202, 683-690. [DOI] [PubMed] [Google Scholar]
- Pasini E., Le DouaironLahaye S., Flati V., Assanelli D., Corsetti G., Speca S., Bernabei R., Calvani R., Marzetti E. (2012) Effects of treadmill exercise and training frequency on anabolic signaling pathways in the skeletal muscle of aged rats. Experimental Gerontology 47(1), 23-28. [DOI] [PubMed] [Google Scholar]
- Paulsen P.G. (2007) Maximal eccentric exercise induces a rapid accumulation of small heat shock proteins on myofibrils and a delayed HSP70 response in humans. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 293, R844-R853. [DOI] [PubMed] [Google Scholar]
- van Ginneken M.M., de Graaf-Roelfsema E., Keizer H.A., van Dam K.G., Wijnberg I.D., van der Kolk J.H., van Breda E. (2006) Effect of exercise on activation of the p38 mitogen-activated protein kinase pathway, c-JunNH2terminalkinase, and heat shock protein27 in equine skeletal muscle. American Journal of Veterinary Research 67(5), 837-844. [DOI] [PubMed] [Google Scholar]
- Vasilaki A., Iwanejko L.M., McArdle F., Broome C.S., Jackson M.J., McArdle A. (2003) Skeletal muscles of aged male mice fail to adapt following contractile activity. Biochemical Society Transactions 31(2), 455-456. [DOI] [PubMed] [Google Scholar]
- Weber M.H., Rocha R.F.D., Schnorr C.E., Schroder R., Moreira J.C. (2012) Changes in lymphocyte HSP70 levels in women handball players throughout 1 year of training: the role of estrogen levels. Journal of Physiology and Biochemistry 68, 365-375. [DOI] [PubMed] [Google Scholar]
- Widegren U., Wretman C., Lionikas A., Hedin G., Henriksson J. (2000) Influence of exercise intensity on ERK/MAP kinase signalling in human skeletal muscle. Pflügers Archive: European Journal of Physiology 41, 317–322. [DOI] [PubMed] [Google Scholar]
- Zembron-Lacny A., Ostapiuk J., Slowinska-Lisowska M., Witkowski K., Szyszka K. (2008) Pro-antioxidant ratio in healthy men exposed to muscle-damaging resistance exercise. Journal of Physiology and Biochemistry 4(1), 27-35. [DOI] [PubMed] [Google Scholar]


