Abstract
Anthropogenic sensory pollution is affecting ecosystems worldwide. Human actions generate acoustic noise, emanate artificial light and emit chemical substances. All of these pollutants are known to affect animals. Most studies on anthropogenic pollution address the impact of pollutants in unimodal sensory domains. High levels of anthropogenic noise, for example, have been shown to interfere with acoustic signals and cues. However, animals rely on multiple senses, and pollutants often co-occur. Thus, a full ecological assessment of the impact of anthropogenic activities requires a multimodal approach. We describe how sensory pollutants can co-occur and how covariance among pollutants may differ from natural situations. We review how animals combine information that arrives at their sensory systems through different modalities and outline how sensory conditions can interfere with multimodal perception. Finally, we describe how sensory pollutants can affect the perception, behaviour and endocrinology of animals within and across sensory modalities. We conclude that sensory pollution can affect animals in complex ways due to interactions among sensory stimuli, neural processing and behavioural and endocrinal feedback. We call for more empirical data on covariance among sensory conditions, for instance, data on correlated levels in noise and light pollution. Furthermore, we encourage researchers to test animal responses to a full-factorial set of sensory pollutants in the presence or the absence of ecologically important signals and cues. We realize that such approach is often time and energy consuming, but we think this is the only way to fully understand the multimodal impact of sensory pollution on animal performance and perception.
Keywords: sensory ecology, multimodal, anthropogenic pollution, animal communication
1. A multimodal view of sensory pollution
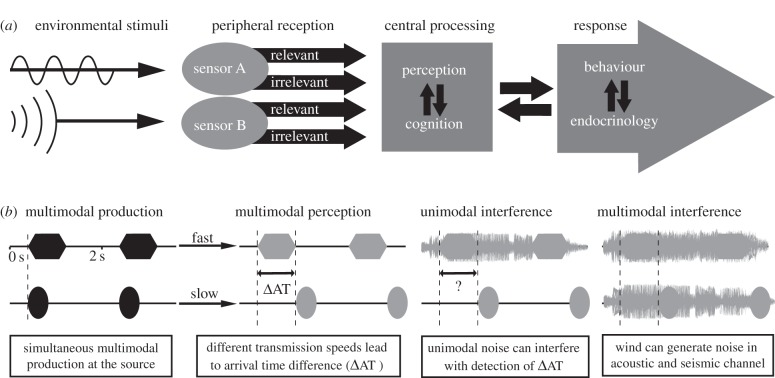
Anthropogenic activities are increasingly affecting the welfare and reproductive success of free-ranging animals [1–3]. Humans emit chemical and physical stimuli into the environment that are received through a range of sensory modalities. These anthropogenic stimuli can decrease animal survival and reproductive success and may ultimately alter populations and ecological communities. To understand and mitigate the effect of these stimuli, it is crucial to study the mechanisms underlying the sensory reception of these pollutants, termed sensory pollution [3–6]. High levels of anthropogenic acoustic noise, for instance, can mask acoustic communication. Chemical emission on the other hand can impair olfactory orientation [5–7]. However, anthropogenic activities often produce stimuli in multiple modalities simultaneously, like the joint emission of acoustic and chemical pollutants by automobile traffic. Furthermore, perception itself is multimodal, and animals sometimes respond in a complex way to the combination of cues from different modalities [8–11]. Finally, sensory pollutants can affect an animal's behaviour as well as its endocrinology. These responses are known to feed back into perceptual and cognitive processes, which further complicate predictions of the potential impact of anthropogenic activities on animal behaviour and reproductive success. We therefore propose an integrated approach to fully understand the multimodal nature of sensory pollution (figure 1). This approach allows us to address how pollutants can disturb animals and interfere with the processing of important signals and cues, how pollutants can affect processes across different modalities, and how the combination of pollutants from different modalities may affect animal performances.
Figure 1.
Multimodal approach to understand the environmental impact on animal perception and performance. (a) Animals can receive all sorts of environmental stimuli, such as light, sounds or chemicals, with a variety of sensors found in the peripheral part of their nervous system. These sensors pass on the received information to the central nervous system for higher level processing in different perceptual and cognitive areas. Environmental stimuli can contain relevant information for an animal, e.g. acoustic signals, or irrelevant information, e.g. acoustic noise (arrow widths do not correspond to amount of processed information). When central processing reaches a decision, a behavioural and/or endocrinal response will follow. Complex interactions between and within reception, processing and response determine the impact of the multimodal environment on animals. (b) Example of how environmental conditions can interfere with the perception of multimodal signals and cues. Two stimuli differing in physical form (e.g. a sound or light) that are simultaneously produced by a source travel with different speeds to a receiver who has to extract the relevant information from the two perceptual streams against a background of environmental noise. For example, a male frog produces an acoustic signal, but at the same time generates cues in other sensory modalities, such as vibrations on the water surface. These multimodal components arrive at the receiver at different times (ΔAT). Perceptual processing involves comparing information across modalities. Receivers must bind different sensory components to the same source across time and space. Unimodal noise, e.g. sounds of other calling males, can interfere with multimodal perceptual binding. Sensory interference can also be multimodal, e.g. wind-induced leaf movements, which simultaneously result in acoustic noise and substrate-borne vibrations.
Whether and how animals are affected by sensory pollution depends on overlap in time and space between stimuli exposure and behavioural activity [2–4]. We will first address how anthropogenic pollutants covary in time and space and how this compares to natural variation in environmental sensory conditions. Next, we will discuss how multimodal signals and cues are produced, what sort of information they contain and how animals perceptually process relevant and irrelevant environmental information. Finally, we will describe the ways in which covariance in anthropogenic sensory conditions can interfere with the processing of signals and cues, how pollutants disturb animals by affecting endocrinology and how animals respond by adjusting their behaviour. We will end our review by outlining how we think multimodal sensory pollution should be addressed and list some of the most important outstanding issues.
2. Environmental sensory conditions and covariance among modalities
Animal sensory systems often operate under challenging conditions and are under strong selection from the environment [12]. How does the sensory environment change across time and space for different modalities? How have animals adapted to these changes? And how do human activities affect the sensory environment? Conditions can often correlate across sensory modalities, but we have little data on actual covariance levels in time and space for natural as well as human-impacted environments.
(a). Temporal and spatial variation in natural sensory conditions
Environmental conditions can show large temporal and spatial fluctuations and often covary across modalities (table 1). Ambient light levels, for example, quickly rise with dawn, rapidly drop at dusk and typically correlate with fluctuations in acoustic background levels caused by biotic activity [13,14,16]. Daily and seasonal changes in climate conditions, such as temperature and wind, can also result in concordant patterns across modalities [17,27]. Wind, for instance, can result in higher noise levels, more substrate vibrations and increased visual motion, thus simultaneously affecting multiple senses [28–30]. Multimodal covariance may also occur across space. A fast-flowing stream will be much noisier as well as turbid compared to a slow-flowing river, resulting in covariance of sound and light levels between these two habitat types.
Table 1.
Examples of covariance in light, sound and chemical levels from natural and anthropogenic impacted environments.
| combination of sensory conditions | correlated levels | examples from natural environments | examples from anthropogenic impacted environments | references |
|---|---|---|---|---|
| sound versus light | + | daylight and birdsong in temperate regions | traffic sounds and streetlights | [13–15] |
| − | moonlight and insect sounds of tropical regions | reduced visibility and increased noise at water locks | [16–18] | |
| sound versus chemicals | + | sound and chemical transmission in air/water currents | emission of sounds and chemicals by traffic | [19–22] |
| − | ? | ? | ||
| light versus chemicals | + | pheromone release in relation to lunar phase | chemical and light pollution of industrial areas | [23] |
| − | reduced visibility and increased CO2 levels due to aquatic vegetation | reduced visibility and increased nitrogen levels due to aquatic eutrophication | [24–26] |
(b). Covariance in anthropogenic sensory pollutants
High levels of chemical and acoustic emission as well as light pollution typically characterize industrial areas, urban city centres and multi-lane highways. Humans can also alter multiple sensory conditions more indirectly. For example, high phosphate levels in the aquatic environment can increase algae growth, and thus affects both the chemical, as well as the visual environment (table 1, [24]). Sensory pollution can occasionally be biased to particular modalities. Remote terrestrial drilling stations for the gas industry, for example, generate high levels of acoustic noise, but relatively low levels of light pollution [31,32]. Bicycle paths, pedestrian areas or long-term parking lots on the other hand are sometimes associated with high levels of light pollution, but low levels of other pollutants [33,34]. Cases in which types of sensory pollution occur independently provide the opportunity to obtain independent correlational data between pollutant and animal performance [31,34].
Sensory pollution also shows temporal fluctuations. Highway noise levels can be higher during the day than at the night and traffic sounds transmit further on cold spring days compared to warm days later on [14]. Artificial light pollution on the other hand is mainly a nocturnal problem [15,34]. Peak levels in noise and light pollution may therefore not overlap in time, but we should keep in mind that nocturnal urban noise and light levels are still substantially higher when compared with natural conditions, in particular in temperate habitats (table 1, [14,33]).
3. Production and perception of multimodal signals and cues under natural conditions
Animals rely on multiple senses to orient and to communicate. These senses can pick up stimuli emitted intentionally (signals) or unintentionally (cues). How signals and cues of different modalities are produced, transmitted and received has important consequences for environmental selection pressures, such as sensory conditions, that act on the behaviour and physiology of animals [8,35,36].
(a). Multimodal signals produced by animal displays
Animals have evolved elaborate displays to attract mates, fend off rivals or deter predators. Most of these displays generate stimuli that can be perceived with a wide variety of sensory modalities [8,35,36]. Sound production often involves inflation and deflation of morphological structures, like the vocal pouch of grouse or vocal sac of frogs, and as a consequence provides a synchronized multimodal display consisting of visual and acoustic components [37–39]. Sexual displays can also combine components that are independently produced, such as fish using body coloration together with pheromones, or spiders drumming vibrations with one leg and waving colourful tufts with another [24,40].
(b). Multimodal cues produced by predators and prey
Animals also emit stimuli detectable through multiple senses that do not serve themselves, but their predators or prey. A mouse rustling among leaves produces acoustic and visual cues that can aid predatory owls [41]. Vibrations combined with the flow of warm air produced by foraging cattle provide aphids a multimodal cue to flee from plants [42]. Signal production can also generate unintended cues in a different modality. Frogs that call from water bodies to attract mates induce water surface waves, or ripples, that can be detected by eavesdropping bats that benefit from these relatively slow-travelling prey cues [43].
(c). Multimodal perception and nonlinear effects
Incorporating information from multiple sensory systems can increase perceptual processing and the resulting responses in a linear way [44]. However, multimodal perception often involves more complex processes that may not add-up linearly [44,45]. Many perceptual tasks rely on comparisons of information across sensory systems, for instance, to assess timing between components of multimodal signals and cues [44–47]. Such comparisons rely on the brain to accurately assign different components to the same source, which can be challenging under fluctuating sensory conditions (figure 1b, [46]). Animals tested in psychophysical experiments have been shown to respond in linear as well as nonlinear ways when presented with stimuli from different modalities (see [8,9] for a detailed classification of behavioural responses to multimodal signals and cues). Presenting a stimulus in isolation (e.g. a visual signal) can have no effect on an animal's behaviour, but that same stimulus can modify the response to another stimulus (e.g. an acoustic signal) in a complex way [47]. Multimodal signals and cues can even elicit emergent responses [37,46]. Chickens have been found to ignore chemical and visual warning signals of unpalatable caterpillars when presented in isolation, but were shown to avoid food items when both signals were presented in combination [48].
(d). When do animals rely on multimodal perception?
In general, animals rely on multimodal signals and cues when it increases their chances of detecting important environmental events, when it enhances processing of environmental cues or when it provides them with unique sources of environmental information [9,35,46]. Simultaneously produced components from different modalities arrive with varied time delays at a receiver and can thereby provide unique information on distance to the source (figure 1b, [46]). When multimodal signals or cues provide ambiguous information, animals can ignore information from one modality, or arrive at an intermediate solution [45,49]. For example, when nectar-feeding moths are presented with spatially separated chemical and visual cues they approach the visual ones [49].
(e). Multimodal communication and perceptual interference
Multimodal communication can be affected by environmental sensory conditions [7,12]. Animals relying on multimodal signals may benefit from having a signal component that serves a back-up function when interference levels impair processing in one sensory modality [7]. Torrent frogs living next to noisy streams in the rainforest make sounds and wave their legs at the same time [50]. Perceptual information from the two sensory components can be redundant, for instance, both the acoustic and the visual display allowing the detection or recognition of the signaller. Such redundancy can make multimodal signals robust to fluctuations in sensory conditions [50]. On the other hand, multimodal signals will be more susceptible to environmental interference when animals rely on the comparison between sensory components for unique information, such as for estimating the distance from a signaller (figure 1b). Finally, multimodal signals may suffer from multimodal interference when sensory conditions covary across modalities (figure 1b), for example, when wind generates visual and seismic noise and thereby hampers multimodal perception of a spiders' drumming display [40,51].
4. Multimodal impact of anthropogenic pollution on behaviour and physiology
Anthropogenic pollutants are known to disturb animals and to interfere with perceptual processing of important signals and cues. How sensory pollution affects individuals and how their behaviour changes in response depend on the modalities involved, covariance in sensory pollutants, perceptual mechanisms and response plasticity.
(a). Multimodal disturbance by anthropogenic sensory pollution
Anthropogenic noise and artificial lights are well known to disturb animals and often co-occur. However, most studies to date have focused on the impact of one pollutant, or assessed which pollutant was most predictive of a behavioural or endocrinal response and ignored potential additive effects [15,33]. Traffic noise has been associated with increased stress levels and light pollution has been linked to shifts in circadian rhythms [34,52]. So, both pollutants apparently can thus affect endocrine processes and it would be interesting to assess in more detail how noise and light pollution covary and whether their combined impact is similar, increased, or decreased compared to the impact of each pollutant in isolation (figure 2).
Figure 2.
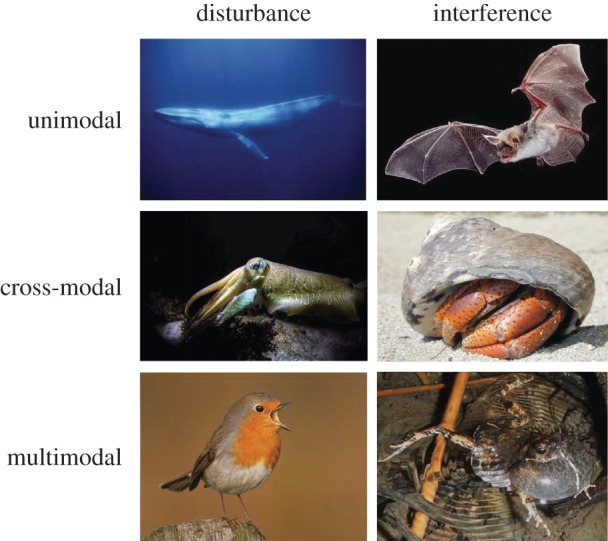
Impact of sensory pollution on animal responses across taxa. Sensory pollution can cause general disturbance of behaviour and endocrinology, or interfere with detection and processing of signals and cues. Sensory pollution can be restricted to a single sensory domain (unimodal), affect processes and responses in a different domain (cross-modal), or arrive at the brain through multiple sensory systems (multimodal). Exposure to anthropogenic noise disturbs blue whales and masks acoustic prey cues used by bats [53,54]. Acoustic noise also disturbs visual signalling in squids and may interfere with visual processing in hermit crabs [55,56]. Combined light and noise pollution may increasingly disturb a robin's song behaviour and anthropogenic noise travelling through air and along the water surface may interfere with multimodal communication in frogs (see also figure 1b). (Online version in colour.)
(b). Multimodal interference by anthropogenic sensory pollution
Anthropogenic pollutants can directly interfere with the detection of signals and cues. Traffic noise is known to mask acoustic signals as well as cues used by a wide range of taxa, including birds, mammals, insects and fish [53,57–59], a process we refer to as unimodal interference (figure 2). Anthropogenic noise can also result in multimodal interference, for example, when sounds induce surface vibrations on a leaf or a water surface, resulting in covarying noise levels that may hamper the use of signals and cues in the acoustic and seismic domain at the same time (figure 2, [51]). Pollution with artificial lights has not been linked to impaired detection of visual signals, but may interfere with the use of spatial cues during navigation [60,61]. Animals also rely on acoustic and olfactory cues for orientation, and covariance levels between sound, light and chemicals may consequently result in multimodal interference with spatial navigation cues [6,19]. Indirect anthropogenic effects on aquatic environments may also provide interesting cases for multimodal interference of visual and chemical signals and cues [36].
(c). Cross-modal interference of anthropogenic pollutants
The processing of irrelevant environmental information in one sensory modality may hinder the processing of information in another modality (figure 2, [11]). Such so-called cross-modal interference has been assumed to be responsible for an impact of anthropogenic acoustic noise on the processing speed by hermit crabs that rely on visual cues to detect a predatory threat [56]. Anthropogenic noise has also been linked to an impact on higher level processing of visual information, such as spatial visual orientation [62]. The effect of anthropogenic noise on processing of information in other modalities or cognitive domains can operate via different routes. Noise may induce increased vigilance as a response to a decreased ability to detect acoustic predatory cues and thereby affect the amount of time spent on a visual task [62,63]. Noise may also limit cognitive attention or reduce perceptual processing capacity [56,64]. Finally, noise may induce endocrinal changes, such as increased stress hormone levels, that can cause an indirect effect of the noise on behaviour or feedback to perceptual and cognitive processes (figure 1). Examples of cross-modal interference of other modalities are lacking as far as we know, but a recent study on moths suggests that light pollution may reduce responses to ultrasonic bat calls [65].
(d). Multimodal and cross-modal behavioural responses
Animals are well known to adjust their behaviour in response to interference from sensory pollutants. Some birds can almost instantly change their songs when exposed to noise [66,67]. Whether multimodal signals show similar behavioural flexibility in response to multimodal interference is not known [68]. However, anthropogenic pollutants have been shown to have cross-modal impacts on signalling [55,69]. Noise-exposed squids and chemical-polluted fish change their visual signals, whereas light-exposed birds adjust their acoustic signals [34,55]. These signal adjustments probably reflect a disturbing impact of sensory pollution on endocrinology, e.g. through a link with stress hormones or an impact on circadian rhythms (figure 2). Nevertheless, these examples illustrate that anthropogenic pollution can have consequences for selection pressures acting on signals from different modalities.
5. Anticipating nonlinear impact of multimodal sensory pollution
There is limited experimental data showing how the combination of sensory pollutants alters the behaviour and physiology of animals. Do pollutants enhance or mitigate each other's impact? What happens when one sensory pollutant interferes with signal or cue detection, while another sensory pollutant disturbs an animal, increasing its stress levels? We realize that such questions require extensive testing of animals in a series of stimulus combinations. For example, the full-factorial combination between relevant signals and cues and irrelevant pollutants arriving through two sensory systems results in 16 different experimental treatments (e.g. modality A: signal (yes/no) × pollutant (yes/no) = 4 treatments; modality A × B = 16 treatments). Nevertheless, we hope to have provided the conceptual background to encourage researchers to start addressing some of the most interesting outstanding issues.
(a). Additive effects of multimodal sensory pollution
To our knowledge, only one study with hermit crabs addressed multimodal sensory pollution and that study found that anti-predator response was affected most when crabs were exposed to boat noise and boat lights simultaneously [56]. Future studies should aim to address whether the combination of sensory pollutants can have additive, linear or nonlinear, impacts on animal behaviour and physiology by using a full-factorial design in which each pollutant is also tested in isolation.
(b). Modulation effects between sensory pollutants
Sensory pollutants may have a modulating effect on each other via multiple routes (figure 1). A pollutant may not affect behaviour when presented in isolation, but can enhance or reduce the behavioural impact of a pollutant from another modality. These modulation effects can be mediated via an endocrine route, where the modulating pollutant increases stress hormone levels that consequently increase the behavioural impact of the other pollutant. Disentangling different modulation routes again requires testing animals on the full-factorial combination of signals, cues and sensory pollutants.
(c). Emergent multimodal sensory pollution
One of the most interesting outstanding questions in this field concerns the possible impacts of anthropogenic activities that emerge only when sensory pollution is multimodal. Evidence of such hidden impacts would not be found when sensory pollutants are tested in isolation, but could have a substantial effect on animal performances and ultimately on populations or even whole ecosystems considering the potentially high levels of covariance among sensory pollutants.
7. Conclusion and final remarks
The environment is filled with stimuli differing in physical forms, and animals have evolved a variety of sensory systems to make sense of this multimodal world. Likewise, pollution is not restricted to a particular modality. We argue that we need an integrated multimodal approach to appreciate the full ecological impact of human activities on animal performance and perception. We have outlined how anthropogenic stimuli from multiple modalities can co-occur in time and space, and how, across time and space, we need a detailed assessment of multimodal covariance levels to assess potential impact. We have described unimodal, cross-modal and multimodal impacts of sensory pollutants on animal behaviour and physiology, and argue that additive effects can become increasingly complex. We describe sensory disturbance and interference, using examples from a wide range of taxa and sensory domains and think that these concepts are widely applicable to other cases. Recent years have seen a wide body of literature addressing the importance of multimodality in understanding the sensory ecology of animal behaviour. We now add sensory pollution to the concept of multimodality and, in doing so, invoke a number of interesting, outstanding issues that we think should receive considerable attention in the years to come.
Acknowledgements
We are grateful to Rachel Page and two anonymous referees for their feedback on previous versions of our manuscript. Adam Dunn, Daniel T. Blumstein, Alvin Chan, Jens Stahl, Mariusz Kluzniak, Dietmar Nill and Mike Johnson provided the right to use their photos for figure 2.
Conflict of interest
We report no conflict of interest.
References
- 1.Slabbekoorn H, Bouton N, van Opzeeland I, Coers A, ten Cate C, Popper AN. 2010. A noisy spring: the impact of globally rising underwater sound levels on fish. Trends Ecol. Evol. 25, 419–427. ( 10.1016/j.tree.2010.04.005) [DOI] [PubMed] [Google Scholar]
- 2.Barber JR, Crooks KR, Fristrup KM. 2009. The costs of chronic noise exposure for terrestrial organisms. Trends Ecol. Evol. 25, 180–189. ( 10.1016/j.tree.2009.08.002) [DOI] [PubMed] [Google Scholar]
- 3.Longcore T, Rich C. 2004. Ecological light pollution. Front. Ecol. Environ. 2, 191–198. ( 10.1890/1540-9295(2004)002[0191:ELP]2.0.CO;2) [DOI] [Google Scholar]
- 4.Francis CD, Barber JR. 2013. A framework for understanding noise impacts on wildlife: an urgent conservation priority. Front. Ecol. Environ. 11, 305–313. ( 10.1890/120183) [DOI] [Google Scholar]
- 5.Briffa M, de la Haye K, Munday PL. 2012. High CO2 and marine animal behaviour: potential mechanisms and ecological consequences. Mar. Pollut. Bull. 64, 1519–1528. ( 10.1016/j.marpolbul.2012.05.032) [DOI] [PubMed] [Google Scholar]
- 6.Lürling M, Scheffer M. 2007. Info-disruption: pollution and the transfer of chemical information between organisms. Trends Ecol. Evol. 22, 374–379. ( 10.1016/j.tree.2007.04.002) [DOI] [PubMed] [Google Scholar]
- 7.Brumm H, Slabbekoorn H. 2005. Acoustic communication in noise. In Advances in the study of behavior (eds Slater PJB, Snowdon CT, Roper TJ, Brockmann HJ, Naguib M.), vol. 35, pp. 151–209. San Diego, CA: Academic Press. [Google Scholar]
- 8.Partan S, Marler P. 1999. Behavior—communication goes multimodal. Science 283, 1272–1273. ( 10.1126/science.283.5406.1272) [DOI] [PubMed] [Google Scholar]
- 9.Munoz NE, Blumstein DT. 2012. Multisensory perception in uncertain environments. Behav. Ecol. 23, 457–462. ( 10.1093/beheco/arr220) [DOI] [Google Scholar]
- 10.Hebets EA, Papaj DR. 2005. Complex signal function: developing a framework of testable hypotheses. Behav. Ecol. Sociobiol. 57, 197–214. ( 10.1007/s00265-004-0865-7) [DOI] [Google Scholar]
- 11.Chan AAY-H, Blumstein DT. 2011. Attention, noise, and implications for wildlife conservation and management. Appl. Anim. Behav. Sci. 131, 1–7. ( 10.1016/j.applanim.2011.01.007) [DOI] [Google Scholar]
- 12.Endler JA. 1992. Signals, signal conditions, and the direction of evolution. Am. Nat. 139, S125–S153. ( 10.1086/285308) [DOI] [Google Scholar]
- 13.Cuthill IC, Macdonald WA. 1990. Experimental manipulation of the dawn and dusk chorus in the blackbird Turdus merula. Behav. Ecol. Sociobiol. 26, 209–216. ( 10.1007/BF00172088) [DOI] [Google Scholar]
- 14.Halfwerk W, Holleman LJM, Lessells CM, Slabbekoorn H. 2011. Negative impact of traffic noise on avian reproductive success. J. Appl. Ecol. 48, 210–219. ( 10.1111/j.1365-2664.2010.01914.x) [DOI] [Google Scholar]
- 15.Fuller RA, Warren PH, Gaston KJ. 2007. Daytime noise predicts nocturnal singing in urban robins. Biol. Lett. 3, 368–370. ( 10.1098/rsbl.2007.0134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang AB, Kalko EK, Römer H, Bockholdt C, Dechmann DK. 2006. Activity levels of bats and katydids in relation to the lunar cycle. Oecologia 146, 659–666. ( 10.1007/s00442-005-0131-3) [DOI] [PubMed] [Google Scholar]
- 17.Wiley RH, Richards DG. 1978. Physical constraints on acoustic communication in atmosphere: implications for evolution of animal vocalizations. Behav. Ecol. Sociobiol. 3, 69–94. ( 10.1007/BF00300047) [DOI] [Google Scholar]
- 18.van der Sluijs I, et al. 2011. Communication in troubled waters: responses of fish communication systems to changing environments. Evol. Ecol. 25, 623–640. ( 10.1007/s10682-010-9450-x) [DOI] [Google Scholar]
- 19.Montgomery JC, Jeffs A, Simpson SD, Meekan M, Tindle C. 2006. Sound as an orientation cue for the pelagic larvae of reef fishes and decapod crustaceans. Adv. Mar. Biol. 51, 143–196. ( 10.1016/S0065-2881(06)51003-X) [DOI] [PubMed] [Google Scholar]
- 20.Dahl J, Nilsson PA, Pettersson LB. 1998. Against the flow: chemical detection of downstream predators in running waters. Proc. R. Soc. Lond. B 265, 1339–1344. ( 10.1098/rspb.1998.0439) [DOI] [Google Scholar]
- 21.Brönmark C, Hansson LA. 2000. Chemical communication in aquatic systems: an introduction. Oikos 88, 103–109. ( 10.1034/j.1600-0706.2000.880112.x) [DOI] [Google Scholar]
- 22.David C, Kennedy J, Ludlow A. 1983. Finding of a sex pheromone source by gypsy moths released in the field. Nature 303, 804–806. ( 10.1038/303804a0) [DOI] [Google Scholar]
- 23.Herborg LM, Bentley MG, Clare AS, Last KS. 2006. Mating behaviour and chemical communication in the invasive Chinese mitten crab Eriocheir sinensis. J. Exp. Mar. Biol. Ecol. 329, 1–10. ( 10.1016/j.jembe.2005.08.001) [DOI] [Google Scholar]
- 24.Heuschele J, Mannerla M, Gienapp P, Candolin U. 2009. Environment-dependent use of mate choice cues in sticklebacks. Behav. Ecol. 20, 1223–1227. ( 10.1093/beheco/arp123) [DOI] [Google Scholar]
- 25.Candolin U. 2009. Population responses to anthropogenic disturbance: lessons from three-spined sticklebacks Gasterosteus aculeatus in eutrophic habitats. J. Fish Biol. 75, 2108–2121. ( 10.1111/j.1095-8649.2009.02405.x) [DOI] [PubMed] [Google Scholar]
- 26.Vann CD, Megonigal JP. 2003. Elevated CO2 and water depth regulation of methane emissions: comparison of woody and non-woody wetland plant species. Biogeochemistry 63, 117–134. ( 10.1023/A:1023397032331) [DOI] [Google Scholar]
- 27.Richards DG, Wiley RH. 1980. Reverberations and amplitude fluctuations in the propagation of sound in a forest: implications for animal communication. Am. Nat. 115, 381–399. ( 10.1086/283568) [DOI] [Google Scholar]
- 28.Ord TJ, Peters RA, Clucas B, Stamps JA. 2007. Lizards speed up visual displays in noisy motion habitats. Proc. R. Soc. B 274, 1057–1062. ( 10.1098/rspb.2006.0263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penna M, Pottstock H, Velasquez N. 2005. Effect of natural and synthetic noise on evoked vocal responses in a frog of the temperate austral forest. Anim. Behav. 70, 639–651. ( 10.1016/j.anbehav.2004.11.022) [DOI] [Google Scholar]
- 30.McNett GD, Luan LH, Cocroft RB. 2010. Wind-induced noise alters signaler and receiver behavior in vibrational communication. Behav. Ecol. Sociobiol. 64, 2043–2051. ( 10.1007/s00265-010-1018-9) [DOI] [Google Scholar]
- 31.Francis CD, Ortega CP, Cruz A. 2009. Cumulative consequences of noise pollution: noise changes avian communities and species interactions. Curr. Biol. 19, 1415–1419. ( 10.1016/j.cub.2009.06.052) [DOI] [PubMed] [Google Scholar]
- 32.Bayne EM, Habib L, Boutin S. 2008. Impacts of chronic anthropogenic noise from energy-sector activity on abundance of songbirds in the boreal forest. Conserv. Biol. 22, 1186–1193. ( 10.1111/j.1523-1739.2008.00973.x) [DOI] [PubMed] [Google Scholar]
- 33.Arroyo-Solis A, Castillo JM, Figueroa E, Lopez-Sanchez JL, Slabbekoorn H. 2013. Experimental evidence for an impact of anthropogenic noise on dawn chorus timing in urban birds. J. Avian Biol. 44, 288–296. ( 10.1111/j.1600-048X.2012.05796.x) [DOI] [Google Scholar]
- 34.Kempenaers B, Borgstrom P, Loes P, Schlicht E, Valcu M. 2010. Artificial night lighting affects dawn song, extra-pair siring success, and lay date in songbirds. Curr. Biol. 20, 1735–1739. ( 10.1016/j.cub.2010.08.028) [DOI] [PubMed] [Google Scholar]
- 35.Higham J, Hebets E. 2013. An introduction to multimodal communication. Behav. Ecol. Sociobiol. 67, 1381–1388. ( 10.1007/s00265-013-1590-x) [DOI] [Google Scholar]
- 36.Partan S. 2013. Ten unanswered questions in multimodal communication. Behav. Ecol. Sociobiol. 67, 1523–1539. ( 10.1007/s00265-013-1565-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Narins PM, Hödl W, Grabul DS. 2003. Bimodal signal requisite for agonistic behavior in a dart-poison frog, Epipedobates femoralis. Proc. Natl Acad. Sci. USA 100, 577–580. ( 10.1073/pnas.0237165100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whalen C, Brown MB, McGee J, Powell LA, Smith JA, Walsh EJ. 2014. The acoustic characteristics of greater prairie-chicken vocalizations. J. Acoust. Soc. Am. 136, 2073 ( 10.1121/1.4899443) [DOI] [Google Scholar]
- 39.Taylor RC, Klein BA, Stein J, Ryan MJ. 2008. Faux frogs: multimodal signalling and the value of robotics in animal behaviour. Anim. Behav. 76, 1089–1097. ( 10.1016/j.anbehav.2008.01.031) [DOI] [Google Scholar]
- 40.Uetz GW, Roberts JA. 2002. Multisensory cues and multimodal communication in spiders: insights from video/audio playback studies. Brain Behav. Evol. 59, 222–230. ( 10.1159/000064909) [DOI] [PubMed] [Google Scholar]
- 41.Knudsen EI. 2002. Instructed learning in the auditory localization pathway of the barn owl. Nature 417, 322–328. ( 10.1038/417322a) [DOI] [PubMed] [Google Scholar]
- 42.Ben-Ari M, Inbar M. 2014. Aphids link different sensory modalities to accurately interpret ambiguous cues. Behav. Ecol. 25, 627–632. ( 10.1093/beheco/aru033) [DOI] [Google Scholar]
- 43.Halfwerk W, Jones P, Taylor R, Ryan M, Page R. 2014. Risky ripples allow bats and frogs to eavesdrop on a multisensory sexual display. Science 343, 413–416. ( 10.1126/science.1244812) [DOI] [PubMed] [Google Scholar]
- 44.Calvert G, Spence C, Stein BE. 2004. The handbook of multisensory processes. Cambridge, MA: MIT press. [Google Scholar]
- 45.McGurk H, Macdonald J. 1976. Hearing lips and seeing voices. Nature 264, 746–748. ( 10.1038/264746a0) [DOI] [PubMed] [Google Scholar]
- 46.Halfwerk W, Page RA, Taylor RC, Wilson PS, Ryan MJ. 2014. Crossmodal comparisons of signal components allow for relative distance assessment. Curr. Biol. 24, 1751–1755. ( 10.1016/j.cub.2014.05.068) [DOI] [PubMed] [Google Scholar]
- 47.Taylor RC, Ryan MJ. 2013. Interactions of multisensory components perceptually rescue Túngara frog mating signals. Science 341, 273–274. ( 10.1126/science.1237113) [DOI] [PubMed] [Google Scholar]
- 48.Rowe C, Guilford T. 1996. Hidden colour aversions in domestic clicks triggered by pyrazine odours of insect warning displays. Nature 383, 520–522. ( 10.1038/383520a0) [DOI] [Google Scholar]
- 49.Goyret J, Markwell PM, Raguso RA. 2007. The effect of decoupling olfactory and visual stimuli on the foraging behavior of Manduca sexta. J. Exp. Biol. 210, 1398–1405. ( 10.1242/jeb.02752) [DOI] [PubMed] [Google Scholar]
- 50.Preininger D, Boeckle M, Freudmann A, Starnberger I, Sztatecsny M, Hödl W. 2013. Multimodal signaling in the small torrent frog (Micrixalus saxicola) in a complex acoustic environment. Behav. Ecol. Sociobiol. 67, 1449–1456. ( 10.1007/s00265-013-1489-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gordon SD, Uetz GW. 2012. Environmental interference: impact of acoustic noise on seismic communication and mating success. Behav. Ecol. 23, 707–714. ( 10.1093/beheco/ars016) [DOI] [Google Scholar]
- 52.Tempel DJ, Gutiérrez R. 2004. Factors related to fecal corticosterone levels in California spotted owls: implications for assessing chronic stress. Conserv. Biol. 18, 538–547. ( 10.1111/j.1523-1739.2004.00372.x) [DOI] [Google Scholar]
- 53.Siemers BM, Schaub A. 2011. Hunting at the highway: traffic noise reduces foraging efficiency in acoustic predators. Proc. R. Soc. B 278, 1646–1652. ( 10.1098/rspb.2010.2262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goldbogen JA, et al. 2013. Blue whales respond to simulated mid-frequency military sonar. Proc. R. Soc. B 280, 20130657 ( 10.1098/rspb.2013.0657) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kunc HP, Lyons GN, Sigwart JD, McLaughlin KE, Houghton J. 2014. Anthropogenic noise affects behavior across sensory modalities. Am. Nat. 184, E93–E100. ( 10.1086/677545) [DOI] [PubMed] [Google Scholar]
- 56.Chan AAY-H, Giraldo-Perez P, Smith S, Blumstein DT. 2011. Anthropogenic noise affects risk assessment and attention: the distracted prey hypothesis. Biol. Lett. 6, 458–461. ( 10.1098/rsbl.2009.1081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Halfwerk W, Bot S, Buikx J, van der Velde M, Komdeur J, ten Cate C, Slabbekoorn H. 2011. Low songs lose potency in urban noise conditions. Proc. Natl Acad. Sci. USA 108, 14 549–14 554. ( 10.1073/pnas.1109091108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vasconcelos RO, Amorim MCP, Ladich F. 2007. Effects of ship noise on the detectability of communication signals in the Lusitanian toadfish. J. Exp. Biol. 210, 2104–2112. ( 10.1242/jeb.004317) [DOI] [PubMed] [Google Scholar]
- 59.Schmidt R, Morrison A, Kunc HP. 2014. Sexy voices–no choices: male song in noise fails to attract females. Anim. Behav. 94, 55–59. ( 10.1016/j.anbehav.2014.05.018) [DOI] [Google Scholar]
- 60.Stone EL, Jones G, Harris S. 2009. Street lighting disturbs commuting bats. Curr. Biol. 19, 1123–1127. ( 10.1016/j.cub.2009.05.058) [DOI] [PubMed] [Google Scholar]
- 61.Poot H, Ens BJ, de Vries H, Donners MA, Wernand MR, Marquenie JM. 2008. Green light for nocturnally migrating birds. Ecol. Soc. 13, 47. [Google Scholar]
- 62.Simpson SD, Purser J, Radford AN. 2014. Anthropogenic noise compromises antipredator behaviour in European eels. Glob. Change Biol. 21, 586–593. ( 10.1111/gcb.12685) [DOI] [PubMed] [Google Scholar]
- 63.Quinn JL, Whittingham MJ, Butler SJ, Cresswell W. 2006. Noise, predation risk compensation and vigilance in the chaffinch Fringilla coelebs. J. Avian Biol. 37, 601–608. ( 10.1111/j.2006.0908-8857.03781.x) [DOI] [Google Scholar]
- 64.Purser J, Radford AN. 2011. Acoustic noise induces attention shifts and reduces foraging performance in three-spined sticklebacks (Gasterosteus aculeatus). PLoS ONE 6, e17478 ( 10.1371/journal.pone.0017478) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Minnaar C, Boyles JG, Minnaar IA, Sole CL, McKechnie AE. 2014. Stacking the odds: light pollution may shift the balance in an ancient predator–prey arms race. J. Appl. Ecol. 52, 522–531. ( 10.1111/1365-2664.12381) [DOI] [Google Scholar]
- 66.Brumm H, Todt D. 2002. Noise-dependent song amplitude regulation in a territorial songbird. Anim. Behav. 63, 891–897. ( 10.1006/anbe.2001.1968) [DOI] [Google Scholar]
- 67.Halfwerk W, Slabbekoorn H. 2009. A behavioural mechanism explaining noise-dependent frequency use in urban birdsong. Anim. Behav. 78, 1301–1307. ( 10.1016/j.anbehav.2009.09.015) [DOI] [Google Scholar]
- 68.Gordon SD, Uetz GW. 2011. Multimodal communication of wolf spiders on different substrates: evidence for behavioural plasticity. Anim. Behav. 81, 367–375. ( 10.1016/j.anbehav.2010.11.003) [DOI] [Google Scholar]
- 69.Arellano-Aguilar O, Garcia CM. 2008. Exposure to pesticides impairs the expression of fish ornaments reducing the availability of attractive males. Proc. R. Soc. B 275, 1343–1351. ( 10.1098/rspb.2008.0163) [DOI] [PMC free article] [PubMed] [Google Scholar]