Abstract
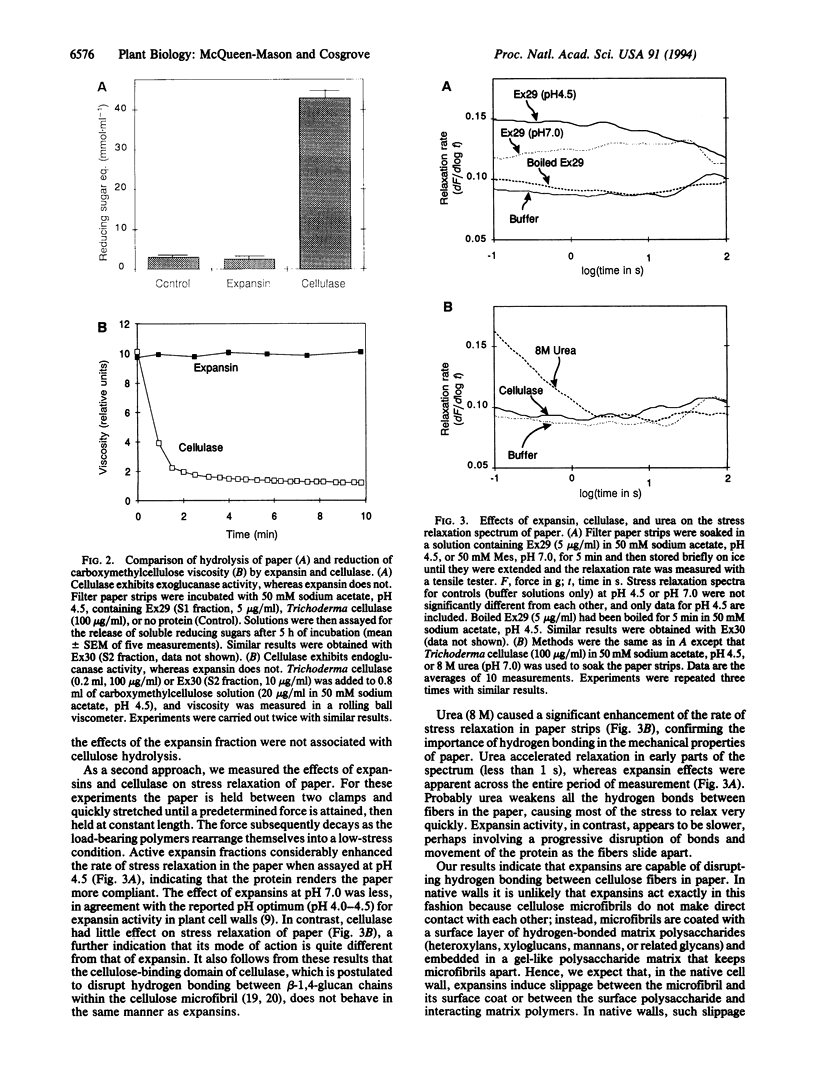
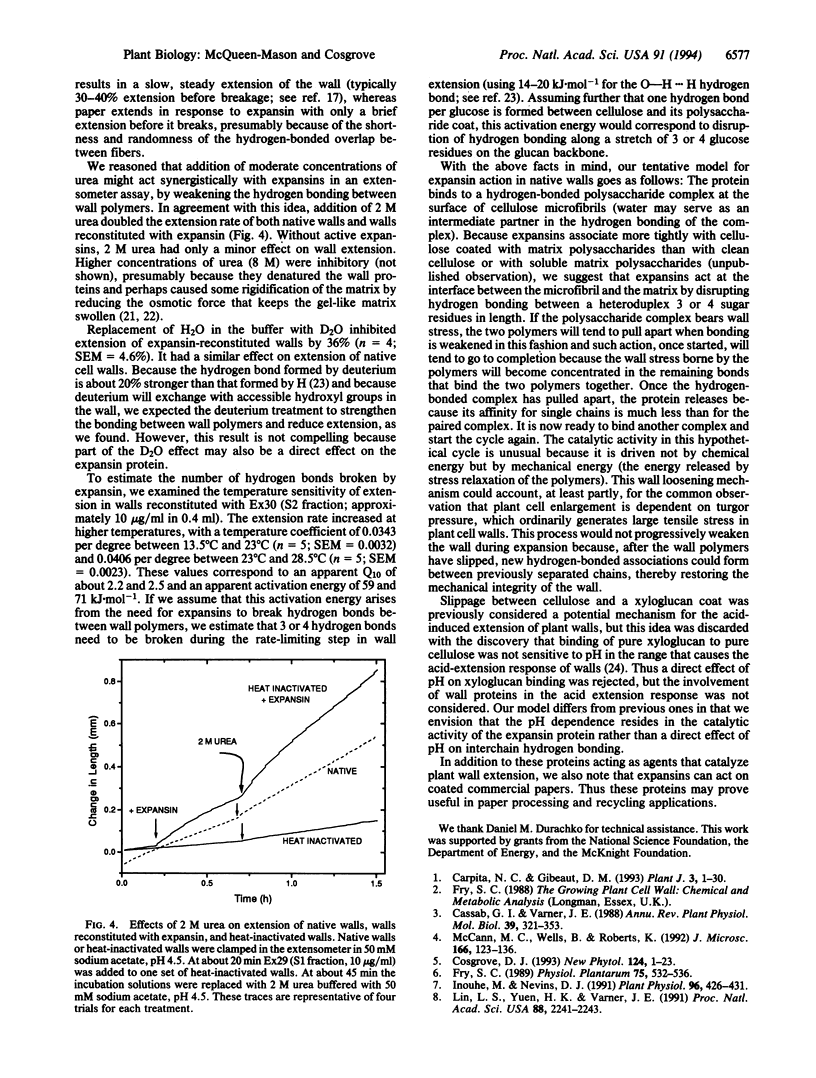
Plant cell enlargement is controlled by the ability of the constraining cell wall to expand. This ability has been postulated to be under the control of polysaccharide hydrolases or transferases that weaken or rearrange the loadbearing polymeric networks in the wall. We recently identified a family of wall proteins, called expansins, that catalyze the extension of isolated plant cell walls. Here we report that these proteins mechanically weaken pure cellulose paper in extension assays and stress relaxation assays, without detectable cellulase activity (exo- or endo- type). Because paper derives its mechanical strength from hydrogen bonding between cellulose microfibrils, we conclude that expansins can disrupt hydrogen bonding between cellulose fibers. This conclusion is further supported by experiments in which expansin-mediated wall extension (i) was increased by 2 M urea (which should weaken hydrogen bonding between wall polymers) and (ii) was decreased by replacement of water with deuterated water, which has a stronger hydrogen bond. The temperature sensitivity of expansin-mediated wall extension suggests that units of 3 or 4 hydrogen bonds are broken by the action of expansins. In the growing cell wall, expansin action is likely to catalyze slippage between cellulose microfibrils and the polysaccharide matrix, and thereby catalyze wall stress relaxation, followed by wall surface expansion and plant cell enlargement.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carpita N. C., Gibeaut D. M. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993 Jan;3(1):1–30. doi: 10.1111/j.1365-313x.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Cosgrove D. J. Characterization of long-term extension of isolated cell walls from growing cucumber hypocotyls. Planta. 1989;177:121–130. [PubMed] [Google Scholar]
- Cosgrove D. J. How do plant cell walls extend? Plant Physiol. 1993 May;102(1):1–6. doi: 10.1104/pp.102.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D. J. Wall extensibility: its nature, measurement and relationship to plant cell growth. New Phytol. 1993 May;124(1):1–23. doi: 10.1111/j.1469-8137.1993.tb03795.x. [DOI] [PubMed] [Google Scholar]
- Fry S. C., Smith R. C., Renwick K. F., Martin D. J., Hodge S. K., Matthews K. J. Xyloglucan endotransglycosylase, a new wall-loosening enzyme activity from plants. Biochem J. 1992 Mar 15;282(Pt 3):821–828. doi: 10.1042/bj2820821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouhe M., Nevins D. J. Inhibition of auxin-induced cell elongation of maize coleoptiles by antibodies specific for cell wall glucanases. Plant Physiol. 1991 Jun;96(2):426–431. doi: 10.1104/pp.96.2.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L. S., Yuen H. K., Varner J. E. Differential scanning calorimetry of plant cell walls. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2241–2243. doi: 10.1073/pnas.88.6.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen-Mason S. J., Fry S. C., Durachko D. M., Cosgrove D. J. The relationship between xyloglucan endotransglycosylase and in-vitro cell wall extension in cucumber hypocotyls. Planta. 1993;190(3):327–331. doi: 10.1007/BF00196961. [DOI] [PubMed] [Google Scholar]
- McQueen-Mason S., Durachko D. M., Cosgrove D. J. Two endogenous proteins that induce cell wall extension in plants. Plant Cell. 1992 Nov;4:1425–1433. doi: 10.1105/tpc.4.11.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani K., Tominaga R. Endo-xyloglucan transferase, a novel class of glycosyltransferase that catalyzes transfer of a segment of xyloglucan molecule to another xyloglucan molecule. J Biol Chem. 1992 Oct 15;267(29):21058–21064. [PubMed] [Google Scholar]
- Rayle D. L., Cleland R. E. The Acid Growth Theory of auxin-induced cell elongation is alive and well. Plant Physiol. 1992 Aug;99(4):1271–1274. doi: 10.1104/pp.99.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T. Gels. Sci Am. 1981 Jan;244(1):124-36, 138. doi: 10.1038/scientificamerican0181-124. [DOI] [PubMed] [Google Scholar]
- Valent B. S., Albersheim P. The structure of plant cell walls: v. On the binding of xyloglucan to cellulose fibers. Plant Physiol. 1974 Jul;54(1):105–108. doi: 10.1104/pp.54.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]