Abstract
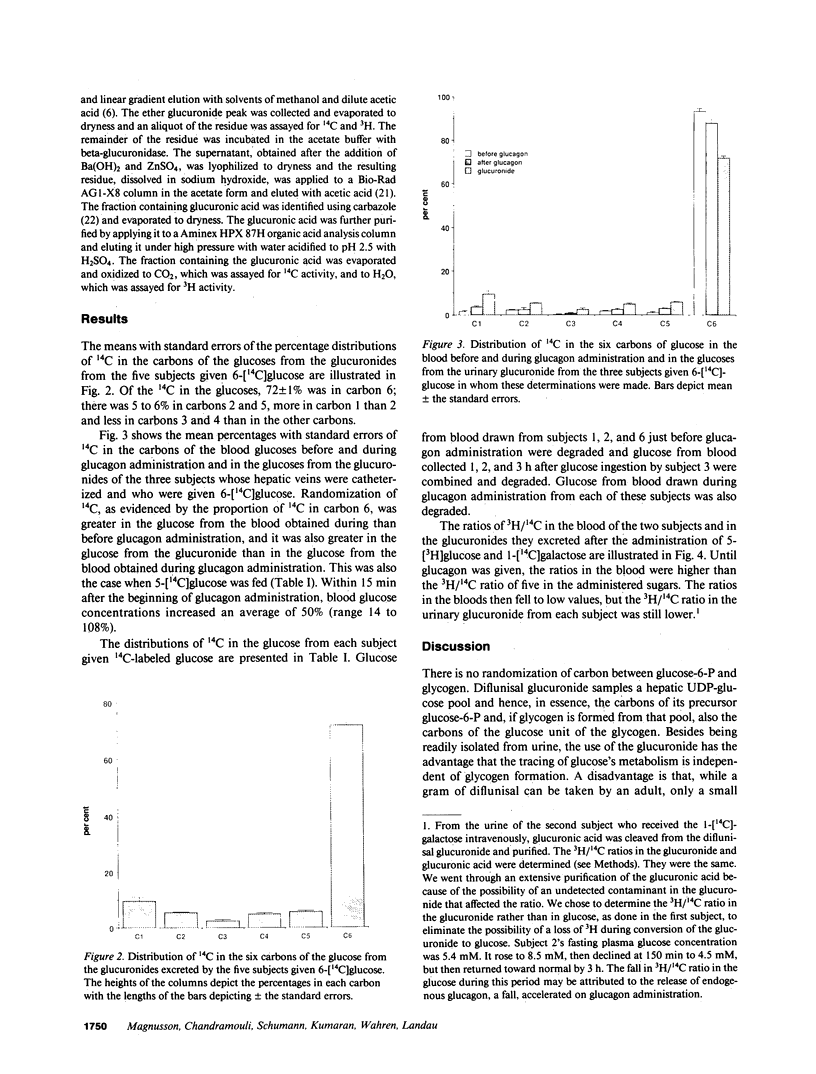
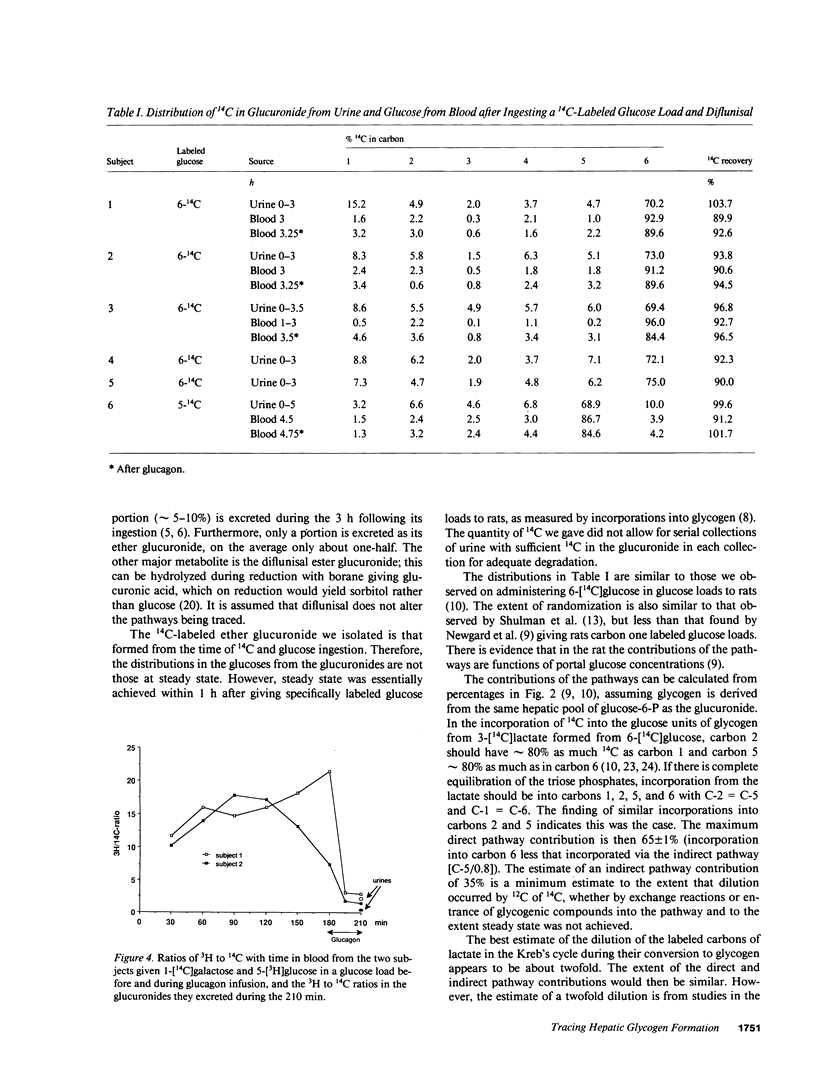
Diflunisal, 5-(2',4'-difluorophenyl)salicylic acid, excreted in urine as its glucuronide, was given to normal humans (n = 6) along with a glucose load specifically labeled with 14C. Glucuronide excreted by each subject was reduced to its glucoside and glucose from it degraded to yield the distribution of 14 C in its six carbons. Randomization of the 14C from the specifically labeled glucose was taken as a measure of the extent to which glucose was deposited indirectly (i.e., glucose----lactate----glucose----6-P----glycogen), rather than directly (i.e., glucose----glucose-6-P----glycogen). The maximum contribution to glycogen formation by the direct pathway was estimated to be 65 +/- 1%, on the assumption that glucuronide and glycogen are derived from the same hepatic pool of glucose-6-P in liver. Evidence that supports that assumption was obtained by comparing the randomization of 14C in the urinary glucuronide with that in glucose in blood from the hepatic vein of four of the subjects before and after they were given glucagon. Other evidence supporting the assumption was obtained by comparing in two subjects 3H/14C ratios in glucose from hepatic vein blood before and after glucagon administration with that in urinary glucuronide, having labeled the uridine diphosphate (UDP)-glucose in their livers with 14C by giving them 1-[14C]galactose and their circulating glucose with 3H by giving a 5-[3H]glucose-labeled load. It is concluded that glucuronide formation in humans can be used to trace glucose metabolism in the liver, and that in humans the indirect pathway of glucose metabolism is active.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker N. Measurement of glucose recycling and liver glycogen synthesis in mice using doubly labeled substrates. Fed Proc. 1977 Feb;36(2):253–258. [PubMed] [Google Scholar]
- Felig P., Wahren J., Hendler R. Influence of oral glucose ingestion on splanchnic glucose and gluconeogenic substrate metabolism in man. Diabetes. 1975 May;24(5):468–475. doi: 10.2337/diab.24.5.468. [DOI] [PubMed] [Google Scholar]
- Ferrannini E., Bjorkman O., Reichard G. A., Jr, Pilo A., Olsson M., Wahren J., DeFronzo R. A. The disposal of an oral glucose load in healthy subjects. A quantitative study. Diabetes. 1985 Jun;34(6):580–588. doi: 10.2337/diab.34.6.580. [DOI] [PubMed] [Google Scholar]
- Firth R. G., Bell P. M., Marsh H. M., Hansen I., Rizza R. A. Postprandial hyperglycemia in patients with noninsulin-dependent diabetes mellitus. Role of hepatic and extrahepatic tissues. J Clin Invest. 1986 May;77(5):1525–1532. doi: 10.1172/JCI112467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinsky R. E., Levy G. Dose- and time-dependent elimination of acetaminophen in rats: pharmacokinetic implications of cosubstrate depletion. J Pharmacol Exp Ther. 1981 Oct;219(1):14–20. [PubMed] [Google Scholar]
- Hart S., Calder I., Ross B., Tange J. Renal metabolism of paracetamol: studies in the isolated perfused rat kidney. Clin Sci (Lond) 1980 May;58(5):379–384. doi: 10.1042/cs0580379. [DOI] [PubMed] [Google Scholar]
- Hellerstein M. K., Greenblatt D. J., Munro H. N. Glycoconjugates as noninvasive probes of intrahepatic metabolism: pathways of glucose entry into compartmentalized hepatic UDP-glucose pools during glycogen accumulation. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7044–7048. doi: 10.1073/pnas.83.18.7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetler K. Y., Williams H. R., Shreeve W. W., Landau B. R. Conversion of specifically 14 C-labeled lactate and pyruvate to glucose in man. J Biol Chem. 1969 Apr 25;244(8):2075–2077. [PubMed] [Google Scholar]
- Kalderon B., Gopher A., Lapidot A. Metabolic pathways leading to liver glycogen repletion in vivo, studied by GC-MS and NMR. FEBS Lett. 1986 Aug 11;204(1):29–32. doi: 10.1016/0014-5793(86)81381-3. [DOI] [PubMed] [Google Scholar]
- Katz J. Determination of gluconeogenesis in vivo with 14C-labeled substrates. Am J Physiol. 1985 Apr;248(4 Pt 2):R391–R399. doi: 10.1152/ajpregu.1985.248.4.R391. [DOI] [PubMed] [Google Scholar]
- Katz J., McGarry J. D. The glucose paradox. Is glucose a substrate for liver metabolism? J Clin Invest. 1984 Dec;74(6):1901–1909. doi: 10.1172/JCI111610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwajima M., Golden S., Katz J., Unger R. H., Foster D. W., McGarry J. D. Active hepatic glycogen synthesis from gluconeogenic precursors despite high tissue levels of fructose 2,6-bisphosphate. J Biol Chem. 1986 Feb 25;261(6):2632–2637. [PubMed] [Google Scholar]
- Lowenthal D. T., Oie S., Van Stone J. C., Briggs W. A., Levy G. Pharmacokinetics of acetaminophen elimination by anephric patients. J Pharmacol Exp Ther. 1976 Mar;196(3):570–578. [PubMed] [Google Scholar]
- Musson D. G., Lin J. H., Lyon K. A., Tocco D. J., Yeh K. C. Assay methodology for quantification of the ester and ether glucuronide conjugates of diflunisal in human urine. J Chromatogr. 1985 Feb 8;337(2):363–378. doi: 10.1016/0378-4347(85)80049-9. [DOI] [PubMed] [Google Scholar]
- Newgard C. B., Hirsch L. J., Foster D. W., McGarry J. D. Studies on the mechanism by which exogenous glucose is converted into liver glycogen in the rat. A direct or an indirect pathway? J Biol Chem. 1983 Jul 10;258(13):8046–8052. [PubMed] [Google Scholar]
- Newgard C. B., Moore S. V., Foster D. W., McGarry J. D. Efficient hepatic glycogen synthesis in refeeding rats requires continued carbon flow through the gluconeogenic pathway. J Biol Chem. 1984 Jun 10;259(11):6958–6963. [PubMed] [Google Scholar]
- PACKHAM M. A., BUTLER G. C. Studies of the biological synthesis of glucuronides. J Biol Chem. 1952 Jan;194(1):349–357. [PubMed] [Google Scholar]
- Pang K. S., Gillette J. R. Complications in the estimation of hepatic blood flow in vivo by pharmacokinetic parameters. The area under the curve after the concomitant intravenous and intraperitoneal (or intraportal) administration of acetaminophen in the rat. Drug Metab Dispos. 1978 Sep-Oct;6(5):566–576. [PubMed] [Google Scholar]
- Partridge S. M. Filter-paper partition chromatography of sugars: 1. General description and application to the qualitative analysis of sugars in apple juice, egg white and foetal blood of sheep. with a note by R. G. Westall. Biochem J. 1948;42(2):238–250. doi: 10.1042/bj0420238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radziuk J. Sources of carbon in hepatic glycogen synthesis during absorption of an oral glucose load in humans. Fed Proc. 1982 Jan;41(1):110–116. [PubMed] [Google Scholar]
- Scofield R. F., Kosugi K., Chandramouli V., Kumaran K., Schumann W. C., Landau B. R. The nature of the pentose pathway in liver. J Biol Chem. 1985 Dec 15;260(29):15439–15444. [PubMed] [Google Scholar]
- Scofield R. F., Kosugi K., Schumann W. C., Kumaran K., Landau B. R. Quantitative estimation of the pathways followed in the conversion to glycogen of glucose administered to the fasted rat. J Biol Chem. 1985 Jul 25;260(15):8777–8782. [PubMed] [Google Scholar]
- Shulman G. I., Rothman D. L., Smith D., Johnson C. M., Blair J. B., Shulman R. G., DeFronzo R. A. Mechanism of liver glycogen repletion in vivo by nuclear magnetic resonance spectroscopy. J Clin Invest. 1985 Sep;76(3):1229–1236. doi: 10.1172/JCI112078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocco D. J., Breault G. O., Zacchei A. G., Steelman S. L., Perrier C. V. Physiological disposition and metabolism of 5-(2',4'-difluorophenyl)salicyclic acid, a new salicylate. Drug Metab Dispos. 1975 Nov-Dec;3(6):453–466. [PubMed] [Google Scholar]
- Watari N., Hanawa M., Iwai M., Kaneniwa N. Pharmacokinetic study of the enterohepatic circulation of acetaminophen glucuronide in rats. J Pharmacobiodyn. 1984 Nov;7(11):811–819. doi: 10.1248/bpb1978.7.811. [DOI] [PubMed] [Google Scholar]
- Watari N., Iwai M., Kaneniwa N. Pharmacokinetic study of the fate of acetaminophen and its conjugates in rats. J Pharmacokinet Biopharm. 1983 Jun;11(3):245–272. doi: 10.1007/BF01061867. [DOI] [PubMed] [Google Scholar]





