Abstract
Elevated plasma creatine kinase (CK) activity is often used as an indicator of exercise-induced muscle damage. Our aim was to study effects of contraction type, sex and age on CK efflux from isolated skeletal muscles of mice. The soleus muscle (SOL) of adult (7.5-month old) female C57BL/6J mice was subjected to either 100 passive stretches, isometric contractions or eccentric contractions, and muscle CK efflux was assessed after two-hour incubation in vitro. SOL of young (3-month old) male and female mice was studied after 100 eccentric contractions. For adult females, muscle CK efflux was larger (p < 0.05) after eccentric contractions than after incubation without exercise (698 ± 344 vs. 268 ± 184 mU·h−1, respectively), but smaller (p < 0.05) than for young females after the same type of exercise (1069 ± 341 mU·h−1). Eccentric exercise-induced CK efflux was larger in muscles of young males compared to young females (2046 ± 317 vs 1069 ± 341 mU · h−1, respectively, p < 0.001). Our results show that eccentric contractions induce a significant increase in muscle CK efflux immediately after exercise. Isolated muscle resistance to exercise-induced CK efflux depends on age and sex of mice.
Key points.
Muscle lengthening contractions induce the highest CK efflux in vitro compared with similar protocol of isometric contractions or passive stretches.
Muscle CK efflux in vitro is applicable in studying changes of sarcolemma permeability/integrity, a proxy of muscle damage, in response to muscle contractile activity.
Isolated muscle resistance to exercise-induced CK efflux is greater in female compared to male mice of young age and is further increased in adult female mice.
Key words: Skeletal muscle, eccentric contractions, muscle damage, CK activity
Introduction
Elevated plasma creatine kinase (CK) activity is often used as a marker of muscle injury in myopathies, cardiomyopathies and encephalopathies (Brancaccio et al., 2007). Muscle exercise can also trigger an efflux of various molecules, including CK, from skeletal muscles (Reihmane et al., 2013). Muscle CK efflux occurs after various types of exercise, but is particularly large after eccentric contractions (Newham et al., 1986). However, the relationship between muscle CK efflux and exercise characteristics is often complicated by the inflammatory response which can lead to secondary muscle damage after exercise (McHugh, 2003: Tidball, 1995). Indeed, plasma CK activity shows no or only a small increase after exercise and peaks after 1-5 days of recovery which often coincides with the time of peak muscle soreness (Ahmadi et al., 2007; Armstrong et al., 1984). Interestingly, no evidence of damage was detected in skeletal muscle fibres showing significant swelling after eccentric exercise (Yu et al. 2013). It appears that plasma CK activity is influenced by the interplay between muscle damage, osmotic factors and CK clearance from the body fluids after exercise (McHugh, 2003; Tidball, 1995). It would be beneficial to separate these factors in order to gain a better understanding of the physiological mechanisms responsible for muscle CK efflux. Isolated muscles might provide a good model for such studies as the effects of secondary muscle damage and CK clearance can be minimized. CK efflux from the isolated muscles increases after muscle injury induced by chemical agents, such as calcium ionophore A23187 and dinitrophenol, supporting the feasibility of this experimental approach (Jackson et al., 1987).
Sex and age might affect susceptibility to muscle damage and loss of muscle proteins after exercise (Amelink et al., 1990; Lynch et al., 2008). However, the findings from the human studies are conflicting. When compared to women, men showed higher plasma CK activity after marathon running (Rogers et al., 1985), but the opposite was true for 50 maximal eccentric arm flexions (Miles et al., 1994). It appears that susceptibility to exercise-induced muscle damage increases from the pre-adolescent age to adulthood (Chen et al., 2014). However, children show greater impairment in muscle voluntary activation and this could affect comparison of muscle damage indicators in children and adult after exercise (Streckis et al. 2007). Most of the mouse studies of muscle damage focused on the very old animals (Brooks et al., 2001; Lynch et al., 2008). Old age is associated with impairments in motor coordination, reduced levels of physical activity and significant loss of muscle mass (Brooks et al., 2001; Wolfe et al., 2006). It is unclear if muscle susceptibility to damage changes from young age to adulthood when animals reach high levels of muscle strength.
The first aim of the present study was to examine if CK efflux from skeletal muscles is indeed affected by the type of muscle exercise. Thus we assessed CK efflux from the isolated soleus muscle (SOL) of adult female mice after either passive stretching, isometric contractions or eccentric contractions in vitro. The second aim of the study was to investigate if muscle CK efflux is dependent on age and sex of animals. We compared muscle CK efflux in young animals of both sexes and female adults at rest and after eccentric exercise.
Methods
Animals and experiments
All procedures involving mice were approved by the Lithuanian Republic Alimentary and Veterinary Public Office (Nr. 0223). As in our previous studies (Baltusnikas et al., 2015; Kilikevicius et al., 2013; Ratkevicius et al., 2010), C57BL/6J mice were housed in standard cages, one to three mice per cage at a temperature of 22-24° C and 40-60 % humidity with the normal 12/12-h light/dark cycle. Animals were fed standard chow diet and received tap water ad libitum. We assessed CK efflux from SOL in adult (7.5 month old) females after 100 non-stimulated stretches (n = 8), 100 isometric contractions (n = 9), 100 lengthening contractions (n = 10) and after passive incubation (n = 10). We have also studied CK efflux from SOL of young (3-month old) females and males after 100 eccentric contractions (n = 11 and n = 8, respectively) and without any exercise (n = 9 and n = 6, respectively).
Experimental procedures
Mice were euthanized by cervical dislocation. Assessment of contractile properties and CK efflux from SOL was performed using similar methods as in our recent study (Baltusnikas et al., 2015). Silk sutures were attached to the proximal and distal tendons of SOL from left leg. The muscle was then excised and fixed between two platinum plate electrodes in 50 ml Radnotti tissue bath filled with Tyrode solution (121 mM NaCl, 5 mM KCl, 0.5 mM MgCl2, 1.8 mM CaCl2, 0.4 mM NaH2PO4, 0.1 mM NaEDTA, 24 mM NaHCO3, 5.5 mM glucose, pH adjusted to 7.4) bubbled with 95% O2 and 5% CO2 at room (~25 °C) temperature. The distal tendon of the muscle was attached to a hook and the proximal end was tied directly to the lever of muscle test system (1200A-LR Muscle Test System, Aurora Scientific Inc., Aurora, Canada). The muscle was then left to equilibrate in the Tyrode solution at a slight pre-tension (~20 – 30 mN) for 7 min in order to minimize heterogeneity of sarcomere lengths that might have occurred as a result of muscle manipulations during the dissection. Afterwards, muscle length was increased in steps every 2 min and the muscle was stimulated supramaximally at 150 Hz for 3 s. This procedure was continued until no further increase in muscle force was seen with the increase in muscle length. Our measurements showed that SOL reaches its peak isometric force at 150 Hz of electrical stimulation. Therefore we used this stimulation frequency to set optimal muscle length (L0), and muscles were kept at this length during the subsequent procedures.
Afterwards, tetanus contraction time was assessed as the time from the beginning of the force development to 90% of the peak force. Then SOL was subjected to one of the three protocols of muscle manipulations which were repeated every 10 s. The typical force recordings from these experiments are presented in Figure 1. For the protocol of passive stretches, the muscle was subjected to a ramp 3.5-mm stretch corresponding to approximately 30% of muscle length over 200 ms followed by return to L0 in 200 ms. For isometric contraction protocol, SOL was stimulated at 150 Hz for 700 ms. For the eccentric exercise, SOL was also stimulated at 150 Hz for 700 ms, but subjected to 3.5 mm ramp stretch during the last 200 ms of this stimulation which was followed by return to the optimal length in another 200 ms. In less than 10 s after completion of all three protocols, SOL was removed from the tissue bath and incubated in 2 ml of Tyrode solution for 2 h at room temperature. In control experiment, muscle was stretched to optimal length and left intact (no electrical stimulation) in Tyrode solution bubbled with aforementioned gas mixture for 20 min after which it was incubated in Tyrode solution for 2 h. Our pilot experiments have shown that peak tetanic force of SOL decreases by less than 5% during such incubation (unpublished observation). In all experiments, muscle CK efflux was assessed by measuring CK activity in 250 µl of the Tyrode solution using biochemical analyzer (Spotchem™ EZ SP-4430, Menarini Diagnostics, Winnersh-Wokingham, UK) with the reagent strips (Arkray Factory, Inc., Shiga, Japan). Coefficient of variation for CK assay was less than 8 % as calculated from the ratio of standard deviation (S.D.) to the mean in repeated measurements (n = 8) of the same sample.
Figure 1.
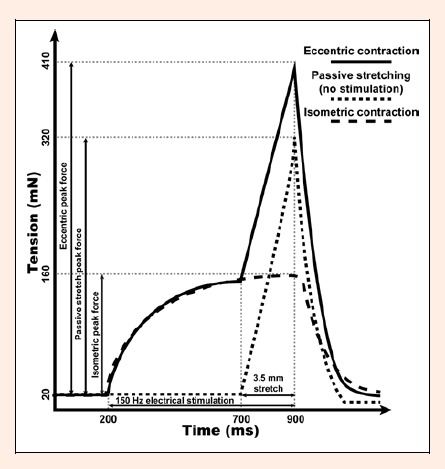
The typical recording of soleus muscle force generated during passive stretching, isometric contraction and eccentric contraction, respectively.
Statistical analysis
All data analysis was performed using Prism 5.0 software software. One factor analysis of variance (ANOVA) was used to assess effects of different muscle manipulations on muscle CK efflux in adult female mice. The two factor ANOVA was applied when analyzing effects of eccentric exercise and sex or age, respectively. Repeated measures ANOVA was used for data on muscle force during exercise. The post hoc testing was carried out using t-tests with a Bonferroni correction for multiple comparisons. The unpaired t-tests were also performed to assess differences in body massand muscle contractile properties. Data are shown as mean with S.D. Significance level was set at p < 0.05.
Results
Body mass and muscle contractile properties vary depending on the age and sex
The data on body mass and contractile properties of SOL in the studied mice are presented in Table 1. Young males were heavier than young females (p < 0.001), and weighed more (p < 0.05) than adult females. Peak tetanic force of young mice did not differ between sexes, but was lower than in the adult females (p < 0.01). Tetanus contraction time of female mice did not differ between the age groups and was longer than in the young male mice (p < 0.001).
Table 1.
Body mass and contractile properties of soleus muscle (SOL) in young (3 month old) mice of both sexes and adult (7.5 month old) female mice. Data are shown as means (±SD).
| Male mice | Female mice | ||
|---|---|---|---|
| 3-month old | 3-month old | 7.5-month old | |
| Body mass (g) | 24.6 (1.9) [n = 14] |
20.0 ± 0.7 *** [n = 20] |
23.4 ± 1.3 *
### [n = 37] |
| Peak tetanic force (mN) | 140(11) [n = 8] |
144 ± 12 [n = 11] |
165 ± 18 ***
### [n = 19] |
| Tetanus contraction time (ms) | 356(45) [n = 14] |
494 ± 69 *** [n = 19] |
542 ± 82 *** [n = 8] |
* p < 0.05,
** p < 0.01,
*** p < 0.001 compared to young males.
### p < 0.001 compared to young females.
Muscle CK efflux depends on the type of exercise
We used SOL of adult female mice to study the effects of different exercises on muscle CK efflux. Data from these experiments are presented in Figure 2. ANOVA showed that muscle CK efflux depended (p < 0.05) on the type of muscle manipulation, but only the eccentric exercise induced a significantly greater (p < 0.05) muscle CK efflux than the control experiment. The repeated measures ANOVA was used to assess effects of the contraction type on the peak isometric force during the two experimental protocols involving muscle contractions, i.e. isometric and eccentric exercise. Peak isometric force decreased (p < 0.001) during these exercises and was greater for the isometric exercise compared to the eccentric exercise (p < 0.001). There was also a significant interaction between the type of exercise and extent of force loss (p < 0.001) as peak isometric force did not differ between the exercise protocols after ten contractions, but decreased to the lower level by the end of the eccentric exercise compared to the isometric exercise (41.0 ± 4.9 versus 72.7 ± 4.2 % of initial force, respectively, p < 0.001).
Figure 2.
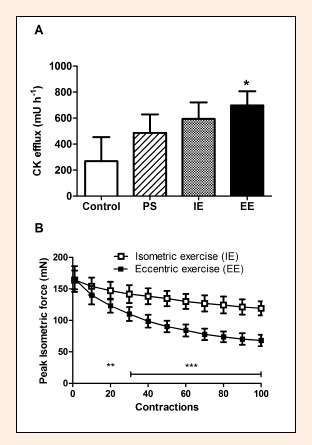
A. Creatine kinase (CK) efflux from soleus muscle in the control experiment without any muscle manipulation (control), after 100 passive stretches (PS), after isometric exercise (IE) and eccentric exercise (EE) consisting of 100 contractions each, respectively. B. Peak isometric force during isometric exercise (IE) and eccentric exercise (EE). Data are shown as means and SD. * p < 0.05 EE compared to control; ** p < 0.01 EE compared to IE; *** p < 0.001 EE compared to IE.
Muscle CK efflux after eccentric exercise depends on sex and age
Data on peak isometric force during the eccentric exercise and muscle CK efflux are presented in Figure 3. The two-factor repeated measures ANOVA showed that peak isometric force decreased during the exercise (p < 0.001) and depended on the sex of the young mice (p < 0.001). There was also an interaction between these factors (p < 0.001) as muscles of young females showed greater (p < 0.01) force drop after performing more than forty contractions and experienced force loss to lower levels by the end of the exercise compared to young males (23.9 ± 5.2 versus 35.5 ± 6.0 % of initial, respectively, p < 0.01). By the end of exercise, muscles of young females showed force decrease to the lower also when compared to adult mice as well (23.9 ± 5.2 % versus 41.0 ± 4.9 % of initial force, p < 0.001, respectively).
Figure 3.
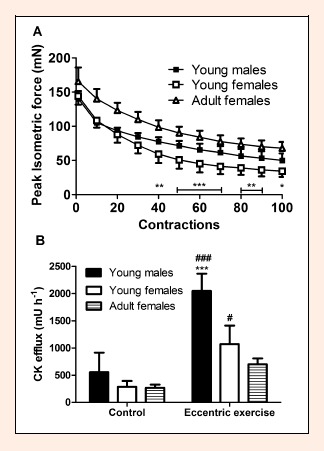
A. Peak isometric force during eccentric exercise of soleus muscle from young mice of both sexes and adult female mice. B. Creatine kinase (CK) efflux from soleus muscle in young mice of both sexes and adult females in the control experiment and after eccentric exercise. Data are shown as means and SD. * p < 0.05, ** p < 0.01, *** p < 0.001 young males compared to young females; # p < 0.05, ### p < 0.001 young males and young females compared to adult females.
The two-factor ANOVA showed that muscle CK efflux depended on the sex of young mice (p < 0.001) and was larger after eccentric exercise compared to the control experiment (p < 0.001). There was an interaction between these two factors (p < 0.01) as young males did not differ from young females in the control experiment, but eccentric exercise triggered larger muscle CK efflux in the males compared to females (p < 0.001). ANOVA also showed that muscle CK efflux was lower for adult females compared to young females (p < 0.05). Adult females also showed lower muscle CK efflux than young males (p < 0.001) after eccentric exercise (p < 0.05), but not in the control experiment.
Discussion
The aim of our study was twofold. Firstly, we tested the hypothesis that muscle CK efflux depends on the type of muscle exercise. Secondly, we investigated effects of age and sex on muscle CK efflux after eccentric exercise. The results of the study confirm that muscle CK efflux does depend on the type of exercise, but only eccentric contractions induce a larger CK efflux than the control experiment without exercise. After eccentric exercise muscles of adult females showed smaller CK efflux compared to young animals of both sexes while young females had reduced muscle CK efflux compared to young males. These results show that muscle resistance to exercise-induced CK efflux depends on age and sex of mice even when studying isolated muscles.
Muscle mass could be one of the factors affecting amount of CK released from the muscles in vitro. We did not assess muscle mass, but young male and female mice did not differ in peak isometric force. This suggests that muscle mass did not differ significantly between sexes and could not account for two fold higher CK efflux from muscles of young males compared to young females. It is even less likely that muscle mass played any role in determining age effects on muscle CK efflux as peak isometric force was higher while CK efflux was lower in adult mice compared to young mice.
We assessed efflux of CK from skeletal muscles of mice. Molecular weight of CK (~80 kDa) is larger than molecular weight of many other myocellular proteins, such as ~18 kDa myoglobin, ~35 kDa lactate dehydrogenase etc., and CK efflux is likely to reflect loss of various proteins from the skeletal muscles (Brancaccio et al., 2007). There was some CK efflux from skeletal muscles even in the control experiment when no exercise was performed. It is likely that this protein efflux was at least partially due to increased membrane permeability associated with osmotic stress which occurs when muscles are incubated in solutions without proteins and other molecules binding ions under in vivo conditions of blood plasma (Skrzypiec-Spring et al., 2007). Muscle CK efflux can be reduced by application of agents modifying membrane lipids (Jackson et al., 1987). However, the negligible decline in muscle force suggests that muscle fibres maintain their structural integrity during in vitro experiments (Plant et al., 2001). Indeed, skeletal muscles can withstand significant osmotic stress since exercise often leads to an increase in muscle intracellular and extracellular water content even under in vivo conditions (Sjøgaard et al., 1985).
Human studies show that unaccustomed exercise leads to efflux of muscle proteins coupled with prolonged depression of force generating capacity and muscle soreness (Armstrong, 1984). We studied CK efflux from the isolated mouse soleus muscle. Human soleus muscle is predominated by type I fibers (Ochs et al., 1977). Mouse soleus muscle, however, contains approximately equal proportions of type I and IIX fibers and very few IIB fibres (DeNies et al., 2014; Kilikevicius et al., 2013) and thus resembles human quadriceps muscle which has often been used in studies of exercise-induced muscle damage (Newham et al., 1983; Harrison et al., 2011; Snieckus et al., 2013). Our experiments (unpublished observation) and other studies (Plant et al. 2001) showed little decline in contractile response of soleus muscle during two-hour incubation in vitro. It appears that soleus muscle is well suited for experiments involving repetitive exercise. Our results demonstrate that differences between exercise protocols in muscle CK efflux were relatively small and only eccentric exercise induced a significant increase in muscle CK efflux during the period immediately after exercise. Interestingly, human in vivo studies show that the largest increase in plasma CK activity is usually observed several days after exercise (Newham et al., 1986) when there are signs of secondary muscle damage due to tissue inflammation (Pizza et al., 2002) or muscle fibre swelling (Yu et al. 2013). Thus, plasma CK activity is influenced by both local muscular and systemic factors and is unlikely to be an accurate indicator of exercise effects on skeletal muscles.
We studied soleus muscle of adult female which showed smaller eccentric exercise-induced muscle CK efflux compared to young mice. Muscles of adult female mice generated larger forces than muscles of young mice. Thus, the relatively low muscle CK efflux from skeletal muscles of adult female mice cannot be due to smaller mechanical load generated by muscle during exercise. Hormonal changes and variations in physical activity might play a role here, but the associated mechanisms are unclear (Guo et al., 2012; Messier et al., 2011; Velders and Diel, 2013). Structural proteins, such as titin, modulate muscle resistance to stretching, but it is unclear if concentration and/or properties of these proteins change in the adulthood (Monroy et al., 2012). Human studies suggest that muscle aging leads to slowing of skeletal muscle contractions which increases force output in eccentric exercise (Vandervoort, 2002). However, tetanus contraction time did not differ between young and adult female mice. Thus modification of the contractile properties is unlikely to be of major significance in our study. Our findings about age effects apply to female mice, but it is unlikely that these effects would be qualitatively different between the sexes. Extensor digitorum longus muscle (EDL) of both female B6D2F1 mice (Brooks et al., 2001) and male rats (Lynch et al., 2008) show an increased susceptibility to exercise-induced muscle damage at very old age compared to the respective adult muscles. It appears that skeletal muscles of rodents undergo changes in resistance to exercise-induced muscle damage during the entire life span and have the highest resistance around the adult as compared to young and old age. These findings disagree with data from human studies which suggest that adults have lower resistance to exercise-induced muscle damage than children (Chen et al., 2014; Marginson et al., 2005). The data from studies of old volunteers is contradictory showing both reduced and similar susceptibility to exercise-induced muscle damage in old volunteers compared to young adults (Clarkson and Dedrick, 1988; Gorianovas et al., 2013). However, these human studies have been conducted using repetitive voluntary contractions where older volunteers and children show reduced power output and thus experience smaller muscle overloading compared to adults. Human studies with electrical stimulation of skeletal muscles are needed to attest if the age-related changes in susceptibility to muscle damage are qualitatively different between humans and rodents.
Muscle CK efflux depended on sex of mice as female mice showed smaller eccentric exercise-induced muscle CK efflux compared to male mice. Similar findings have been reported for rat soleus muscle in vivo (Amelink et al., 1990). It is thought that high levels of oestradiol might act to improve resistance to exercise-induced damage of skeletal muscles in females (Amelink et al., 1990). However, differences in fiber type composition between male and female muscles might also be of importance. Muscles of male mice had faster twitch contraction time compared to female mice. Indeed, soleus muscle of female C57BL/6J mice as well as other mouse strains has higher content of slow type I fibers compared to male mice (Carroll et al., 2012; DeNies et al., 2014). Studies of human muscles show that high intensity eccentric contractions induce protein efflux primarily from type II fibers (Chapman et al., 2013). Thus lower content of damage-sensitive type II fibers might be reflected in reduced muscle CK efflux after eccentric exercise in females. Interestingly, female mice showed greater decrease in muscle force during the exercise than male mice. Eccentric muscle contractions were separated by 10 s periods to minimize metabolic inhibition which is often associated with accumulation of inorganic phosphate in muscle fibres (Allen et al., 1995). However, eccentric contractions induce impairment in excitation-contraction coupling as well (Allen, 2001; Warren et al., 1993). This might result in inactivation of muscles fibres. It appears that muscles of female mice experienced greater inactivation of muscle fibres which might have contributed to less pronounced muscle damage during exercise compared to male mice.
Conclusion
Our results show that muscle CK efflux tends to increase with increase in forces generated during muscle exercise and eccentric exercise is particularly effective in inducing muscle CK efflux. Muscle resistance to eccentric exercise-induced CK efflux is partially determined by the intrinsic muscular factors which are dependent on age and sex of mice. Studies of male mice are needed to attest if effects of age are sex specific. Variations in muscle mass also need to be assessed as a possible factor modifying protein efflux after exercise.
Acknowledgments
This research was funded by a grant (No. MIP-067/2012) from the Research Council of Lithuania. We are thankful to Petras Jeneckas and Audrius Čapskas for their technical assistance.
Biographies
Juozas BALTUSNIKAS
Employment
Junior Research Fellow, Institute of Sports Sciences and Innovation. Lithuanian Sports University.
Degree
MSc
Research interests
Systemic targeted delivery of drugs, transcriptional gene regulation, muscle fibers plasticity and diversity, muscle hypertrophy.
E-mail: juozas.baltusnikas@lsu.lt
Tomas VENCKUNAS
Employment
Senior Research Fellow, Institute of Sports Sciences and Innovation. Lithuanian Sports University.
Degree
PhD
Research interests
Adaptation of cardiac and skeletal muscle to exercise training.
E-mail: tomas.venckunas@lsu.lt
Audrius KILIKEVICIUS
Employment
Research Fellow, Institute of Sports Sciences and Innovation.Lithuanian Sports University.
Degree
PhD
Research interests
Genetic basis of skeletal muscle traits and myopathies.
E-mail: audrius.kilikevicius@lsu.lt
Andrej FOKIN
Employment
PhD student. Lithuanian Sports University.
Degree
MSc
Research interests
Cellular etiology and genetic background of muscle properties, mitochondrial biology, cell physiology.
E-mail: fokinandrej@yahoo.com
Aivaras RATKEVICIUS
Employment
Senior Research Fellow, Institute of Sports Sciences and Innovation, Lithuanian Sports University, Kaunas, Lithuania. Lecturer, School of Medical Sciences, AHS building, Foresterhill, University of Aberdeen, Scotland, UK.
Degree
PhD
Research interests
Genetics and molecular biology of skeletal muscles in health and disease
E-mail: a.ratkevicius@abdn.ac.uk
References
- Allen D.G., Lännergren J., Westerblad H. (1995) Muscle cell function during prolonged activity: cellular mechanisms of fatigue. Experimental Physiology 80(4), 497-527. [DOI] [PubMed] [Google Scholar]
- Allen D.G. (2001) Eccentric muscle damage: mechanisms of early reduction of force. Acta Physiologica Scandinavica 171(3), 311-319. [DOI] [PubMed] [Google Scholar]
- Ahmadi S., Sinclair P.J., Foroughi N., Davis G.M. (2007) Electromyographic activity of the biceps brachii after exercise-induced muscle damage. Journal of sports science and medicine 6(4), 461-470. [PMC free article] [PubMed] [Google Scholar]
- Amelink G.J., Koot R.W., Erich W.B., Van Gijn J., Bär P.R. (1990) Sex-linked variation in creatine kinase release, and its dependence on oestradiol, can be demonstrated in an in vitro rat skeletal muscle preparation. Acta physiologica Scandinavica 138(2), 115-124. [DOI] [PubMed] [Google Scholar]
- Armstrong R.B. (1984) Mechanisms of exercise-induced delayed onset muscular soreness: a brief review. Medicine and Science in Sports and Exercise 16(6), 529-538. [PubMed] [Google Scholar]
- Arnett M.G., Hyslop R., Dennehy C.A., Schneider C.M. (2000) Age-related variations of serum CK and CK MB response in females. Canadian Journal of Applied Physiology 25(6), 419-429. [DOI] [PubMed] [Google Scholar]
- Baltusnikas J., Kilikevicius A., Venckunas T., Fokin A., Lionikas A., Ratkevicius A. (2015) Regenerated soleus muscle shows reduced creatine kinase efflux after contractile activity in vitro. Applied Physiology, Nutrition, and Metabolism 40, 129-133. [DOI] [PubMed] [Google Scholar]
- Brancaccio P., Maffulli N., Limongelli F.M. (2007) Creatine kinase monitoring in sport medicine. British Medical Bulletin 81-82, 209-230. [DOI] [PubMed] [Google Scholar]
- Brooks S.V., Opiteck J.A., Faulkner J.A. (2001) Conditioning of skeletal muscles in adult and old mice for protection from contraction-induced injury. Journal of Gerontology: Biological Sciences 56(4), B163-171. [DOI] [PubMed] [Google Scholar]
- Carroll A.M., Palmer A.A., Lionikas A. (2012) QTL analysis of Type I and Type IIA fibers in soleus muscle in a cross between LG/J and SM/J mouse strains. Frontiers in Genetics 2, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman D.W., Simpson J.A., Iscoe S., Robins T., Nosaka K. (2013) Changes in serum fast and slow skeletal troponin I concentration following maximal eccentric contractions. Journal of Science and Medicine in Sport 16(1), 82-85. [DOI] [PubMed] [Google Scholar]
- Chen T.C., Chen H.L., Liu Y.C., Nosaka K. (2014) Eccentric exercise-induced muscle damage of pre-adolescent and adolescent boys in comparison to young men. European Journal of Applied Physiology 114(6), 1183-1195. [DOI] [PubMed] [Google Scholar]
- Clarkson P.M., Dedrick M.E. (1988) Exercise-induced muscle damage, repair, and adaptation in old and young subjects. Journal of Gerontology 43(4), M91-96. [DOI] [PubMed] [Google Scholar]
- DeNies M.S., Johnson J., Maliphol A.B., Bruno M., Kim A., Rizvi A., Rustici K., Medler S. (2014) Diet-induced obesity alters skeletal muscle fiber types of male but not female mice. Physiological Reports 2(1), e00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredsted A., Clausen T., Overgaard K. (2008) Effects of step exercise on muscle damage and muscle Ca2+ content in men and women. Journal of Strength and Conditioning Research (4), 1136-146. [DOI] [PubMed] [Google Scholar]
- Harrison B.C., Allen D.L, Leinwand L.A. (2011) IIb or not IIb? Regulation of myosin heavy chain gene expression in mice and men. Skeletal Muscle 1, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorianovas G., Skurvydas A., Streckis V., Brazaitis M., Kamandulis S., McHugh M.P. (2013) Repeated bout effect was more expressed in young adult males than in elderly males and boys. Biomed Research International 2013, 218970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Wong S., Li M., Liang W., Liesa M., Serra C., Jasuja R., Bartke A., Kirkland J.L., Shirihai O., Bhasin S. (2012) Testosterone plus low-intensity physical training in late life improves functional performance, skeletal muscle mitochondrial biogenesis, and mitochondrial quality control in male mice. PLoS One 7(12), e51180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M.J., Wagenmakers A.J., Edwards R.H. (1987) Effect of inhibitors of arachidonic acid metabolism on efflux of intracellular enzymes from skeletal muscle following experimental damage. Biochemical Journal 241(2), 403-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilikevicius A., Venckunas T., Zelniene R., Carroll A.M., Lionikaite S., Ratkevicius A., Lionikas A. (2013) Divergent physiological characteristics and responses to endurance training among inbred mouse strains. Scandinavian Journal of Medicine and Science in Sports 23(5), 657-668. [DOI] [PubMed] [Google Scholar]
- Lynch G.S., Hinkle R.T., Chamberlain J.S., Brooks S.V., Faulkner J.A. (2001) Force and power output of fast and slow skeletal muscles from mdx mice 6-28 months old. Journal of Physiology 535(Pt 2), 591-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G.S., Faulkner J.A., Brooks S.V. (2008) Force deficits and breakage rates after single lengthening contractions of single fast fibers from unconditioned and conditioned muscles of young and old rats. American Journal of Physiology - Cell Physiology 295(1), C249-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marginson V., Rowlands A.V., Gleeson N.P., Eston R.G. (2005) Comparison of the symptoms of exercise-induced muscle damage after an initial and repeated bout of plyometric exercise in men and boys. Journal of Applied Physiology 99(3): 1174-1181. [DOI] [PubMed] [Google Scholar]
- McHugh M.P. (2003) Recent advances in the understanding of the repeated bout effect: the protective effect against muscle damage from a single bout of eccentric exercise. Scandinavian Journal of Medicine and Science in Sports 13(2), 88-97. [DOI] [PubMed] [Google Scholar]
- Messier V., Rabasa-Lhoret R., Barbat-Artigas S., Elisha B., Karelis A.D., Aubertin-Leheudre M. (2011) Menopause and sarcopenia: A potential role for sex hormones. Maturitas 68(4), 331-336. [DOI] [PubMed] [Google Scholar]
- Miles M.P., Clarkson P.M., Smith L.L., Howell J.N., McCammon M.R. (1994) Serum creatine kinase activity in males and females following two bouts of lengthening exercise. Medicine and Science in Sports and Exercise 26(5), S168. [Google Scholar]
- Monroy J.A., Powers K.L., Gilmore L.A., Uyeno T.A., Lindstedt S.L., Nishikawa K.C. (2012) What is the role of titin in active muscle? Exercise and Sports Sciences Reviews 40(2), 73-78. [DOI] [PubMed] [Google Scholar]
- Newham D.J., Jones D.A., Edwards R.H. (1986) Plasma creatine kinase changes after eccentric and concentric contractions. Muscle and Nerve 9(1), 59-63. [DOI] [PubMed] [Google Scholar]
- Newham D.J., Jones D.A., Edwards R.H. (1983) Large delayed plasma creatine kinase changes after stepping exercise. Muscle and Nerve 6(5), 380-385. [DOI] [PubMed] [Google Scholar]
- Ochs R. M., Smith J. L., Edgerton V. R. (1977) Fatigue characteristics of human gastrocnemius and soleus muscles. Electromyography and Clinical Neurophysiology 17, 297-306. [PubMed] [Google Scholar]
- Pizza F.X., Koh T.J., McGregor S.J., Brooks S.V. (2002) Muscle inflammatory cells after passive stretches, isometric contractions, and lengthening contractions. Journal of Applied Physiology 92(5), 1873-1878. [DOI] [PubMed] [Google Scholar]
- Plant D.R., Gregorevic P., Williams D.A., Lynch G.S. (2001) Redox modulation of maximum force production of fast-and slow-twitch skeletal muscles of rats and mice. Journal of Applied Physiology 90(3), 832-838. [DOI] [PubMed] [Google Scholar]
- Ratkevicius A., Carroll A.M., Kilikevicius A., Venckunas T., McDermott K.T., Gray S.R., Wackerhage H., Lionikas A. (2010) H55N polymorphism as a likely cause of variation in citrate synthase activity of mouse skeletal muscle. Physiological Genomics 42A(2), 96-102. [DOI] [PubMed] [Google Scholar]
- Reihmane D., Jurka A., Tretjakovs P., Dela F. (2013) Increase in IL-6, TNF-α, and MMP-9, but not sICAM-1, concentrations depends on exercise duration. European Journal of Applied Physiology 113(4), 851-858. [DOI] [PubMed] [Google Scholar]
- Rogers M.A., Stull G.A., Apple F.S. (1985) Creatine kinase isoenzyme activities in men and women following a marathon race. Medicine and Science in Sports and Exercise 17(6), 679-682. [DOI] [PubMed] [Google Scholar]
- Roth S.M., Martel G.F., Ivey F.M., Lemmer J.T., Metter E.J., Hurley B.F., Rogers M.A. (2000) High-volume, heavy-resistance strength training and muscle damage in young and older women. Journal of Applied Physiology 88(3), 1112-1118. [DOI] [PubMed] [Google Scholar]
- Sjøgaard G., Adams R.P., Saltin B. (1985) Water and ion shifts in skeletal muscle of humans with intense dynamic knee extension. American Journal of Physiology 248(2), R190-196. [DOI] [PubMed] [Google Scholar]
- Skrzypiec-Spring M., Grotthus B., Szelag A., Schulz R. (2007) Isolated heart perfusion according to Langendorff---still viable in the new millennium. Journal of Pharmacological and Toxicological Methods 55, 113-126. [DOI] [PubMed] [Google Scholar]
- Snieckus A., Kamandulis S., Venckunas T., Brazaitis M., Volungevicius G., Skurvydas A. (2013) Concentrically trained cyclists are not more susceptible to eccentric exercise-induced muscle damage than are stretch-shortening exercise-trained runners. European Journal of Applied Physiology 113(3), 621-628. [DOI] [PubMed] [Google Scholar]
- Streckis V., Skurvydas A., Ratkevicius A. (2007) Children are more susceptible to central fatigue than adults. Muscle Nerve 36(3), 357-363 [DOI] [PubMed] [Google Scholar]
- Tidball J.G. (1995) Inflammatory cell response to acute muscle injury. Medicine and Science in Sports and Exercise 27(7), 1022-1032. [DOI] [PubMed] [Google Scholar]
- Vandervoort A.A. (2002) Aging of the human neuromuscular system. Muscle and nerve 25(1), 17-25. [DOI] [PubMed] [Google Scholar]
- Velders M., Diel P. (2013) How sex hormones promote skeletal muscle regeneration. Sports Medicine (Auckland, N.Z.) 43(11), 1089-1100. [DOI] [PubMed] [Google Scholar]
- Wolfe R.R. (2006) The underappreciated role of muscle in health and disease. Americn Journal of Clinical Nutrition 84, 475-482. [DOI] [PubMed] [Google Scholar]
- Yu J.G., Liu J.X., Carlsson L., Thornell L.E., Stål P.S. (2013) Re-evaluation of sarcolemma injury and muscle swelling in human skeletal muscles after eccentric exercise. PLoS One 8(4), e62056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G.L., Lowe D.A., Hayes D.A., Karwoski C.J., Prior B.M., Armstrong R.B. (1993) Excitation failure in eccentric contraction-induced injury of mouse soleus muscle. Journal of Physiology 468, 487-499. [DOI] [PMC free article] [PubMed] [Google Scholar]


