Abstract
Marine benthic communities face multiple anthropogenic pressures that compromise the future of some of the most biodiverse and functionally important ecosystems in the world. Yet one of the pressures these ecosystems face, night-time lighting, remains unstudied. Light is an important cue in guiding the settlement of invertebrate larvae, and altering natural regimes of nocturnal illumination could modify patterns of recruitment among sessile epifauna. We present the first evidence of night-time lighting changing the composition of temperate epifaunal marine invertebrate communities. Illuminating settlement surfaces with white light-emitting diode lighting at night, to levels experienced by these communities locally, both inhibited and encouraged the colonization of 39% of the taxa analysed, including three sessile and two mobile species. Our results indicate that ecological light pollution from coastal development, shipping and offshore infrastructure could be changing the composition of marine epifaunal communities.
Keywords: artificial light pollution, marine ecosystems, epifaunal communities, larval recruitment, anthropogenic disturbance, light-emitting diodes
1. Background
Assemblages of sessile marine benthic invertebrates act as engineers that support some of the world's most diverse ecosystems, sustain local fisheries, provide coastal protection and attract tourism [1]. Despite these important services, many such assemblages are threatened globally by multiple anthropogenic pressures including bottom fishing, coral bleaching, hypoxia and ocean acidification. Night-time artificial light represents an as yet unexamined disturbance that will probably alter the composition of sessile invertebrate assemblages by interfering with patterns of reproduction and recruitment among their constituent species [2]. The intensity, spectral composition and periodicity of natural light are important cues both for synchronizing the timing of broadcast spawning events [3,4] and in guiding larval recruitment into suitable habitats for post settlement survival and reproduction [5,6]. 22% of the world's coastal regions [2] (excluding Antarctica) are experiencing artificial light at night from a variety of sources, including coastal towns, harbours, offshore infrastructure in the form of oil, gas and renewable energy installations, shipping and light fisheries [2]. Where this artificial light is illuminating shallow benthic communities, it is likely giving rise to a range of unanticipated effects including sub-optimal settlement site selection and a consequent increase in post settlement mortality, and extending the time where light is available to guide the settlement process.
We investigated how nocturnal illumination by white light-emitting diodes (LEDs), a technology forecast to dominate the lighting industry by 2020 [7], influenced the colonization of sessile and mobile temperate invertebrates in newly available habitats. Our results indicate that colonization can be improved or hindered by white LED lighting at intensities encountered in the environment, hence night-time lighting has the potential to re-structure the composition of both existing and recovering assemblages by altering the recruitment of new individuals.
2. Material and methods
We quantified colonization of 36 previously bare 10 × 10 cm roughened grey PVC settlement panels over 12 weeks of deployment from 1 July 2013 on a floating raft in the Menai Strait, UK (53.229507° latitude; –4.153227° longitude). Panels were deployed vertically at 20 cm depth in pairs on 18 separate wooden boards, with each pair of panels treated as one treatment replicate in the analysis to avoid pseudoreplication. Each treatment pair was either not artificially lit (control), or lit to either 19 lux or 30 lux (measured using a ATP DT-1300 LUX meter) at the water's surface using cool white LEDs (n = 6 replicate boards per treatment). These lux levels were comparable to those found at the water's surface adjacent to nearby assemblages of epifaunal invertebrates exposed to night-time lighting (5–21.6 lux). The spectral power distribution of the make and model of cool white LED strips used is provided in Bennie et al. [8]. All lights were powered via a 12 V battery trickle charged using a solar panel (Sunware 24 W), and switched on at dawn and off at dusk using a CellOptick 12 V photocell. The boards were deployed vertically to simulate substrates that would be both suitable for colonization by temperate epifauna and exposed to artificial light (for example pier pilings, vertical rock faces, sea defences, floating pontoons, etc.), and randomly allocated across two rows of nine slots. Light trespass across treatments was avoided by facing panel-fronted boards in the same direction so that any stray light illuminated the back face rather than the experimental face of the neighbouring board (electronic supplementary material, figure S1). A separate sheet of grey PVC was used to guide the light down the experimental face of each board, minimizing light trespass onto adjacent boards (electronic supplementary material, figure S1). At the end of the colonization period, panels were brought back to the laboratory and preserved in 4% formalin pending analysis. The abundance of each taxon (identified to the lowest practicable resolution) was quantified as either the number of individuals, or percentage cover for colonial mat-forming taxa. The composition of the resulting communities was compared separately for percentage cover and numerical abundance data using multivariate analysis of variance (MANOVA, CRAN: Vegan) performed on Bray–Curtis dissimilarity matrices calculated from square root- and log(x + 1)-transformed data, respectively. Differences in numerical abundance and percentage cover were tested individually for each taxon. Percentage cover data were analysed using either a Gaussian generalized linear model (GLM) performed on fourth root-transformed data, or a quasi-binomial GLM performed on raw data where transformation failed to satisfy linear modelling assumptions. Numerical abundance data were fitted with Poisson and negative binomial GLMs, and zero-adjusted Poisson (ZAP) and negative binomial (ZANB) regression models (CRAN: pscl), with the most parsimonious model (that which displayed the lowest AIC) being selected. Individual tests were not performed on species recorded in less than half of the replicates as they were deemed to have occurred too infrequently to draw reliable conclusions using any of the above approaches. Prior to analysis, the paired panels in each replicate treatment board were summed for numerical abundance data and averaged for percentage cover data.
3. Results
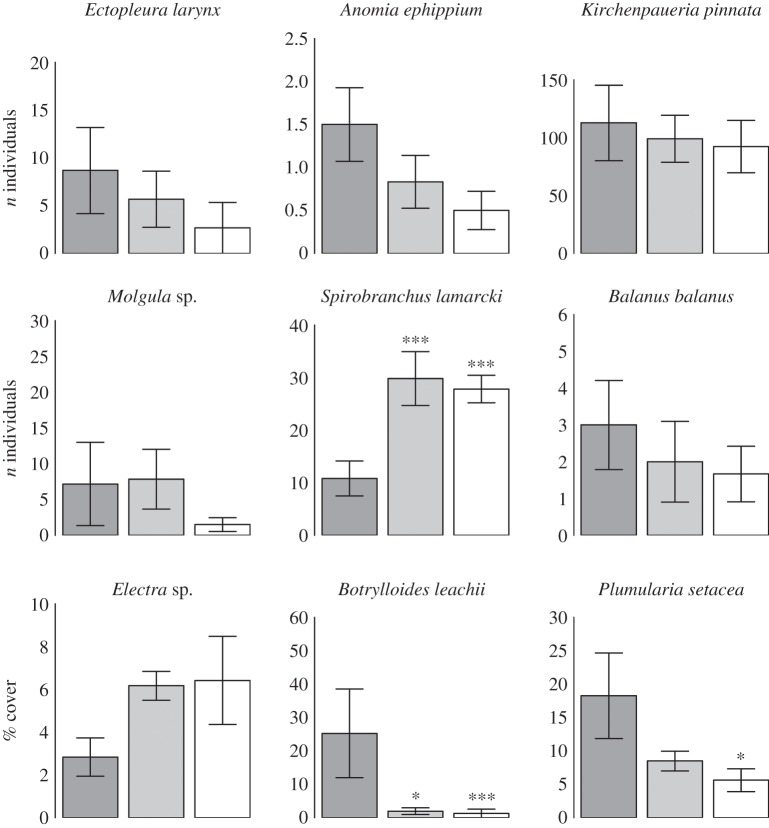
Forty-seven taxa representing seven phyla were identified on the settlement panels. Communities colonized under artificial light were significantly dissimilar to those colonized under control conditions (MANOVA: F2,15 = 2.85, p = 0.005) for taxa quantified using percentage cover. For taxa quantified using numerical abundance, light treatment had no impact on community composition (MANOVA: F2,15 = 1.21, p = 0.252), although this was driven by the influence of one outlying data point with unusually low species richness exerting leverage on the analysis (electronic supplementary material, figure S2). When this data point was omitted, light treatment had a significant impact on the composition of numerically enumerated taxa (MANOVA: F2,14 = 1.79, p = 0.018). This was further supported by independent tests performed on individual taxa (table 1 and figure 1). Of the 47 taxa identified, 13 were present in sufficient abundance for reliable estimates of the impact of LED lighting on abundance to be made. Of these, the abundances of three sessile and two mobile taxa were significantly affected by light treatment (table 1). Colonization by the colonial ascidian Botrylloides leachii was suppressed significantly by both light treatments (figure 1 and table 2), while the hydroid Plumularia setacea displayed significantly reduced colonization under the 30 lux treatment (figure 1 and table 2). By contrast, the abundance of the tube-building polychaete worm Spirobranchus lamarcki was significantly higher on panels colonized under both artificial light treatments (figure 1 and table 2), suggesting that white LED lighting encouraged its colonization. Among the mobile taxa, the abundances of the copepod Metis ignea and Corophium amphipods were significantly higher under the 30 lux treatment (figure 2 and table 2).
Table 1.
The effect of LED lighting on colonization by sessile and mobile benthic species. Significant results indicate that light treatment explained significantly more variation in taxon abundance when compared to a null intercept only model. n denotes data quantified as numerical abundance. % Data quantified as % cover. Species where colonization was significantly affected are in bold.
| higher classification | taxon | mobility | abundance | χ2 or F | p-value |
|---|---|---|---|---|---|
| Arthropoda | |||||
| Amphipoda | Corophium sp.c | mobile | n | 8.46 | 0.015 |
| Cirripedia | Balanus balanusd | sessile | n | 0.83 | 0.661 |
| Copepoda | Laophonte setosad | mobile | n | 0.73 | 0.695 |
| Metis ignead | mobile | n | 6.10 | 0.047 | |
| Ostracoda | Leptocythere pellucidac | mobile | n | 3.21 | 0.200 |
| Bryozoa | |||||
| Cheilostomatida | Electra sp.b | sessile | % | 2.50 | 0.115 |
| Chordata | |||||
| Ascidiacea | Botrylloides leachiia | sessile | % | 8.79 | 0.003 |
| Molgula sp.d | sessile | n | 3.01 | 0.222 | |
| Cnidaria | |||||
| Hydrozoa | Kirchenpaueria pinnatad | sessile | n | 0.27 | 0.875 |
| Plumularia setaceab | sessile | % | 3.68 | 0.050 | |
| Ectopleura larynxf | sessile | n | 5.13 | 0.275 | |
| Mollusca | |||||
| Bivalvia | Anomia ephippiumc | sessile | n | 3.26 | 0.196 |
| Polychaeta | |||||
| Serpulidae | Spirobranchus lamarckid | sessile | n | 19.45 | <0.001 |
aGaussian GLM on fourth root-transformed data.
bQuasi-binomial GLM on raw data.
cPoisson GLM.
dNegative binomial GLM.
eZAP.
fZANB.
Figure 1.
The impact of white LED lighting on the recruitment of sessile marine invertebrates. Dark grey bars are controls, light grey are 19 lux and open are 30 lux at the sea surface. Error bars represent standard errors. Significant differences between each light treatment and controls are denoted for 95% ‘*’, 99% ‘**’ or more than 99% ‘***’ confidence levels. Statistical output for species significantly affected by light treatment is given in table 2.
Table 2.
Differences in colonization between 19 and 30 lux LED lighting compared to controls. Summary results are presented from the models reported in table 1.
| 19 lux |
30 lux |
|||
|---|---|---|---|---|
| taxon | z or t | p-value | z or t | p-value |
| Plumularia setaceab | −1.82 | 0.090 | −2.39 | 0.030 |
| Spirobranchus lamarkid | 4.11 | <0.001 | 3.81 | <0.001 |
| Botrylloides leachiia | −2.45 | 0.027 | −4.17 | <0.001 |
| Metis ignead | −0.15 | 0.883 | 1.97 | 0.049 |
| Corophium sp.c | 0 | 1 | 2.08 | 0.038 |
aGaussian GLM on fourth root transformed data.
bQuasi-binomial GLM on raw data.
cPoisson GLM.
dNegative binomial GLM.
eZAP.
fZANB.
Figure 2.
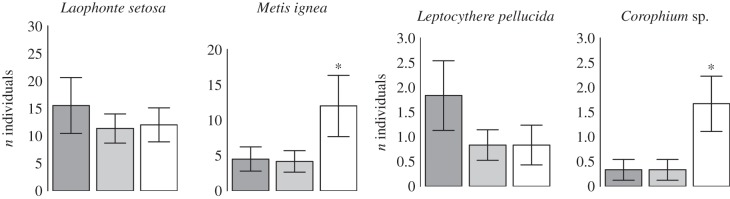
The impact of white LED lighting on colonization by mobile marine invertebrates. Dark grey bars are controls, light grey are 19 lux and open are 30 lux at the sea surface. Error bars represent standard errors. Significant differences between each light treatment and controls are denoted for 95% ‘*’, 99% ‘**’ or more than 99% ‘***’ confidence levels. Statistical output for species significantly affected by light treatment is given in table 2.
4. Discussion
Artificial light at night changes organism behaviour [9,10], re-structures communities [11] and alters trophic interactions [8] in terrestrial ecosystems. Night-time lighting is known to disrupt navigation, increase mortality and alter spatial and temporal activity patterns in marine birds, turtles and fish [12–14], but to our knowledge, the results presented here are the first evidence that it can affect the composition of marine communities. Although a limited number of species were affected in this study, a large proportion (72%) of the taxa colonizing our tiles were present in insufficient abundance to draw reliable conclusions about the impact of LED lighting on recruitment success. LED lighting significantly affected colonization by 39% of the taxa for which tests could be performed, suggesting potentially far-reaching impacts on epifaunal marine invertebrates and their associated mobile species.
Although novel, our results are perhaps unsurprising, given the importance of light in guiding recruitment to sessile invertebrate assemblages [5,6], and the role of this mechanism in optimizing post settlement survival. Light is a key factor structuring shallow marine benthic ecosystems both vertically and horizontally [15,16], and a plethora of studies have documented its importance for larval movement, orientation and recruitment over the twentieth century. The significant responses observed among sessile invertebrates here are consistent with the life-history traits of the species concerned [17–19].
The recent global surge in LED lighting is increasingly illuminating night-time environments with white light. While these lights hold the potential to reduce expenditure and CO2 emissions, their broad spectral output compared with traditional sodium-based technologies encompasses a greater range of wavelengths to which a variety of light-guided behaviours may be sensitive [20], including larval recruitment. LEDs are forecast to take over as the predominant light source in industrial, commercial, residential and architectural lighting applications by 2020 [7], and they are increasingly popular in shipping and the oil and gas industries. We conclude that such lights can alter the recruitment of sessile marine invertebrates, changing the composition of epifaunal communities. The consequences of night-time lighting for a broader range of marine ecosystems and the services they provide are unknown. The breadth of marine species for which light is an important ecological factor, and its role in guiding broadcast spawning, recruitment, diel vertical migration, communication, navigation and predator–prey interactions [2] suggest that widespread impacts of artificial light on the structure and function of marine ecosystems may already be occurring.
Supplementary Material
Supplementary Material
Supplementary Material
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Funding statement
T.W.D.'s employment is funded by the European Research Council under the European Union's Seventh Framework programme (FP7/2007-2013)/ERC grant agreement no. 268504.
Authors' contributions
T.W.D. and S.R.J. conceived the study; M.C., S.R.J. and K.M.G. conducted the data collection. T.W.D. performed the analysis and wrote the first draft. All authors contributed to revisions.
Conflict of interests
We have no competing interests.
References
- 1.Coleman FC, Williams SL. 2002. Overexploiting marine ecosystem engineers: potential consequences for biodiversity. Trends. Ecol. Evol. 17, 40–44. ( 10.1016/S0169-5347(01)02330-8) [DOI] [Google Scholar]
- 2.Davies TW, Duffy JP, Bennie J, Gaston KJ. 2014. The nature, extent, and ecological implications of marine light pollution. Front. Ecol. Environ. 12, 347–355. ( 10.1890/130281) [DOI] [Google Scholar]
- 3.Naylor E. 1999. Marine animal behaviour in relation to lunar phase. Earth Moon Planets 85–86, 291–302. ( 10.1023/a:1017088504226) [DOI] [Google Scholar]
- 4.Harrison PL. 2011. Sexual reproduction of scleractinian corals. In Coral reefs: an ecosystem in transition (eds Dubinsky Z, Stambler N, Harrison PL.), pp. 59–85. London, New York: Springer Science + Business Media. [Google Scholar]
- 5.Thorson G. 1964. Light as an ecological factor in the dispersal and settlement of larvae of marine bottom invertebrates. Ophelia 1, 167–208. ( 10.1080/00785326.1964.10416277) [DOI] [Google Scholar]
- 6.Mundy CN, Babcock RC. 1998. Role of light intensity and spectral quality in coral settlement: implications for depth-dependent settlement? J. Exp. Mar. Biol. Ecol. 223, 235–255. ( 10.1016/S0022-0981(97)00167-6) [DOI] [Google Scholar]
- 7.McKinsey & Company. 2012. Lighting the way: perspectives on the global lighting market, 2nd edn See https://www.mckinsey.com/∼/media/McKinsey/dotcom/client_service/Automotive%20and%20Assembly/Lighting_the_way_Perspectives_on_global_lighting_market_2012.ashx. [Google Scholar]
- 8.Bennie J, Davies TW, Cruse D, Inger R, Gaston KJ. 2015. Cascading effects of artificial light at night: resource-mediated control of herbivores in a grassland ecosystem. Phil. Trans. R. Soc. B 370, 20140131 ( 10.1098/rstb.2014.0131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stone EL, Jones G, Harris S. 2012. Conserving energy at a cost to biodiversity? Impacts of LED lighting on bats. Glob. Change Biol. 18, 2458–2465. ( 10.1111/j.1365-2486.2012.02705.x) [DOI] [Google Scholar]
- 10.Kempenaers B, Borgström P, Loës P, Schlicht E, Valcu M. 2010. Artificial night lighting affects dawn song, extra-pair siring success, and lay date in songbirds. Curr. Biol. 20, 1735–1739. ( 10.1016/j.cub.2010.08.028) [DOI] [PubMed] [Google Scholar]
- 11.Davies TW, Bennie J, Gaston KJ. 2012. Street lighting changes the composition of invertebrate communities. Biol. Lett. 8, 764–767. ( 10.1098/rsbl.2012.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Witherington BE, Bjorndal KA. 1991. Influences of artificial lighting on the seaward orientation of hatchling loggerhead turtles Caretta caretta. Biol. Conserv. 55, 139–149. ( 10.1016/0006-3207(91)90053-C) [DOI] [Google Scholar]
- 13.Merkel FR. 2010. Light-induced bird strikes on vessels in Southwest Greenland. Technical Report No. 84 Pinngortitaleriffik, Greenland: Greenland Insitutute of Natural Resources. [Google Scholar]
- 14.Becker A, Whitfield AK, Cowley PD, Järnegren J, Næsje TF. 2012. Potential effects of artificial light associated with anthropogenic infrastructure on the abundance and foraging behaviour of estuary-associated fishes. J. App. Ecol. 50, 43–50. ( 10.1111/1365-2664.12024) [DOI] [Google Scholar]
- 15.Vermeij MJA, Bak RPM. 2002. How are coral populations structured by light? Marine light regimes and the distribution of Madracis. M. Ecol.-Prog. Ser. 233, 105–116. ( 10.3354/meps233105) [DOI] [Google Scholar]
- 16.Miller RJ, Etter RJ. 2008. Shading facilitates sessile invertebrate dominance in the rocky subtidal Gulf of Maine. Ecology 89, 452–462. ( 10.1890/06-1099.1) [DOI] [PubMed] [Google Scholar]
- 17.Kupriyanova EK, Nishi E, Ten Hove HA, Rzhavsky AV. 2001. Life-history patterns in serpulimorph polychaetes: ecological and evolutionary perspectives. Oceanogr. Mar. Biol. 39, 1–101. [Google Scholar]
- 18.Svane I, Young CM. 1989. The ecology and behaviour of ascidian larvae. Oceanogr. Mar. Biol. 27, 45–90. [Google Scholar]
- 19.Meadows P, Reid A. 1966. The behaviour of Corophium volutator (Crustacea: Amphipoda). J. Zool. 150, 387–399. ( 10.1111/j.1469-7998.1966.tb03013.x) [DOI] [Google Scholar]
- 20.Davies TW, Bennie J, Inger R, de Ibarra NH, Gaston KJ. 2013. Artificial light pollution: are shifting spectral signatures changing the balance of species interactions? Glob. Change Biol. 19, 1417–1423. ( 10.1111/gcb.12166) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.