Abstract
The mechanisms that set the thermal limits to life remain uncertain. Classically, researchers thought that heating kills by disrupting the structures of proteins or membranes, but an alternative hypothesis focuses on the demand for oxygen relative to its supply. We evaluated this alternative hypothesis by comparing the lethal temperature for lizard embryos developing at oxygen concentrations of 10–30%. Embryos exposed to normoxia and hyperoxia survived to higher temperatures than those exposed to hypoxia, suggesting that oxygen limitation sets the thermal maximum. As all animals pass through an embryonic stage where respiratory and cardiovascular systems must develop, oxygen limitation may limit the thermal niches of terrestrial animals as well as aquatic ones.
Keywords: Sceloporus tristichus, critical thermal maximum, oxygen-limited thermal tolerance, temperature
1. Introduction
Why do animals die when they overheat? Until recently, biologists have suggested that heat kills by disrupting molecular structures, such as enzymes and membranes [1,2]. However, in bilaterian animals, heating might first disrupt the functions of cardiovascular and respiratory systems that supply cells with oxygen [2,3]. This oxygen-limitation hypothesis focuses on a fundamentally different scale from the classic hypothesis: organisms die because organ systems fail before cellular structures do. Thus, the oxygen-limitation hypothesis could explain why most animals die at temperatures below the functional limits of proteins or membranes and well below temperatures that cause denaturation ([2] and [4, pp. 410–417]).
The oxygen-limitation hypothesis initially gained support from studies of aquatic animals, but biologists have questioned whether the idea applies to terrestrial animals [5]. If warming causes oxygen demand to exceed supply, the lethal temperature should depend on factors limiting oxygen supply. Accordingly, a hypoxic environment should reduce the lethal temperature. Although this phenomenon has been documented for marine and freshwater animals [2,6,7], no effect of hypoxia on thermal limits has been observed in terrestrial insects [5,8]. Because the concentrations of oxygen are lower in water than in air, aquatic organisms may be more susceptible to oxygen reductions than terrestrial organisms [2,5]. However, terrestrial species still face aerobic challenges, especially when respiratory or cardiovascular systems are developing.
Here, we show that atmospheric oxygen concentration modifies the thermal limit of survival in embryonic plateau fence lizards, Sceloporus tristichus. This species occurs at elevations of 900–2750 m [9] and experiences oxygen supplies equivalent to hypoxia at sea level (15–19% oxygen). At moderate temperatures, Sceloporus embryos grow and develop successfully under severe hypoxia (8%) or hyperoxia (30%) [10]. At normoxia, they survive daily heating to temperatures below 42–44°C (O. Levy, C. D. Smith, D. Kumar, K. Boateng, M. J. Angilletta 2012–2013, unpublished data). By manipulating oxygen concentration and thermal stress, we show that S. tristichus embryos exposed to hypoxia succumb to reduced temperatures. Thus, constraints on oxygen supply during early-life stages can limit the thermal niches of terrestrial species.
2. Material and methods
(a). Lizard collection and general husbandry
Gravid females (N = 8) were collected from Navajo County, AZ (34.207786, −110.101056, elevation ≈ 1000 m) and transported to Arizona State University. Lizards were housed individually in plastic terraria (35 × 20 × 20 cm) with a 12 L : 12 D cycle. Ambient temperature was 33°C during photophase and 20°C during scotophase. Water was available at all times, and four to five crickets were offered as food daily. Females oviposited between 16 and 23 June 2014.
Prior to measures of thermal tolerance, embryos developed under normoxic conditions. Eggs were assigned to plastic containers (12.7 × 12.7 × 12.7 cm) in groups of four, with the constraint that only one egg per mother was assigned per container. Containers held 400 g of sand mixed with 4 g of water; this mixture yields a water potential of −10 kPa [11], which causes eggs to gain water linearly during incubation [12]. We placed egg containers in an incubator (DR-36VL; Percival Scientific, Perry, IA, USA) programmed to maintain 85% relative humidity and a diel cycle of temperature that mimics a natural nest (20.4°C to 34.7°C, mean = 27°C [13]). Containers were weighed weekly to replace evaporated water.
(b). Experimental protocol
After approximately 30 days (range = 27–34 days) or 45% of the mean incubation period [11], we randomly selected four eggs per clutch (N = 32) to measure the effect of atmospheric oxygen on thermal tolerance. To confirm that these eggs were alive, we measured cardiac activity with an infrared monitor (Avitronics, Cornwall, UK). Three eggs without cardiac activity were excluded. The remaining eggs were transferred to airtight chambers (Lock&Lock, Anaheim, CA, USA) in a second programmable incubator. Clutch mates were divided evenly among the chambers, resulting in three or four embryos per chamber. A ROXY-8 oxygen controller (Sable Systems International, Las Vegas, NV, USA) was used to set the concentration of oxygen in each chamber (10, 12, 15, 18, 21, 24, 27 and 30% ± 0.5%).
Eggs were positioned within chambers to maximize contact with the atmosphere while minimizing evaporative water loss. Each egg was placed on a moist piece of cotton in a plastic dish. To maintain high humidity, we placed an open vial with 50 ml of water in each chamber and dispersed an additional 10 ml on the floor of the chambers. Eggs maintained stable mass during the experiment (paired t-test of egg mass before versus after: t28 = 0.54, P = 0.59).
On the first day of the experiment, incubation chambers cycled between 20°C and 38°C, simulating diel fluctuations in natural nests [13] and well within the range of tolerable temperatures [14]. On subsequent days, the temperature cycled in a similar fashion, except that the maximal temperature increased by 1°C d−1. We placed iButton temperature loggers (Maxim Integrated, San Jose, CA, USA) inside the chambers to confirm these temperatures (figure 1). After each thermal cycle, cardiac activity was monitored to establish whether individuals had survived. Prior to returning embryos to their chambers, egg masses were measured and water was added to the cotton to offset evaporation. After nine thermal cycles, all embryos lacked signs of cardiac activity. To verify death, eggs were placed in their original incubation containers and observed for several weeks; embryos neither resumed cardiac activity nor completed development.
Figure 1.
During the experiment, embryos were exposed to higher maximal temperatures each day until all embryos were dead. Temperatures shown were measured within our chambers by data loggers.
(c). Statistical analyses
We compared linear, nonlinear and piecewise models of the relationship between oxygen concentration and lethal temperature. These analyses were conducted with the glm (linear and nonlinear models) or segmented (piecewise model) functions of the R Statistical Software (v. 3.1.1) [15,16]. For linear and quadratic models, we used a Gaussian distribution with the identity link function. For an asymptotic model (lethal temperature ∝ 1/oxygen concentration), we used a Gaussian distribution with an inverse link function, which produces the Michaelis–Menton model [17]. The piecewise model began with a linear fit and used an iterative approach to locate the most parsimonious break point (22.2% oxygen). The most likely model was determined by the Akaike information criterion (AIC). Early in our analyses, we included egg mass (fixed factor), maternal identity (random effect) and their interactions with oxygen concentration; however, these terms were ultimately excluded because they failed to reduce the AIC.
3. Results and discussion
As predicted by the oxygen-limitation hypothesis, embryos exposed to lower concentrations of oxygen died at lower temperatures (figure 2). The asymptotic model was twice as likely to best describe the relationship between oxygen concentration and lethal temperature as the quadratic model was, and three times more likely than the piecewise or linear models (table 1). Embryos exposed to normoxia (21%) survived to temperatures between 42°C and 44°C. Lethal temperature fell sharply with exposure to hypoxia, such that embryos exposed to 10–12% oxygen died between 39°C and 41°C. By contrast, hyperoxia had a small positive effect on lethal temperature; all but one embryo exposed to hyperoxia (23–30%) survived to at least 42°C and two embryos survived to 45°C. In previous studies of S. tristichus at normoxia, every embryo succumbed to temperatures below 45°C (O. Levy, C. D. Smith, D. Kumar, K. Boateng, M. J. Angilletta 2012–2013, unpublished data). The small positive effect of hyperoxia was supported by the higher likelihood of the asymptotic model, in which lethal temperature increases monotonically with increasing oxygen concentration.
Figure 2.
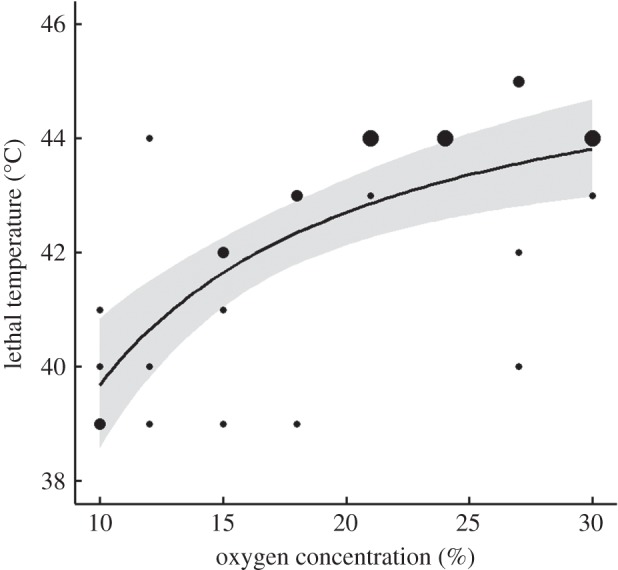
The lethal temperature of lizard embryos increased asymptotically with increasing oxygen. The solid line depicts the most likely model selected from a set of potential models, and the shaded region depicts the 95% confidence interval. Point size scales according to the number of overlapping observations (1–3).
Table 1.
An asymptotic function was more than twice as likely to be the best model of lethal temperature, as judged by the differences between AIC scores (ΔAIC) and the Akaike weights (w).
| model | parameter | d.f. | F | P | ΔAIC | w |
|---|---|---|---|---|---|---|
| asymptotic | oxygen | 1,27 | 24.5 | 0.00003 | 0 | 0.45 |
| quadratic | oxygen | 1,27 | 22.3 | 0.00007 | 1.4 | 0.22 |
| oxygen2 | 1,26 | 2.3 | 0.14 | |||
| linear | oxygen | 1,27 | 21.3 | 0.00009 | 1.9 | 0.17 |
| piecewise | oxygen ≤ 22.2% | 1,27 | 22.4 | 0.00007 | 2.1 | 0.15 |
| oxygen > 22.2% | 1,26 | 3.5 | 0.07 |
We interpret the strong effect of hypoxia on thermal tolerance compared with the weak effect of hyperoxia in two ways. First, hyperoxia might enable embryos to survive to temperatures that affect proteins or membranes, thus factors other than oxygen supply limited survival. However, this explanation seems implausible considering that most enzymes would not denature at 45°C and would regain performance upon cooling [2,4]. By contrast, the weak effect of hyperoxia makes sense when interpreted as a product of symmorphosis—the idea that structural design matches functional demand [18]. If a species never experiences oxygen concentrations above 21%, it should lack the cellular machinery to use additional oxygen (i.e. blood should be fully saturated at normoxia). Consistent with this interpretation, a previous experiment revealed that hyperoxia (30%) had no impact on growth and development in Sceloporus embryos, although hypoxia (8%) slowed growth and development relative to normoxia [10]. As these embryos were not exposed to thermal stress, structural changes in proteins or membranes could not have caused the nonlinear effect of oxygen supply on growth and development.
By focusing on the embryonic stage, we showed that oxygen supply can influence the thermal tolerance of terrestrial animals. Prior work with insects suggested that oxygen supply does not affect the thermal tolerance of terrestrial animals, potentially because air contains more oxygen than water [5]. Our study casts doubt on this simple dichotomy [8] and underscores the fact that each species' ability to acquire oxygen depends on its evolutionary history and developmental stage. Adult insects respire using efficient tracheal systems [19], but the embryos of oviparous amniotes possess a relatively poor respiratory system. These embryos rely on atmospheric oxygen diffusing across the shell and into the bloodstream of the chorioallantoic membrane. Adult insects should be less susceptible to hypoxia than embryonic reptiles, but even insects pass through an embryonic stage. Therefore, lethal temperatures of most animals should depend on oxygen supply at some stage of their life cycle. This interaction has important implications for ecology and evolution, especially given that climate warming pushes species to higher elevations with lower oxygen supplies [20].
Acknowledgements
We thank David Belohlavek and Angela Riley for laboratory assistance.
Ethics statement
Permission for this study was granted from the Arizona Department of Game and Fish (permit no. SP666234) and the Animal Care and Use Committee of Arizona State University (protocol no. 14–1338R).
Data accessibility
Data are available through Dryad (http://dx.doi.org/10.5061/dryad.1h7p0).
Funding statement
Research was supported by grants from the National Science Foundation to M.J.A. (EF-1065856) and L. B. Buckley (EF-1065638).
Authors' contributions
All authors contributed to study design, set-up, interpretation and revision, and approved the final draft. Data were primarily collected by C.S. and analysed by R.S.T. C.S. and R.S.T. co-wrote early drafts.
Conflict of interests
We have no competing interests.
References
- 1.Fields PA. 2001. Review: protein function at thermal extremes: balancing stability and flexibility. Comp. Biochem. Phys. A 129, 417–431. ( 10.1016/S1095-6433(00)00359-7) [DOI] [PubMed] [Google Scholar]
- 2.Pörtner HO. 2002. Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp. Biochem. Physiol. A 132, 739–761. ( 10.1016/S1095-6433(02)00045-4) [DOI] [PubMed] [Google Scholar]
- 3.Pörtner HO. 2001. Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften 88, 137–146. ( 10.1007/s001140100216) [DOI] [PubMed] [Google Scholar]
- 4.Hochachka PW, Somero GN. 1984. Biochemical adaptation. Princeton, NJ: Princeton University Press. [Google Scholar]
- 5.McCue MD, De Los Santos R. 2013. Upper thermal limits of insects are not the result of insufficient oxygen delivery. Physiol. Biochem. Zool. 86, 257–265. ( 10.1086/669932) [DOI] [PubMed] [Google Scholar]
- 6.Pörtner HO, Knust R. 2007. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315, 95–97. ( 10.1126/science.1135471) [DOI] [PubMed] [Google Scholar]
- 7.Verberk WCEP, Sommer U, Davidson RL, Viant MR. 2013. Anaerobic metabolism at thermal extremes: a metabolomic test of the oxygen limitation hypothesis in an aquatic insect. Integr. Comp. Biol. 53, 609–619. ( 10.1093/icb/ict015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klok CJ, Sinclair BJ, Chown SL. 2004. Upper thermal tolerance and oxygen limitation in terrestrial arthropods. J. Exp. Biol. 207, 2361–2370. ( 10.1242/jeb.01023) [DOI] [PubMed] [Google Scholar]
- 9.Persons TB, Leaché AD. 2009. Plateau fence lizard. In Lizards of the American Southwest: a photographic field guide (eds Jones LLC, Lovich RE.), pp. 254–257. Tuscon, AZ: Rio Nuevo Publishers. [Google Scholar]
- 10.Andrews RM. 2002. Low oxygen: a constraint on the evolution of viviparity in reptiles. Physiol. Biochem. Zool. 75, 145–154. ( 10.1086/339388) [DOI] [PubMed] [Google Scholar]
- 11.Oufiero CE, Angilletta MJ. 2006. Convergent evolution of embryonic growth and development in the eastern fence lizard (Sceloporus undulatus). Evolution 60, 1066–1075. ( 10.1111/j.0014-3820.2006.tb01183.x) [DOI] [PubMed] [Google Scholar]
- 12.Angilletta MJ, Winters RS, Dunham AE. 2000. Thermal effects on the energetics of lizard embryos: implications for hatchling phenotypes. Ecology 81, 2957–2968. ( 10.1890/0012-9658(2000)081[2957:Teoteo]2.0.Co;2) [DOI] [Google Scholar]
- 13.Angilletta MJ, Sears MW, Pringle RM. 2009. Spatial dynamics of nesting behavior: lizards shift microhabitats to construct nests with beneficial thermal properties. Ecology 2009, 2933–2939. ( 10.1890/08-2224.1) [DOI] [PubMed] [Google Scholar]
- 14.Angilletta MJ, Zelic MH, Adrian GJ, Hurliman AM, Smith CD. 2013. Heat tolerance during embryonic development has not diverged among populations of a widespread species (Sceloporus undulatus). Conserv. Phys. 1, 1–9. ( 10.1093/conphys/cot018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vito M, Muggeo R. 2008. segmented: an R package to fit regression models with broken-line relationships. R News 8, 20–25. [Google Scholar]
- 16.R Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 17.Ritz C, Streibig JC. 2008. Nonlinear regression with R. New York, NY: Springer Science+Business Media, LLC. [Google Scholar]
- 18.Weibel ER, Taylor CR, Hoppeler H. 1991. The concept of symmorphosis: a testable hypothesis of structure–function relationship. Proc. Natl Acad. Sci. USA 88, 10 357–10 361. ( 10.1073/pnas.88.22.10357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison JF, Kaiser A, VandenBrooks JM. 2010. Atmospheric oxygen level and the evolution of insect body size. Proc. R. Soc. B 277, 1937–1946. ( 10.1098/rspb.2010.0001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parmesan C, Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42. ( 10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available through Dryad (http://dx.doi.org/10.5061/dryad.1h7p0).


