Abstract
Most gymnosperms are wind-pollinated, but some are insect-pollinated, and in Ephedra (Gnetales), both wind pollination and insect pollination occur. Little is, however, known about mechanisms and evolution of pollination syndromes in gymnosperms. Based on four seasons of field studies, we show an unexpected correlation between pollination and the phases of the moon in one of our studied species, Ephedra foeminea. It is pollinated by dipterans and lepidopterans, most of them nocturnal, and its pollination coincides with the full moon of July. This may be adaptive in two ways. Many nocturnal insects navigate using the moon. Further, the spectacular reflection of the full-moonlight in the pollination drops is the only apparent means of nocturnal attraction of insects in these plants. In the sympatric but wind-pollinated Ephedra distachya, pollination is not correlated to the full moon but occurs at approximately the same dates every year. The lunar correlation has probably been lost in most species of Ephedra subsequent an evolutionary shift to wind pollination in the clade. When the services of insects are no longer needed for successful pollination, the adaptive value of correlating pollination with the full moon is lost, and conceivably also the trait.
Keywords: anemophily, entomophily, lunar phases, nocturnal insects
1. Background
The moon influences biological systems. Correlation with the lunar cycle has been described regarding activity, reproduction, communication and navigation for a number of vertebrates and invertebrates [1]. Even eyeless animals such as reef corals correlate their reproduction with the phases of the moon [2]. Therefore, it is not surprising that the moon can influence plants as well, e.g. as a consequence of plant–animal interactions. Pollination biology in Ephedra (Gnetales) has gained renewed interest since a recent study documents variation in pollination syndrome in this small gymnospermous relict [3], which can be traced back to the Early Cretaceous [4]. Insect pollination is conceivably the ancestral state in the Gnetales (figure 1a). The sister species of the remaining Ephedra, Ephedra foeminea [5], is insect-pollinated [3,8,9], as are Welwitschia [10] and Gnetum [11] (Gnetales), and probably also Ephedra aphylla [12]. By contrast, other species of Ephedra are considered wind-pollinated [13] although this has only been rigorously tested for Ephedra distachya [3,9]. As most gymnosperms, Ephedra produces liquid by secretion from the nucellus [6,14], and the liquid is exposed as a pollination drop at the micropylar opening (figure 1b,c). Its main function is to receive and transport pollen to the nucellus [14], but in the Gnetales, the pollination drops are high in sugar [15] and therefore attractive to insects.
Figure 1.
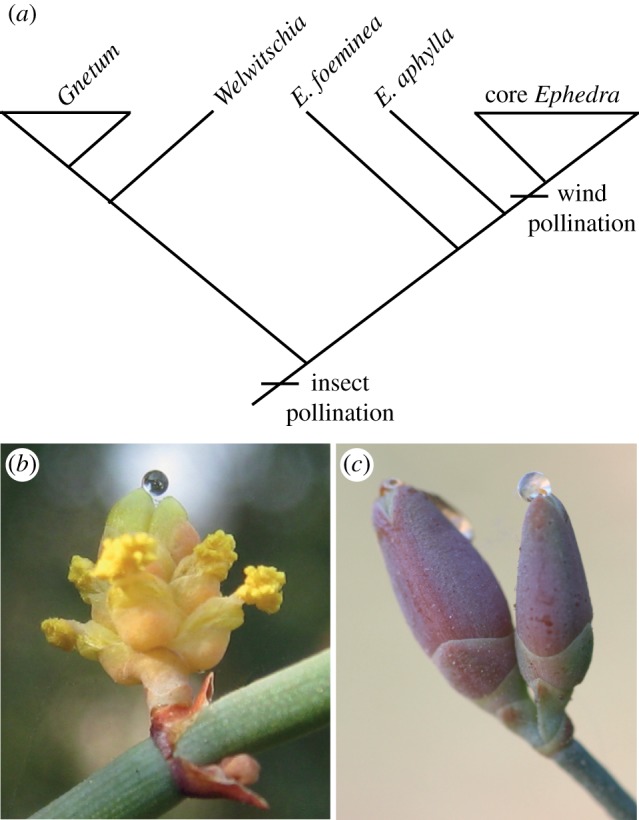
Pollination in the Gnetales. (a) Hypothesis on evolution of pollination syndromes in the Gnetales based on phylogenetic [5–7] and pollination [3,8–12] studies. (b) Ephedra foeminea. Male cone with pollination drops produced by sterile ovules at the distal centre of the cone. (c) Ephedra foeminea. Female cones with pollination drops exposed at the micropylar openings.
The idea of a possible correlation between pollination and lunar phases in E. foeminea was founded owing to mistakes in our prediction of the time for pollination in this species. Considering the relatively stable Mediterranean climate, we had not anticipated substantial phenological variation, and we decided to go through all available data in order to find out why we repeatedly failed to predict pollination time in E. foeminea. The present study describes the results and conclusions.
2. Material and methods
During 2011–2014, populations of E. distachya and E. foeminea were studied in Macedonia (Greece) and Dalmatia (Croatia). Pollination drop production and insect visitations were documented. Information on lunar phases was taken from http://www.fullmoon.info/en/fullmoon-calendar_1900-2050.html. Correlation between the peak in the pollination period and the occurrence of full moon was assessed using regression analysis as implemented in R, v. 3.1.1 [16]. Temperature and precipitation for April–July 2011–2014 were extracted from http://www.wunderground.com/history/, compared using an ANOVA and assessed for correlation with pollination peaks as above. Additional details are available in the electronic supplementary material.
3. Results and discussion
The exact timing of pollination in E. foeminea varied considerably from year to year, but was correlated with the full moon of July (figure 2a) (r2 = 0.999, p = 0.013). To our astonishment, even cones that appeared too young to be pollinated (small and ovules without developed micropylar tubes) secreted pollination drops from a pore-shaped micropylar opening during the peaking period at full moon. One to two weeks earlier, when the moon was new and in its first quarter, we observed the exact opposite; drop secretion was weak to non-existent and pollinators were absent. Not even cones of the appropriate developmental stage produced pollination drops. There are secondary peaks in pollination drop production in association with the full moon of August and September, but with few cones involved.
Figure 2.
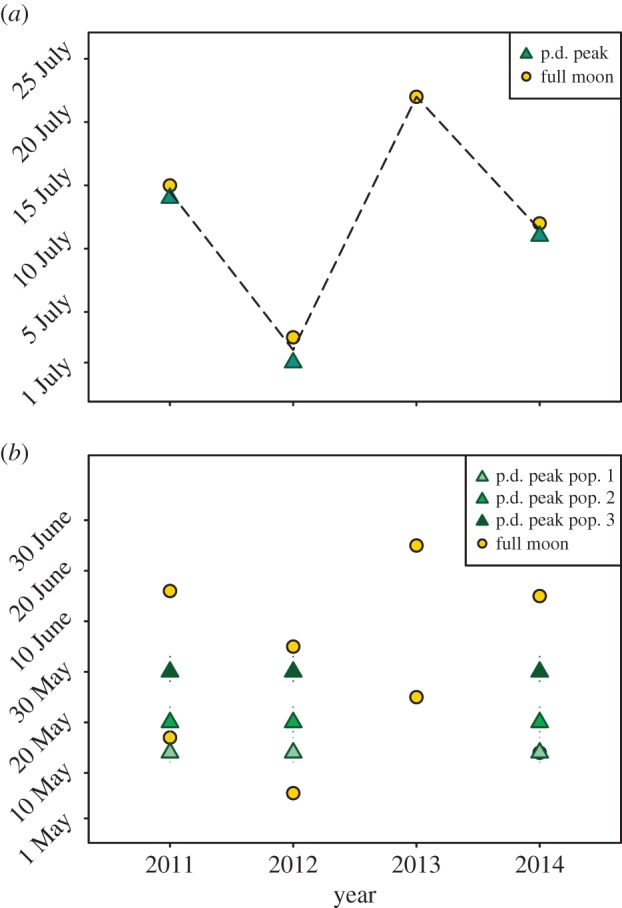
Correlation between peaks in pollination drop production and the full moon. (a) In Ephedra foeminea, the peak in pollination drop (p.d.) production occurs simultaneously in all study populations (green triangles) and is temporally correlated with the full moon of July (yellow circles). (b) In Ephedra distachya, the production peak occurs at different dates in different populations (triangles in different shades of green), and there is no correlation with the full moon (yellow circles).
Only a few studies in the literature provide the necessary details on dates and pollination drop production, but available information [8,12,17] supports our conclusions. Porsch [8] studied reproduction in E. foeminea in Dalmatia (Croatia) in late July to early August 1910. We have been puzzled by how late in the season he apparently found the plants at pollination stage of development, but in the light of the ‘lunar hypothesis’ presented here, Porsch's [8] dates of observations make sense because in 1910, the full moon occurred on 22 July. The lunar hypothesis also explains why we failed to collect pollination drops during the first week of July in 2013 and 2014. The time should have been approximately right according to our observations from 2011 and 2012, and cones were indeed developed, but did not produce pollination drops. In 2014, drop production peaked simultaneously with the full moon of July (12th), as was also the case in 2011 (mid-July) and 2012 (first days of July). In 2013, our last observations from 10 July did not indicate a near start of the pollination period. Although impossible to know now, our guess is that the pollination drop production in 2013 peaked in association with the full moon on 22 July.
Our observations do not support a correlation between pollination and fluctuating weather conditions. The weather is stable in the Balkans; there are only rare exceptions to the otherwise warm and sunny summers with sparse precipitation from May through to September (electronic supplementary material, figure S1). Furthermore, it is not the actual development of the cones that occur at different times or rates. Cones could be at pollination stage of development one–two weeks before the full moon, but did nevertheless not secrete pollination drops of sufficient amount to attract pollinators. Similarly, many cones were small and immature when the moon was full on 12 July 2014, and yet these cones produced pollination drops.
How then, could a correlation between pollination and the moon evolve? And how do the plants identify the lunar phases? Plants can detect moonlight [18], including its different intensities during the phases of the moon [19]. Plants can also detect gravity [20] and can conceivably use both to identify the lunar phases. Pollination in E. foeminea is a low-productive, generalist, entomophilous system [3]. We have identified pollinators of Diptera and Lepidoptera, several of them nocturnal. Nocturnal insects can fluctuate in abundance according to the phase of the moon [21–23], and some navigate using the angle (azimuth) to the moon [24,25]. Dung beetles use polarized moonlight to navigate in straight lines while moving dung [26]. At new moon or cloudy nights, they move around randomly [26]. Insect visitation of E. foeminea cones is not frequent [3] and pollinators caught have a limited number of pollen grains on their bodies. The consequence of poorly navigating pollinators may therefore be a costly deterioration of the pollination process. While some insects can navigate using land marks [27] or stars [25], navigation aided by moonlight is suggested to be widespread among nocturnal animals [21,26] and the system is probably particularly adaptive in open habitats [28]. This fits well with our field sites in the Balkans, where a cloudy sky is rare in the summer and no high trees shade the environment.
The moon may further be crucial for pollinator attraction. While investigated species of Gnetum emit easily detectable scent (C. Rydin 2014, personal observation and [11,29]), Ephedra does not. The colourful cones can attract diurnal insects but at night, field observations reveal only one possible means of attraction of pollinators: the many pollination drops glitter like diamonds in the full-moonlight. A spectacular sight also for the human eye. The full moon is thus important for efficient pollination of E. foeminea both for pollinator navigation in the dark and for attraction to the cones. To experimentally remove the forces of celestial gravitation is difficult [30] but our hypothesis could be further tested by experiments on moonlight detection in plants and by continuing to make predictions followed by targeted observations [30].
By contrast, we find no correlation between the phases of the moon and secretion of pollination drops in the sympatric but wind-pollinated E. distachya (figure 2b) (May: r2 = 0.34, p = 0.39; June: r2 = 0.19, p = 0.44). Instead, its peak in pollination drop secretion occurs at approximately the same dates each year, on 20 to 30 May in Greece (somewhat later in France [17]). The time of the peak differs slightly between nearby localities, a difference that is constant between years and probably correlated with microclimate (see also [17]). Lunar-correlated pollination has probably been lost in E. distachya and other wind-pollinated species of Ephedra. Further studies of insect-pollinated taxa of the Gnetales (the tropical lianas of Gnetum, the Namib Desert endemic Welwitschia and the Mediterranean E. aphylla [10–12]) would, however, be highly interesting.
Supplementary Material
Acknowledgements
We thank Konstantinos Doulkeridis, Kyparissa Doulkeridou, Lena Norbäck-Ivarsson, Anders Rydberg and Olle Thureborn for field assistance, and Aelys Humphreys (Imperial College London) and the reviewers for comments on the text.
Data accessibility
The data reported in this paper are presented in the electronic supplementary material.
Funding statement
The project was funded by grants from the Swedish Research Council to C.R. and from Stiftelsen Extensus and Göransson-Sandviken stipendiefond to K.B.
Author contributions
C.R. conducted fieldwork, developed the hypothesis and wrote the manuscript with comments from K.B. K.B. conducted fieldwork, developed the hypothesis and performed statistical analyses with comments from C.R.
Conflict of interests
We have no competing interests.
References
- 1.Kronfeld-Schor N, Dominoni D, de la Iglesia H, Levy O, Herzog ED, Dayan T, Helfrich-Forster C. 2013. Chronobiology by moonlight. Proc. R. Soc. B 280, 20123088 ( 10.1098/rspb.2012.3088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrison PL, Babcock RC, Bull GD, Oliver JK, Wallace CC, Willis BL. 1984. Mass spawning in tropical reef corals. Science 223, 1186–1189. ( 10.1126/science.223.4641.1186) [DOI] [PubMed] [Google Scholar]
- 3.Bolinder K, Humphreys AM, Ehrlén J, Alexandersson R, Ickert-Bond SM, Rydin C. 2014. Pollination mechanisms in the ancient gymnosperm clade Ephedra (Gnetales). In Pollination in Ephedra (Gnetales) (Licentiate thesis), pp. 30–49. Stockholm, Sweden: Stockholm University. [Google Scholar]
- 4.Rydin C, Pedersen KR, Friis EM. 2004. On the evolutionary history of Ephedra: Cretaceous fossils and extant molecules. Proc. Natl Acad. Sci. USA 101, 16 571–16 576. ( 10.1073/pnas.0407588101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rydin C, Korall P. 2009. Evolutionary relationships in Ephedra (Gnetales), with implications for seed plant phylogeny. Int. J. Plant Sci. 170, 1031–1043. ( 10.1086/605116) [DOI] [Google Scholar]
- 6.Rydin C, Khodabandeh A, Endress PK. 2010. The female reproductive unit of Ephedra (Gnetales): comparative morphology and evolutionary perspectives. Bot. J. Linn. Soc. 163, 387–430. ( 10.1111/j.1095-8339.2010.01066.x) [DOI] [PubMed] [Google Scholar]
- 7.Thureborn O. 2014. New insights into the deep divergences of Ephedra (Gnetales) using molecular data. Master thesis, Stockholm University, Stockholm, Sweden. [Google Scholar]
- 8.Porsch O. 1910. Ephedra campylopoda C.A. Mey., eine entomophile Gymnosperme. Ber. Deutsch. Bot. Ges. 28, 404–412. ( 10.1111/j.1438-8677.1910.tb06911.x) [DOI] [Google Scholar]
- 9.Bolinder K, Niklas KJ, Rydin C. 2015. Aerodynamics and pollen ultrastructure in Ephedra (Gnetales). Am. J. Bot. 102, 457–470. ( 10.3732/ajb.1400517) [DOI] [PubMed] [Google Scholar]
- 10.Pearson HHW. 1909. Further observations on Welwitschia. Phil. Trans. R. Soc. Lond. B 200, 331–402. ( 10.1098/rstb.1909.0009) [DOI] [Google Scholar]
- 11.Kato M, Inoue T, Nagamitsu T. 1995. Pollination biology of Gnetum (Gnetaceae) in a lowland mixed dipterocarp forest in Sarawak. Am. J. Bot. 82, 862–868. ( 10.2307/2445972) [DOI] [Google Scholar]
- 12.Meeuse ADJ, De Meijer AH, Mohr OWP, Wellinga SM. 1990. Entomophily in the dioecious gymnosperm Ephedra aphylla Forssk. (=E. alte C.A. Mey.), with some notes on Ephedra campylopoda C.A. Mey. III. Further anthecological studies and relative importance of entomophily. Isr. J. Bot. 39, 113–123. [Google Scholar]
- 13.Kubitzki K. 1990. Ephedraceae. In The families and genera of vascular plants (ed. Kubitzki K.), pp. 379–382. Berlin, Germany: Springer. [Google Scholar]
- 14.Nepi M, von Aderkas P, Wagner R, Mugnaini S, Coulter A, Pacini E. 2009. Nectar and pollination drops: how different are they? Ann. Bot. 104, 205–219. ( 10.1093/aob/mcp124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziegler H. 1959. Über die Zusammensetzung des ‘Bestäubungstropfens’ und den Mechanismus seiner Sekretion. Planta 52, 587–599. ( 10.1007/BF01914757) [DOI] [Google Scholar]
- 16.R Core Team 2014. R: a language and environment for statistical computing v. 3.1.1. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 17.Moussel B. 1980. Gouttelette réceptrice du pollen et pollinisation chez l'Ephedra distachya L. Observations sur le vivant et en microscopies photonique et électronique. Rev. Cyt. Biol. Vég. 3, 65–89. [Google Scholar]
- 18.Garner WW. 1937. Recent work on photoperiodism. Bot. Rev. 3, 259–275. ( 10.1007/BF02872312) [DOI] [Google Scholar]
- 19.Bünning E, Moser I. 1969. Interference of the moonlight with the photoperiodic measurement of time by plants, and their adaptive reaction. Proc. Natl Acad. Sci. USA 62, 1018–1022. ( 10.1073/pnas.62.4.1018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volkmann D, Baluška F. 2006. Gravity: one of the driving forces for evolution. Protoplasma 229, 143–148. ( 10.1007/s00709-006-0200-4) [DOI] [PubMed] [Google Scholar]
- 21.Danthanarayana W. 1986. Lunar periodicity of insect flight and migration. In Insect flight dispersal and migration (ed. Danthanarayana W.), pp. 88–119. Berlin, Germany: Springer. [Google Scholar]
- 22.Hartland-Rowe R. 1955. Lunar rhythm in the emergence of an ephemeropteran. Nature 176, 657 ( 10.1038/176657a0)13265805 [DOI] [Google Scholar]
- 23.Bidlingmayer WL. 1964. The effect of moonlight on the flight activity of mosquitoes. Ecology 45, 87–94. ( 10.2307/1937110) [DOI] [Google Scholar]
- 24.Papi F, Pardi L. 1963. On the lunar orientation of sandhoppers (Amphipoda Talitridae). Biol. Bull. 124, 97–105. ( 10.2307/1539571) [DOI] [Google Scholar]
- 25.Sotthibandhu S, Baker RR. 1979. Celestial orientation by the large yellow underwing moth, Noctua pronuba L. Anim. Behav. 27, 786–800. ( 10.1016/0003-3472(79)90015-0) [DOI] [Google Scholar]
- 26.Dacke M, Nilsson D-E, Scholtz CH, Byrne M, Warrant EJ. 2003. Insect orientation to polarized moonlight. Nature 424, 33 ( 10.1038/424033a) [DOI] [PubMed] [Google Scholar]
- 27.Warrant EJ, Kelber A, Gislén A, Greiner B, Ribi W, Wcislo WT. 2004. Nocturnal vision and landmark orientation in a tropical halictid bee. Curr. Biol. 14, 1309–1318. ( 10.1016/j.cub.2004.07.057) [DOI] [PubMed] [Google Scholar]
- 28.Roach J. 2003. Dung beetles navigate by the moon, study says. In National Geographic News (National Geographic Society). [Google Scholar]
- 29.Endress PK. 1996. Structure and function of female and bisexual organ complexes in Gnetales. Int. J. Plant Sci. 157, S113–S125. ( 10.1086/297407) [DOI] [Google Scholar]
- 30.Barlow PW, Fisahn J. 2012. Lunisolar tidal force and the growth of plant roots, and some other of its effects on plant movements. Ann. Bot. 110, 301–318. ( 10.1093/aob/mcs038) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data reported in this paper are presented in the electronic supplementary material.


