Abstract
Fungus-farming ant colonies vary four to five orders of magnitude in size. They employ compounds from actinomycete bacteria and exocrine glands as antimicrobial agents. Atta colonies have millions of ants and are particularly relevant for understanding hygienic strategies as they have abandoned their ancestors' prime dependence on antibiotic-based biological control in favour of using metapleural gland (MG) chemical secretions. Atta MGs are unique in synthesizing large quantities of phenylacetic acid (PAA), a known but little investigated antimicrobial agent. We show that particularly the smallest workers greatly reduce germination rates of Escovopsis and Metarhizium spores after actively applying PAA to experimental infection targets in garden fragments and transferring the spores to the ants' infrabuccal cavities. In vitro assays further indicated that Escovopsis strains isolated from evolutionarily derived leaf-cutting ants are less sensitive to PAA than strains from phylogenetically more basal fungus-farming ants, consistent with the dynamics of an evolutionary arms race between virulence and control for Escovopsis, but not Metarhizium. Atta ants form larger colonies with more extreme caste differentiation relative to other attines, in societies characterized by an almost complete absence of reproductive conflicts. We hypothesize that these changes are associated with unique evolutionary innovations in chemical pest management that appear robust against selection pressure for resistance by specialized mycopathogens.
Keywords: mutualism, symbiosis, Attini, Escovopsis, actinomycetes, entomopathogens
1. Introduction
Larger social groups tend to have higher burdens of disease, requiring compensatory measures for prophylaxis and control [1–4]. Human social evolution has seen major increases in disease burden since the dawn of our evolutionary history. Diseases were particularly harmful during the Neolithic agricultural revolution [5] and subsequent urbanization [6], and have only been effectively countered by cultural evolution during the last two centuries [7,8]. The fungus-growing ants (Attini) live in agrarian societies. Like other social animals [5,9], they are under threat from various disease agents (e.g. [10]), but they have also had millions of years of evolutionary time to adapt via natural selection. Across genera and species, attine colony sizes vary from several dozen to millions of individuals and the extent of worker polymorphism increases with colony size [10–12], providing interesting opportunities to understand how disease management strategies covary with social complexity [13–15].
The fungus-growing ants have two obligate symbionts, the basidiomycete cultivar lineages on which they depend for nutrition, and the ascomycete cultivar pathogen, Escovopsis; neither symbiont has been found free-living, excepting cultivars from some basal attines [10–12]. To generally control infections, attines have evolved diverse prophylactic and public health tactics, which involve individual behaviours [13–17], collective behaviours [18] and an array of antimicrobial compounds [14,19,20]. Primary sources of these compounds are mutualistic microorganims, including Pseudonocardia and other actinomycete bacteria [19–23], and exocrine gland secretions, particularly from the metapleural glands (MGs) [14,15,24–26].
The paired MGs have clusters of secretory cells connected to a storage reservoir with a narrow opening [12,25–27]. Attine taxa differ in their reliance on bacteria-produced or glandular antimicrobials: species (or genera) with visible cuticular actinomycetes appear to apply MG secretions primarily to protect brood, while MG secretions are used more generally against fungus garden, brood and adult infections in ants that lack these bacteria [14,15]. The significance of MG-secretion use in disease control has largely been inferred from behavioural observations of MG grooming, combined with correlative data on pathogen growth inhibition ([14,15], but see [27–29]). The transfer of MG secretions to infected tissues has not been confirmed by target-specific chemical assays, and few studies have tested the efficacy of particular compounds as antibiotics [30]. In this study, we explicitly test the hypothesis that a single abundant component of MG secretion plays a key role in disease prophylaxis and infection control in Atta leaf-cutting ants.
In vivo, MG secretions of attine ants are known to suppress germination rates of conidia [14,30] and to increase survival of infected workers [31]. In vitro, conidia and mycelia of Escovopsis and other microorganisms show different sensitivity to an array of MG compounds of Acromyrmex leaf-cutting ants [30], but this cannot be expected to apply in the same way in Atta where workers have lost cuticular actinomycete cultures. In Atta cephalotes and Atta sexdens, phenylacetic acid (PAA) is known to be the primary component of MG secretions, accounting for more than 80% and 57.3% of the total mixture, respectively [32,33], whereas the MGs of Acromyrmex workers are not known to produce PAA [33], and MG secretions are rarely used for grooming during Escovopsis infections [14].
We hypothesized that fungal pathogens are sensitive to PAA secreted by Atta leaf-cutting ants, and that this form of chemical defence evolved to replace ancestral biological control via antibiotics derived from cuticular actinomycete cultures [14,20,28]. For PAA to have evolved as a targeted disease management adaptation against specialized garden mycopathogens, including Escovopsis, we expected ants to have a series of correlated behaviours to ensure that the use of PAA is specific and precise, so that the probability that resistance evolves remains as low as possible. We present in vivo and in vitro assays on the pharmacology of PAA in the MG secretion of A. cephalotes to test the extent to which this compound is instrumental in inhibiting Escovopsis, using other generalist pathogens as controls. We discuss the results of our study in relation to complementary information on disease control in fungus-growing ants in general, and Acromyrmex and other Atta leaf-cutting ants in particular. Finally, we address some of the evolutionary factors that may prevent resistance problems in chemical disease control by fungus-farming ants.
2. Material and methods
(a). Collections and hygienic response behaviours
For all experiments, we used subcolonies of A. cephalotes and 15 other attine ant species created from field-collected colonies taken from Soberania National Park or near Gamboa in central Panama between 2004 and 2010 (see the electronic supplementary material). Voucher specimens of the ants are deposited in the Museo de los Invertebrados, Universidad de Panamá. Subcolonies were used to monitor four specific disease defence behaviours: (i) fungal grooming, which involves ants ‘licking’ the garden surface to remove particles that are presumably contaminated [17]; (ii) MG grooming, which occurs when a worker extends her legs to raise the body from the substrate, and flexes a foreleg along the femoro-tibial joint to bring the posterior surface of the metatarsus into contact with the opening of one of the MGs; (iii) cultivar planting, which involves a worker cutting a piece of healthy fungus garden and transplanting it to an infected garden area to swamp pathogen growth with compensatory growth of the fungal mutualist; and (iv) weeding, which occurs when an ant removes a piece of garden and places it in a garbage dump [15,17]. Some prophylactic behaviours result in the accumulation of waste particles in the infrabuccal pockets of worker ants, which are then discarded in the dump [15,16].
Five colonies of A. cephalotes were collected to quantify prophylactic behaviour. From each colony, we established five subcolonies with 1.0 g of fungus garden and 20 medium-sized workers (headwidth (HW) = 1.2–1.6 mm). These subcolonies were subjected to one of four experimental treatments or were left unmanipulated as controls (cf. [28]). The treatments involved single inoculations with dry conidia from the insect pathogens Beauveria bassiana or Metarhizium brunneum, or from one of two strains of Escovopsis isolated from fungus gardens of A. cephalotes. Each experimental subcolony was inoculated with ca 1.5 × 106 dry conidia [14,15], and controls were sham-inoculated by rubbing a sterile piece of paper on the fungus garden. Subcolonies were observed for 60 min after inoculation, and the frequency of each prophylactic behaviour recorded. For fungal and MG grooming behaviours, which were relatively frequent, behavioural frequencies were then converted to rates (behaviours per minute) and analysed using a linear mixed model, with experimental treatment as a fixed main effect and colony as a random effect.
(b). Identification of phenylacetic acid in metapleural gland secretion and its distribution in gardens
We selected five small (HW ≈ 1.0 mm) and five large workers (HW > 1.8 mm) from each of five colonies. Workers with full MG reservoirs were selected by the visible presence of milky liquid on the MG bulla, the externally visible cover of the gland reservoir. A fine capillary tube was inserted through the meatus of the bulla, which allowed us to collect ca 0.5–1.0 µl of accumulated secretion from both MGs of each ant [33]. MG secretions of the five workers in each size class were pooled for each nest in a 0.5 ml vial, and dissolved in 20 µl of pentane. We injected 2 µl of each sample into a gas chromatograph–mass spectrometer (GC–MS) to confirm the presence and relative abundance of PAA in MG secretions. We identified PAA by comparison of the mass spectra, gas chromatographic retention indices and retention times with those of a pure reference sample (Sigma-Aldrich). Relative abundance was estimated from the peak area of PAA relative to the sum of all MG components. Full GC–MS protocols are given in the electronic supplementary material.
We confirmed that PAA is not naturally present in the ants’ basidiomycete fungal symbiont or in M. brunneum and B. bassiana cultures. We sampled fungal symbionts from pure cultivars isolated from three colonies of A. cephalotes, using nine samples of symbiont per colony, and 10 samples from pure cultivars of each pathogen. We collected ca 0.05 g of fungus from Petri dishes and placed these fragments directly in a vial with HPLC-grade pentane for GC–MS analyses. To determine whether PAA was present in the fungus gardens after MG grooming, we used three colonies of A. cephalotes, constructing 20 subcolonies with 1 g of fungus garden, 20 medium workers and six pupae from each colony. We inoculated the fungus gardens of 10 of these with ca 1.5 × 106 dry conidia of M. brunneum before adding the workers, and in the remaining 10 a sterile piece of paper was rubbed on the fungus garden as a control treatment before adding workers [14]. Three hours later, we froze the Petri dishes at −20°C for 20 min, and then placed 0.05 g of the fungus garden in a vial with solvent for GC–MS analyses.
(c). Phenylacetic acid transfer during metapleural gland grooming
To determine whether PAA is transferred from the ants' MG to infection targets in the garden, we compared extracts from the surface of the tarsi of workers from infected and uninfected A. cephalotes subcolonies using GC–MS. Twenty subcolonies from each of three A. cephalotes colonies were established with 15 medium workers and 1.0 g of fungus garden each (total subcolonies, N = 60). Subsequently, 30 subcolonies were used as unmanipulated controls and 30 other subcolonies were each inoculated with ca 2.5 × 106 dry conidia of M. brunneum. We increased the concentrations of conidia in this experiment to ensure that almost all ants groomed their MG after the infection [28]. Sixty minutes after infection, the subcolonies were transferred to a −20°C freezer for approximately 20 min to kill all ants. For each infected and control subcolony, we randomly selected six workers, removed their fore-, middle- and hind-legs and placed these in separate vials with pentane. After allowing to stand for 5 min, 2 µl of the supernatant was injected into the GC–MS to determine the presence of PAA. Presence–absence data were analysed using a contingency table.
To assess the quantitative transfer of PAA to pathogens, we created nine subcolonies from each of four A. cephalotes colonies, each one containing 1.0 g of fungus garden, three pupae and 30 randomly selected media workers. Three subcolonies were each infected with ca 1.5 × 106 dry conidia of M. brunneum, B. bassiana or Escovopsis, as described above. The Escovopsis used was derived from a separate colony of A. cephalotes collected from the same field site as the experimental colonies. After inoculation with fungal conidia, workers groomed the fungus garden so that detritus was accumulated in the infrabuccal pockets and subsequently discarded as infrabuccal pellets [15]. Three hours after inoculation, the subcolonies were frozen for 20 min after which 30 intact pellets were collected and pooled in a single vial that was stored at −20°C prior to chemical analysis. The chemical composition of pellets was assayed by placing the 30 pellets per treatment in 25 µl pentane, containing 0.001 µg µl−1 pentadecane as an internal standard, and allowed to stand for 5 min. We then injected 2 µl of this extract into the GC–MS and estimated absolute PAA concentration by comparison to the internal standard. Concentrations of PAA were compared using a linear mixed model with pathogen type (M. brunneum, B. bassiana or Escovopsis) as a fixed main effect, and colony, and colony by pathogen interaction as random effects. PAA concentrations were log-transformed to homogenize within-group variance.
(d). Inhibitory effects on pathogen spore germination in Atta
Eight colonies of A. cephalotes were used to quantify the inhibitory effect of MG secretions on germination rates of conidia that ant workers had accumulated in their infrabuccal pellets after infection. Four colonies were exposed to M. brunneum and four to Escovopsis isolated from a separate A. cephalotes colony from the same collection site. To assess whether ants reduced the viability of pathogenic conidia, we recorded the germination of infrabuccal pellets following infection for four HW size classes of ants: minims (HW less than 0.8 mm), minors (HW 1.0–1.2 mm), media (HW 1.2–1.6 mm) and majors (HW more than 1.8 mm). From each colony, we created 12 subcolonies (three replicates for each HW size class), each having 1 g of fungus garden, 20 workers, three larvae and three pupae, and exposed them to ca 2.5 × 106 dried conidia of M. brunneum or Escovopsis, a high enough dose for the ants to rapidly generate and discard infrabuccal pellets with fungal pathogen conidia.
Three hours after infection, all infrabuccal pellets deposited by ants were collected from the subcolonies with a sterile needle and plated on Petri dishes with potato dextrose agar (PDA, 19.5 g per 500 ml distilled water) [28]. Seventy-two hours later, we counted the number of pellets showing fungal germination consistent with the morphology of the pathogen used. Germination rates were compared using a generalized linear mixed model using binomial errors. Main effects were pathogen type (M. brunneum or Escovopsis), worker size class and their interaction. Colony was included as a random variable nested within pathogen type, because different colonies were treated with different pathogens.
(e). Comparative analyses of phenylacetic acid production and inhibitory efficiency across attine ants
To determine the distribution of PAA in MG secretions across the attine ants, we assayed 16 representative species: Mycocepurus smithii, Apterostigma collare, Ap. goniodes, Myrmicocrypta ednaella, Cyphomyrmex longiscapus, Trachymyrmex sp. 10, Trachymyrmex sp. 3, T. cornetzi, T. zeteki, Sericomyrmex amabilis, S.cf. amabilis, Acromyrmex echinatior, Ac. octospinosus, A. cephalotes, A. sexdens and A. colombica. Colonies were maintained in the laboratory for at least two weeks prior to experiments (see the electronic supplementary material). We sampled five nests for each species and created one subcolony from each of them with 0.5 g of fungus garden, three pupae and 20 workers. These subcolonies were infected with ca 1.5 × 106 dry conidia of M. brunneum and 4–7 h later infrabuccal pellets deposited by the workers were collected (N = 25 pellets per subcolony for leaf-cutting ants and N = 50 pellets from non-leaf-cutting species, to obtain a similar amount of pellet material). The pellets were placed in a vial and frozen at −20°C until GC–MS analyses, when we added 20 µl of pentane to each vial, and gently shaken until the pellet dissolved. For each sample, 2 µl of this solution was injected into the GC–MS. We used data from an earlier study [34] to compare the frequency of MG grooming with the presence of PAA in the infrabuccal pellets.
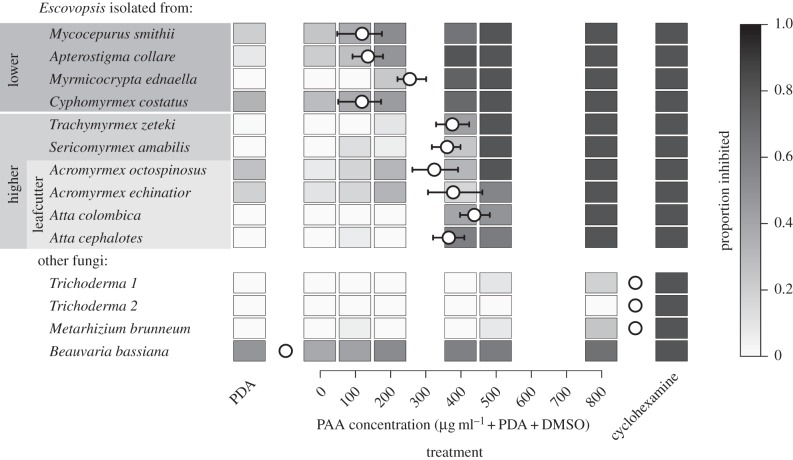
We measured the inhibitory effect of PAA on Escovopsis strains isolated from the gardens of 10 attine species, representing eight genera (Myc. smithii, Ap. pilosum, Myr. ednaella, C. costatus, S. amabilis, T. zeteki, Ac. echinatior, Ac. octospinosus, A. cephalotes and A. colombica). Macromorphology, conidia colour and growth rates on plates were used to distinguish Escovopsis morphotypes [35–38], which confirmed that we worked with a good representation of the overall phylogenetic diversity (electronic supplementary material, figure S1). In addition to Escovopsis, we also tested the sensitivity towards PAA of two generalist entomopathogens, M. brunneum, B. bassiana and two weedy Trichoderma contaminants collected from Atta fungus gardens (see the electronic supplementary material for mycological methods).
Using PDA as a growth medium in Petri dishes (15 × 60 mm), we added different concentrations (100, 200, 400, 500 and 800 µg ml−1) of analytical standard PAA (Sigma-Aldrich), using the polar aprotic solvent dimethyl sulfoxide (DMSO) as a carrier. Three days later, we inoculated an 8-mm diameter plug of pure culture from each fungus on three replicate dishes for each PAA concentration and added three controls (PDA only, PDA + DMSO and 100 µg ml−1 of the fungicide cycloheximide as a positive control). Ten days after treatment, we used a Nikon Coolpix L110 camera to photograph each dish to determine the extent of fungal growth, after which we calculated growth areas (cm2) using the ImageJ v. 1.37v software (following [39]) (electronic supplementary material, figure S1). The effect of PAA concentration on fungal growth was modelled by fitting a generalized linear model with binomial errors for each Escovopsis strain, based on the proportional cover out of the total available medium area (21 cm2). The PAA concentration at which the fungal growth was restricted to 50% of the dish area (ID50) was estimated by inverse prediction from the fitted models.
3. Results
(a). Phenylacetic acid as defensive compound to control infection with fungal pathogens
The frequency of fungal grooming and MG grooming by A. cephalotes workers was significantly different between the infection treatment groups and controls (one-way ANOVA, F4,16 = 8.71, p < 0.001 and F4,16 = 4.44, p = 0.013, respectively; electronic supplementary material, table S1), but these frequencies did not differ across the four types of pathogen challenges (Tukey post hoc tests p > 0.05). No differences across treatments were observed in either cultivar planting or weeding behaviours shortly after infection (both p > 0.5; electronic supplementary material, table S1).
PAA was present in all samples of A. cephalotes MG secretions collected (figure 1), but the collection procedure meant that many samples were contaminated with compounds typically found in haemolymph. The four samples without such contamination confirmed that PAA was the dominant compound in the MG secretion in both large (relative abundance: 54 and 88%) and small (78 and 81%) workers. PAA was also detected in all pellets produced by the four worker size classes. No PAA was detected in pure cultures of the fungal symbiont or any of the pathogens, but PAA was detected in a total of five out of 30 fungus gardens of subcolonies following infection with M. brunneum (electronic supplementary material, table S2). No PAA was detected on fore-, mid- or hind-legs prior to infection, and after infection PAA was found only on the forelegs (electronic supplementary material, table S2; N = 171, χ2 = 20.03, d.f. = 2, p < 0.0001) implying that worker ants transferred PAA to point sources of infection by grooming the MG opening with their forelegs and then contacting the target.
Figure 1.
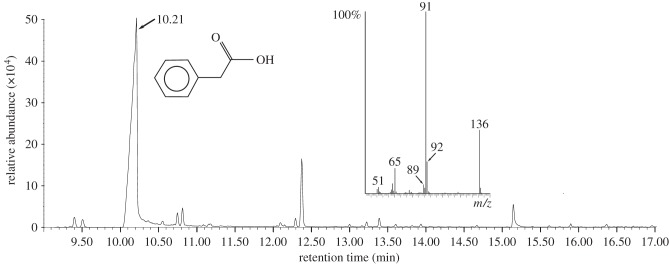
Typical gas chromatogram profile of the secretion of the metapleural gland of an A. cephalotes worker. The large peak at a retention time of 10.21 min was identified as PAA (structure shown to the right of the peak) based on its mass spectrum (inset).
Colonies did not differ significantly in their inhibitory effect against pathogens (random effect; Z = 1.55, p = 0.122) but there were clear differences among worker size classes (figure 2; F3,88 = 42.2, p < 0.001). The smallest workers more strongly inhibited conidia germination of pellets of both M. brunneum and Escovopsis sp. than medium and larger workers (figure 2). All four worker-size classes inhibited growth from Escovopsis pellets significantly more than growth from M. brunneum pellets (figure 2; F1,7 = 49.6, p < 0.001), with differences in inhibition efficiency across size classes also being highly significant (size class × fungus type interaction: F3,88 = 6.87, p < 0.001).
Figure 2.
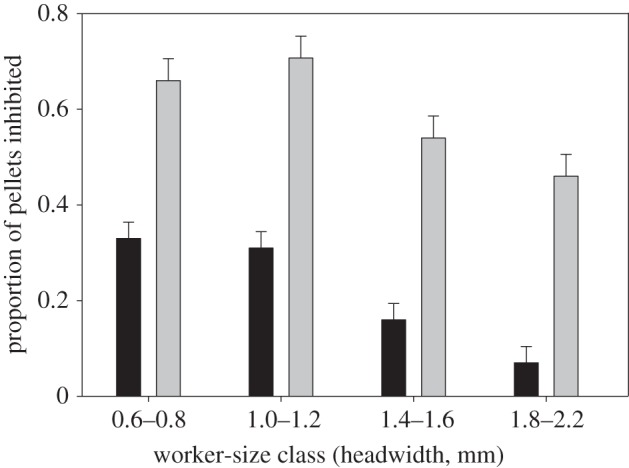
Use of PAA from metapleural glands of different worker size classes of A. cephalotes, showing the mean (+s.e.) proportion of infrabuccal pellets that did not germinate. Minim workers (HW = 0.6–0.8 mm) and minor workers (HW = 1.0–1.2 mm) more strongly inhibited germination of M. brunneum spores (black bars) than Escovopsis sp. (isolated from an A. cephalotes nest) (grey bars) relative to media (HW = 1.4–1.6 mm) and major workers (HW = 1.8–2.2 mm).
Medium-sized workers of A. cephalotes did not differ in the quantity of PAA transferred to pellets following infections with different pathogens (figure 3; F2,6 = 0.375, p = 0.702), and there was no effect of colony of origin (treated as a random effect) in this analysis (F3,6 = 0.576, p = 0.652), suggesting that colonies use PAA for a wide range of fungal infections. There was, however, a significant pathogen by colony interaction (F6,24 = 4.15, p = 0.005, figure 3), suggesting that colonies may respond differently to different fungal infections.
Figure 3.
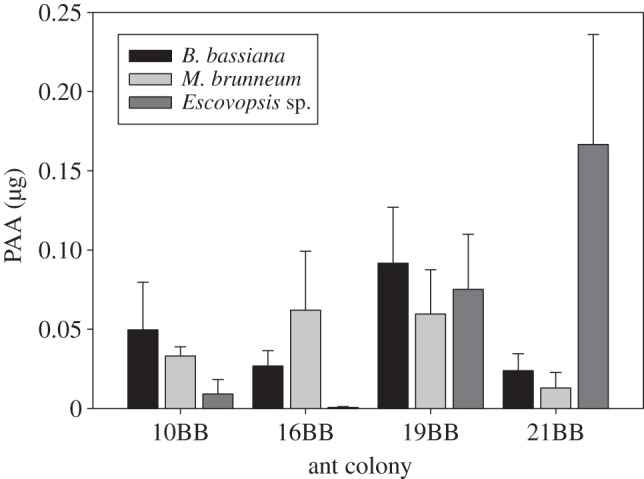
Mean quantity of PAA + s.e. extracted from infrabuccal pellets produced by A. cephalotes workers when challenged with three different fungal pathogens across four test colonies.
(b). Phenylacetic acid as an evolutionarily derived substance for chemical disease control in Atta
PAA was found in all fungal pellets deposited by workers of A. colombica, A. sexdens and A. cephalotes, but was never detected across a representative selection of sympatric attine species from seven other genera, including species such as Trachymyrmex sp. 10 and S. amabilis that are known to have high rates of MG grooming [14,15,34] (electronic supplementary material, table S3) and few if any actinomycete bacteria as alternative defence. PAA was also absent in two species of the sister leafcutter ant genus, Ac. echinatior and Ac. octospinosus, that have cuticular actinomycetes and intermediate rates of MG grooming when their nests are infected with M. brunneum (electronic supplementary material, table S3).
Bioassays showed that PAA inhibited the growth of Escovopsis morphotypes to a different extent. Morphotypes obtained from lower attine species were more sensitive (ID50; i.e. the concentration required for 50% inhibition: mean ± s.e. = 156.8 ± 32.8 μg ml−1), while morphotypes isolated from higher attines (including leaf-cutting ants) required higher concentrations for inhibition (figure 4: ID50 = 373.6 ± 15.1 μg ml−1; t8 = 6.76, p < 0.001). Within the higher attines, however, there was no significant difference between the sensitivity of morphotypes from leaf-cutting and non-leaf-cutting ants (t4 = −0.235, p = 0.826). No Escovopsis morphotype grew at PAA concentrations of 800 μg ml−1, but the entomopathogen M. brunneum and the two strains of Trichoderma showed less than 50% inhibition at this high concentration. Beauveria bassiana showed generally low growth in the Petri dishes, but was rather insensitive to PAA concentration (range of inhibition always 50–85% across the 0–800 μg ml−1 range).
Figure 4.
Inhibitory effects of PAA on Escovopsis strains taken from the fungus gardens of different species of attine ants and on other more general fungal pathogens. Inhibition was measured as the proportion of the Petri dish area with PDA medium that remained free of Escovopsis growth after 10 days. Circles mark the estimated ID50 (concentration of PAA that restricted growth to 50% of the plate area) for each fungal strain, with error bars showing the 95% confidence limits of the estimate. Estimated ID50 values for non-Escovopsis fungi are either less than 0 or more than 800 µg ml−1. The attine ants from which Escovopsis strains were isolated are grouped according to the type of fungal agriculture they practice [10].
4. Discussion
This study documents the use of an antimicrobial agent by Atta leaf-cutting ants for controlling both specialized and generalized mycoparasites. Atta fungus-growing ants have evolved truly large-scale farming and ‘organismal’ colonies, characterized by extreme caste differentiation and almost complete absence of the typical reproductive conflicts that characterize many eusocial Hymenoptera [40,41]. Our results suggest that they have also evolved unique innovations in disease management. Advances in disease management have also characterized the cultural evolution of large-scale human societies [5], so it is of interest to explore the extent of analogy across these two domains of social evolution. We address how our study complements earlier work on leaf-cutting ants and their close relatives, what makes Atta disease management unique and how even the most sophisticated insect societies continue to face threats from rapidly evolving diseases.
(a). Unravelling the details of prophylactic and acute disease control in leaf-cutting ants
Results confirmed the hypothesis that PAA has antimicrobial activity [42], and that it is the most abundant MG compound in A. cephalotes [33]. PAA has previously been shown to be the most abundant MG compound in A. sexdens [24,33], and second most abundant in A. laevigata [25]. While it has previously been inferred that the less abundant organic acid Myrmicacin (3-hydroxydecanoic acid) is used to control fungus garden ‘weeds’ by Atta [32], we did not detect this compound in the secretions of A. cephalotes. We estimated that a medium-sized worker can produce 0.45 µg of PAA during an infection, which is considerably less than the 1.4 µg of PAA that was estimated to be produced by an ‘average sized’ A. sexdens worker [12]. Heuristically, using our lower value, a mature colony of A. cephalotes with 1 million medium workers could thus produce roughly 0.5 g of PAA. Future studies are needed to determine the effective in vivo dosage of PAA to inhibit the spread of Escovopsis and other pathogens within the garden, and whether this massive production helps explain why there is little evidence that Escovopsis regularly kills large colonies of any Atta species, even though chronic infections are widely prevalent [43]. Earlier studies have reported that PAA is only found in Atta, but not in other genera of attine ants [24,25,32,33,44], a result that we confirmed across a substantial selection of Panamanian attine ants. This study complements our earlier findings that Atta has adopted synthesized chemical disease control, in contrast to its sister taxon of leaf-cutting ants, Acromyrmex, which has maintained ancestral cuticular cultures of Pseudonocardia actinomycetes to control Escovopsis infections [14,45]. However, a shift from biological to chemical control of a specialized pathogen is not necessarily more sustainable [14,28], unless enhanced care and flexibility of use outweigh possible higher risks of resistance evolution over time.
The results of our study confirm that the cocktail of behavioural and chemical tools that Atta workers employ largely lives up to common-sense sustainability criteria [14,15]. The main active control component PAA appears to be dynamically transferred from the MGs to the forelegs of workers to infection targets of both insect and fungus garden pathogens, after which a series of other grooming behaviours ensures that infective particles accumulate in the infrabuccal pockets of (particularly small and medium size) workers, where they are killed prior to permanent removal from the nest. Our study thus provides experimental evidence that has so far been lacking [27,28] in showing that Atta workers can control conidia germination rates of specialized (Escovopsis strains) and generalized (B. bassiana and Metarhizium anisopliae) fungal pathogens, but that they are especially efficient in controlling different morphotypes of Escovopsis, the only known pathogen that specializes on attine fungus gardens. Due to this specialization, it is therefore most likely to show coevolutionary responses to increased control efficiency.
The in vivo experimental results confirmed that A. cephalotes workers increased their frequency of MG grooming when exposed to a challenge by fungal conidia, but that this increase was independent of the fungal species used, as observed in Acromyrmex when workers were inoculated with conidia of Metarhizium and Escovopsis [29], indicating that PAA is active against both specialized and generalist pathogens. Our finding that the amount of PAA transferred may be different for different colonies exposed to different pathogens suggests that Atta workers can adjust both the quantity and quality of their MG secretion in response to the type of disease challenge, as was recently shown for sympatric Acromyrmex [29]. Two studies have now shown that MG compounds and secretions from Acromyrmex species can reduce mycelial growth or inhibit germination of Escovopsis conidia [29,30], but Acromyrmex workers only occasionally target Escovopsis infections behaviourally with their MG secretions [14], consistent with their actinomycetes being the main control strategy [14,45]. Our in vitro assays further showed that the PAA concentrations required to control Escovopsis were lower than those needed to control other pathogens, consistent with the hypothesis that these MG secretions, and PAA in particular, are alternative functional defences that have replaced actinomycete-bacteria-produced antibiotics used by Acromyrmex and most sympatric Trachymyrmex to control Escovopsis [14,15,28].
(b). Can chemical pest control in large-scale ant farms meet resistance problems?
Atta and Acromyrmex both process live vegetation for fungus farming, and their alternative systems of chemical and biological pest control have likely been evolutionarily elaborated since the genera split about 10 Mya [10]. The hypothesis that Atta would not have been able to overtake Acromyrmex in terms of ecological footprint (and pest-ant status) unless it had evolved ways to prevent or severely limit the evolution of resistance against chemical pest control seems reasonable. Understanding how this was achieved is important, because of the analogies with human large-scale farming that is plagued by problems of resistance to pesticides after only a few decades of use [46]. It is interesting that higher concentrations of PAA are required to inhibit other pathogens (figure 4) with the exception of B. bassiana, which suggests that PAA synthesis could have evolved because Escovopsis is particularly vulnerable to this compound. It is also noteworthy that Escovopsis strains from lower attine species (i.e. more basal attine ant genera) were more susceptible to PAA (ID50 around 160 µg ml−1) than Escovopsis isolated from the gardens of higher attines (ID50 around 370 µg ml−1). This result agrees with previous studies of Escovopsis isolates from Atta showing that these strains are generally different from those found in non-leaf-cutting higher attine ants [36] and even more different from those normally found in lower attines [37,38]. Only Escovopsis strains from the basal attine genus Apterostigma are highly variable, with some strains being similar to those found in Atta [36–38].
Differential patterns of susceptibility and resistance are often inferred to be the result of an evolutionary arms race in which natural antagonists have achieved a dynamic tug-of-war equilibrium. As Atta and Acromyrmex are likely to share the same strains of Escovopsis [35] and rear very similar strains of the symbiotic cultivar [47,48], it is not surprising that Escovopsis strains isolated from Atta and Acromyrmex were equally sensitive to PAA. However, horizontal transmission of fungus garden strains between sympatric colonies of Atta and Acromyrmex does not appear to occur at our Panamanian study site [48], so that any horizontally transmitted Escovopsis strain parasitizing both Atta and Acromyrmex will face the challenge of having to cope with both actinomycete bacterial antibiotics and ant PAA across generations. This may make it harder for Escovopsis to evolve higher virulence and raises the question of why Escovopsis lineages do not seem to have evolved specificity for either Atta or Acromyrmex [35].
The PAA-based chemical control practices of Atta have many characteristics that may help to maintain sustainability even if there would be no sympatric Acromyrmex hosts to co-infect. First, the results of our present study show that PAA is never used prophylactically (electronic supplementary material, table S1), i.e. it is not detected in gardens or on the legs of ants unless there is an infection. Second, PAA application is extremely precisely targeted only to sources of infection and reinforced by elaborate complementary behaviours such as active weeding, manual grooming and concentrated treatment in the infrabuccal cavity (i.e. away from the garden) to reduce germination success of spores of pathogenic fungi. Remarkably, the infrabuccal pellet treatment was considerably less effective against Metarhizium, a generalist insect pathogen that will never evolve resistance [49], than against the specialist garden pathogen Escovopsis (figure 2). Although Escovopsis spores in deposited pellets can still partly germinate in vitro (figure 2), this would hardly be a selection factor for resistance unless these hyphae can find their way back to a fungus garden to sporulate, which seems highly unlikely as they will be unattractive for foraging workers. Further research in the dynamic interactions between Atta, Acromyrmex and their cultivar and Escovopsis symbionts will remain highly rewarding for understanding the general emergence or avoidance of resistance problems in farming symbioses.
(c). The comparative perspective of disease management in fungus-growing ants
Despite substantial progress, we lack sufficient comparative data to fully understand the parallel transitions in public health systems that co-occurred with evolutionary transitions in attine fungiculture and social organization [10,11,14,15,17]. We know that dramatic changes in the sources of antimicrobial compounds have occurred across the attine phylogenetic tree, such that actinomycete-bacteria-produced antimicrobials appear to be key for controlling Escovopsis in many species of Trachymyrmex and Acromyrmex, and in several genera of basal attine ants, while MG secretions fulfil this role in Atta, Sericomyrmex [14] and possibly in some Trachymyrmex species [15] that lack visible actinomycetes. However, the interactions among different antimicrobials are incompletely understood. For example, we do not know if other MG components, other exocrine products or bacterial metabolites may enhance the inhibitory effect of PAA or constrain responses due to trade-offs with other vital functions. Furthermore, information about the antimicrobial compounds produced by the cuticular actinomycetes associated with Acromyrmex and Trachymyrmex species is very scarce [21–23], limiting our understanding of the biocontrol system that ancestral Atta species abandoned when evolving PAA-based chemical control.
Finding that the smallest Atta workers have the greatest inhibitory effect on pellet germination is of interest. These tiny workers are actively recruited by larger workers to sites of infection [50], which suggests they have a special role in disease management, consistent with their MGs being disproportionately large compared with those of larger workers [51]. Comparative studies between attine and non-attine ants, and between leaf-cutting versus non-leaf-cutting ants, have shown that Atta and Acromyrmex both have significantly enlarged MGs across all worker castes [52,53]. Relative MG-sizes also vary between patrilines in the same colony of two Panamanian Acromyrmex species [54]. MG size is thus heritable in the sterile castes but not in queens, posing intriguing questions about trade-offs with other costly performance traits, both in general and for disease management in particular [54].
Relative abundance of visible actinomycetes on ants' exoskeletons [14] and MG-grooming are key traits, but we still know rather little about the pathogen pressure on leaf-cutting ant nests in the field ([20,28], but see [55]), seasonal variation in disease pressure and defence [56], and the possible roles of myrmecophilous arthropods in disease transmission or control [12]. Detection and cognition are also likely to be of key significance, as the smallest Atta worker castes invest more biomass in their brains relative to larger nest-mates [57,58], but it remains unknown whether they have a heightened sensitivity to cues indicating infection. Large workers of Ac. echinatior are better at discriminating between fungal symbiont strains than medium and small workers, consistent with division of labour in cognitive tasks across worker castes [59] and with disease removal tasks that require significant time and metabolic efforts being delegated to the small worker castes.
Finally, it is intriguing that PAA has evolved repeatedly as an antimycotic compound in microorganisms (e.g. [42,60]) and that some actinomycete bacteria of the genus Streptomyces also produce PAA [42] while other strains of this actinomycete genus have been isolated from the cuticle of Acromyrmex ants producing other antibiotics [22,23]. However, nothing is known about in vivo effective dosages of PAA when used as an antimycotic by attine ants, and little is known about the quantitative production of PAA by bacteria (e.g. [42,60]). In humans, PAA is a very potent compound that controls pathogens at concentrations of 2 µg ml−1 [60], further adding to the unresolved question why this compound is not more common among attines if it is so potent for fungal disease control.
Supplementary Material
Acknowledgements
Facilities and logistic support came from the Center for Social Evolution (CSE) and the CODICES programme at the Department of Biology, University of Copenhagen, and the Smithsonian Tropical Research Institute (STRI), and INDICASAT. We are grateful to the ANAM, Republic of Panama, for research and export permits.
Funding statement
Funding was provided by a STRI Tupper Postdoctoral fellowship, and a postdoctoral fellowship, SNI and Collaboration grant no. 09–038 from SENACYT to H.F.M., the Danish Natural Research Foundation (grant no. DNRF57 to J.J.B.) and STRI general research funds to W.T.W.
References
- 1.Anderson RM, May RM. 1979. Population biology of infection diseases. Part I. Nature 280, 361–367. ( 10.1038/280361a0) [DOI] [PubMed] [Google Scholar]
- 2.Schmid-Hempel P. 1998. Parasites in social insects. Princeton, NJ: Princeton University Press. [Google Scholar]
- 3.Cremer S, Sixt M. 2009. Analogies in the evolution of individual and social immunity. Phil. Trans. R. Soc. B 364, 129–142. ( 10.1098/rstb.2008.0166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strassmann JE. 1981. Parasitoids, predators, and group size in the paper wasp, Polistes exclamans. Ecology 62, 1225–1233. ( 10.2307/1937287) [DOI] [Google Scholar]
- 5.Harper K, Armelagos G. 2010. The changing disease-scape in the third epidemiological transition. Int. J. Environ. Res. Public Health 7, 675–697. ( 10.3390/ijerph7020675) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnes I, Duda A, Pybus OG, Thomas MG. 2010. Ancient urbanization predicts genetic resistance to tuberculosis. Evolution 65, 842–848. ( 10.1111/j.1558-5646.2010.01132.x) [DOI] [PubMed] [Google Scholar]
- 7.Alexander RD. 1974. The evolution of social behavior. Annu. Rev. Ecol. Syst. 5, 325–383. ( 10.1146/annurev.es.05.110174.001545) [DOI] [Google Scholar]
- 8.Hamilton WD. 1987. Kinship, recognition, disease, and intelligence: constraints of social evolution. In Animal societies: theories and facts (eds Itô Y, Brown JL, Kikkawa J.), p. 291 Tokyo, Japan: Japan Scientific Societies Press. [Google Scholar]
- 9.Wilson-Rich N, Spivak M, Fefferman NH, Starks PT. 2009. Genetic, individual and group facilitation of disease resistance in insect societies. Annu. Rev. Entomol. 54, 405–423. ( 10.1146/annurev.ento.53.103106.093301) [DOI] [PubMed] [Google Scholar]
- 10.Schultz TR, Brady SG. 2008. Major evolutionary transitions in ant agriculture. Proc. Natl Acad. Sci. USA 105, 5435–5440. ( 10.1073/pnas.0711024105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller UG, Rehner SA, Schultz TS. 1998. The evolution of agriculture in ants. Science 281, 2034–2038. ( 10.1126/science.281.5385.2034) [DOI] [PubMed] [Google Scholar]
- 12.Hölldobler B, Wilson EO. 1990. The ants. Cambridge, MA: Harvard University Press. [Google Scholar]
- 13.Morelos-Juárez C, Walker TN, Lopes JFS, Hughes WOH. 2010. Ant farmers practice proactive personal hygiene to protect their fungus crop. Curr. Biol. 20, R553–R554. ( 10.1016/j.cub.2010.04.047) [DOI] [PubMed] [Google Scholar]
- 14.Fernández-Marín H, Zimmerman JK, Nash DR, Boomsma JJ, Wcislo WT. 2009. Reduced biological control and enhanced chemical pest management in the evolution of fungus-farming in ants. Proc. R. Soc. B 276, 2263–2269. ( 10.1098/rspb.2009.0184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernández-Marín H, Bruner G, Gomez EB, Nash DR, Boomsma JJ, Wcislo WT. 2013. Dynamic disease management in Trachymyrmex fungus-growing ants (Attini: Formicidae). Am. Nat. 181, 571–582. ( 10.1086/669664) [DOI] [PubMed] [Google Scholar]
- 16.Bass M, Cherrett JM. 1994. The role of leaf-cutting ant workers (Hymenoptera: Formicidae) in fungus garden maintenance. Ecol. Entomol. 19, 215–220. ( 10.1111/j.1365-2311.1994.tb00412.x) [DOI] [Google Scholar]
- 17.Currie CR, Stuart AE. 2001. Weeding and grooming of pathogens in agriculture by ants. Proc. R. Soc. Lond. B 268, 1033–1039. ( 10.1098/rspb.2001.1605) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bot ANM, Currie CR, Hart AG, Boomsma JJ. 2001. Waste management in leaf-cutting ants. Ethol. Ecol. Evol. 13, 225–237. ( 10.1080/08927014.2001.9522772) [DOI] [Google Scholar]
- 19.Currie CR, Scott JA, Summerbell RC, Malloch D. 1999. Fungus-growing ants use antibiotic producing bacteria to control garden parasites. Nature 398, 701–704. ( 10.1038/19519) [DOI] [Google Scholar]
- 20.Sen R, Ishak HD, Estrada D, Dowd SD, Hong E, Mueller UG. 2009. Generalized antifungal activity and 454-screening of Pseudonocardia and Amycolatopsis bacteria in nests of fungus-growing ants. Proc. Natl Acad. Sci. USA 106, 17 805–17 810. ( 10.1073/pnas.0904827106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh D-C, Poulsen M, Currie CR, Clardy J. 2009. Dentigerumycin: a bacterial mediator of an ant–fungus symbiosis. Nat. Chem. Biol. 5, 391–393. ( 10.1038/nchembio.159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haeder S, Wirth R, Herz H, Spitellera D. 2009. Candicidin-producing Streptomyces support leaf-cutting ants to protect their fungus garden against the pathogenic fungus Escovopsis. Proc. Natl Acad. Sci. USA 106, 4742–4746. ( 10.1073/pnas.0812082106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schoenian I, Spiteller M, Ghaste M, Wirth R, Herz H, Spiteller D. 2011. Chemical basis of the synergism and antagonism in microbial communities in the nests of leaf-cutting ants. Proc. Natl Acad. Sci. USA 108, 1955–1960. ( 10.1073/pnas.1008441108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maschwitz U, Koob K, Schildknecht H. 1970. Ein beitrag zur funktion der metathoracaldrüse der ameisen. J. Insect Physiol. 16, 387–404. ( 10.1016/0022-1910(70)90180-0) [DOI] [Google Scholar]
- 25.Vieira AS, Morgan ED, Drijfhout FP, Camargo-Mathias MI. 2012. Chemical composition of metapleural gland secretions of fungus-growing and non-fungus-growing ants. J. Chem. Ecol. 38, 1289–1297. ( 10.1007/s10886-012-0185-8) [DOI] [PubMed] [Google Scholar]
- 26.Hölldobler B, Engel-Siegel H. 1985. On the metapleural gland in ants. Psyche 91, 201–224. ( 10.1155/1984/70141) [DOI] [Google Scholar]
- 27.Yek SH, Mueller UG. 2011. The metapleural gland of ants. Biol. Rev. 86, 774–791. ( 10.1111/j.1469-185X.2010.00170.x) [DOI] [PubMed] [Google Scholar]
- 28.Fernández-Marín H, Zimmerman JK, Rehner SA, Wcislo WT. 2006. Active use of the metapleural glands by ants in controlling fungal infection. Proc. R. Soc. B 273, 1689–1695. ( 10.1098/rspb.2006.3492) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yek SH, Nash DR, Jensen AB, Boomsma JJ. 2012. Regulation and specificity of antifungal metapleural gland secretion in leaf-cutting ants. Proc. R. Soc. B 279, 4215–4222. ( 10.1098/rspb.2012.1458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bot ANM, Orthius-Lechner D, Finster K, Maile R, Boomsma JJ. 2002. Variable sensitivity of fungi and bacteria to compounds produced by the metapleural glands of leaf-cutting ants. Insect Soc. 49, 363–370. ( 10.1007/PL00012660) [DOI] [Google Scholar]
- 31.Poulsen M, Bot ANM, Nielsen MG, Boomsma JJ. 2002. Experimental evidence for the cost and hygienic significance of the antibiotic metapleural gland secretion in leaf cutting ants. Behav. Ecol. Sociobiol. 52, 151–157. ( 10.1007/s00265-002-0489-8) [DOI] [Google Scholar]
- 32.Schildknecht H, Koob K. 1971. Myrmicacin, the first insect herbicide. Angew. Chem. Int. Ed. Engl. 10, 124–125. ( 10.1002/anie.197101241) [DOI] [PubMed] [Google Scholar]
- 33.do Nascimento RR, Schoeters E, Morgan ED, Billen J, Stradling D. 1996. Chemistry of metapleural gland secretions of three attine ants, Atta sexdens rubrupilosa, Atta cephalotes and Acromyrmex octospinosus (Hymenoptera: Formicidae). J. Chem. Ecol. 22, 987–1000. ( 10.1007/BF02029949) [DOI] [PubMed] [Google Scholar]
- 34.Armitage SAO, Fernández-Marín H, Wcislo WT, Boomsma JJ. 2012. Secondary evolution of cocoon-like protection? An evaluation of the possible adaptive function of fungal brood covering by attine ants. Evolution 66, 1966–1975. ( 10.1111/j.1558-5646.2011.01568.x) [DOI] [PubMed] [Google Scholar]
- 35.Taerum SJ, Cafaro MJ, Little AEF, Schultz TR, Currie CR. 2007. Low host–pathogen specificity in the leaf-cutting ant–microbe symbiosis. Proc. R. Soc. B 274, 1971–1978. ( 10.1098/rspb.2007.0431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerardo NM, Caldera EJ. 2007. Labile associations between fungus-growing ant cultivars and their garden pathogens. ISME 1, 373–384. ( 10.1038/ismej.2007.57) [DOI] [PubMed] [Google Scholar]
- 37.Masiulionis VE, Cabello MN, Seifert KA, Rodrigues A, Pagnocca FC. 2015. Escovopsis trichodermoides sp. nov., isolated from a nest of the lower attine ant Mycocepurus goeldii. Antonie Van Leeuwenhoek 107, 731–740. ( 10.1007/s10482-014-0367-1) [DOI] [PubMed] [Google Scholar]
- 38.Meirelles LA, Montoya QV, Solomon SE, Rodrigues A. 2015. New light on the systematics of fungi associated with attine ant gardens and the description of Escovopsis kreiselii sp. nov. PLoS ONE 10, e0112067 ( 10.1371/journal.pone.0112067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Bael SA, Fernández-Marín H, Valencia CM, Rojas EI, Wcislo WT, Herre AE. 2009. Two fungal symbioses collide: endophytic fungi are not welcome in leaf-cutting ant gardens. Proc. R. Soc. B 276, 2419–2426. ( 10.1098/rspb.2009.0196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dijkstra MB, Boomsma JJ. 2006. Are workers of Atta leafcutter ants capable of reproduction? Insectes Soc. 53, 136–140. ( 10.1007/s00040-005-0848-3) [DOI] [Google Scholar]
- 41.Boomsma JJ. 2013. Beyond promiscuity: mate-choice commitments in social breeding. Phil. Trans. R. Soc. B 368, 20120050 ( 10.1098/rstb.2012.0050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hwang BK, Lim SW, Kim BS, Yeop J, Lee JY, Moon SS. 2001. Isolation and in vivo and in vitro antifungal activity of phenylacetic acid and sodium phenylacetate from Streptomyces humidus . Appl. Environ. Microbiol. 67, 3739–3745. ( 10.1128/AEM.67.8.3739-3745.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Currie CR, Mueller UG, Malloch D. 1999. The agricultural pathology of ant fungus gardens. Proc. Natl Acad. Sci. USA 96, 7998–8002. ( 10.1073/pnas.96.14.7998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ortius-Lechner D, Maile R, Morgan ED, Boomsma JJ. 2000. Metapleural gland secretion of the leaf-cutter ant Acromyrmex octospinosus: new compounds and their functional significance. J. Chem. Ecol. 26, 1667–1683. ( 10.1023/A:1005543030518) [DOI] [Google Scholar]
- 45.Andersen SB, Hansen LH, Sapountzis P, Sørensen JS, Boomsma JJ. 2013. Specificity and stability of the Acromyrmex–Pseudonocardia symbiosis. Mol. Ecol. 22, 4307–4321. ( 10.1111/mec.12380) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davies J, Davies D. 2010. Origins and evolution of antibiotic resistance. Microb. Mol. Biol. Rev. 74, 417–433. ( 10.1128/MMBR.00016-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mueller UG, Mikheyev AS, Solomon SE, Cooper M. 2011. Frontier mutualism: co-evolutionary patterns at the northern range limit of the leafcutter ant–fungus symbiosis. Proc. R. Soc. B 278, 3050–3059. ( 10.1098/rspb.2011.0125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kooij PW. 2013. Fungal adaptations to mutualistic life with ants. PhD thesis, Copenhagen University, Copenhagen, Denmark. [Google Scholar]
- 49.Boomsma JJ, Jensen AB, Meyling NV, Eilenberg J. 2014. Evolutionary interaction networks of insect pathogenic fungi. Annu. Rev. Entomol. 59, 467–485. ( 10.1146/annurev-ento-011613-162054) [DOI] [PubMed] [Google Scholar]
- 50.Gerstner AT, Poulsen M, Currie CR. 2011. Recruitment of minor workers for defense against a specialized parasite of Atta leaf-cutting ant fungus gardens. Ethol. Ecol. Evol. 23, 61–75. ( 10.1080/03949370.2010.529828) [DOI] [Google Scholar]
- 51.Bot ANM, Boomsma JJ. 1996. Variable metapleural gland size-allometries in Acromyrmex leafcutter ants (Hymenoptera: Formicidae). J. Kansas Entomol. Soc. 69(Suppl.), 375–383. [Google Scholar]
- 52.Hughes WOH, Pagliarini R, Madsen HB, Dijkstra MB, Boomsma JJ. 2008. Antimicrobial defence shows an abrupt evolutionary transition in the fungus-growing ants. Evolution 62, 1252–1257. ( 10.1111/j.1558-5646.2008.00347.x) [DOI] [PubMed] [Google Scholar]
- 53.Vieira AS, Bueno OC, Camargo-Mathias MI. 2012. Morphophysiological differences between the metapleural glands of fungus-growing and non-fungus-growing ants (Hymenoptera, Formicidae). PLoS ONE 7, e43570 ( 10.1371/journal.pone.0043570) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hughes WOH, Bot ANM, Boomsma JJ. 2010. Caste-specific expression of genetic variation in the size of antibiotic-producing glands of leaf-cutting ants. Proc. R. Soc. B 277, 609–615. ( 10.1098/rspb.2009.1415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hughes WOH, Thomsen L, Eilenberg J, Boomsma JJ. 2004. Diversity of entomopathogenic fungi near leaf-cutting ant nests in a neotropical forest, with particular reference to Metarhizium anisopliae var. anisopliae. J. Inv. Pathol. 85, 46–53. ( 10.1016/j.jip.2003.12.005) [DOI] [PubMed] [Google Scholar]
- 56.Diehl E, Junquiera LK. 2001. Seasonal variations of metapleural secretion in the leaf-cutting ant Atta sexdens piriventris Santschi (Myrmicinae: Attini), and lack of fungicide effect on Beauveria bassiana (Bals.) Vuillemin. Neotrop. Entomol. 30, 517–522. ( 10.1590/S1519-566X2001000400002) [DOI] [Google Scholar]
- 57.Seid M, Castillo A, Wcislo WT. 2011. The allometry of brain miniaturization in ants. Brain Behav. Evol. 77, 5–13. ( 10.1159/000322530) [DOI] [PubMed] [Google Scholar]
- 58.Riveros AJ, Seid M, Wcislo WT. 2012. Evolution of brain size in class-based societies of fungus-growing ants (Attini). Anim. Behav. 83, 1043–1049. ( 10.1016/j.anbehav.2012.01.032) [DOI] [Google Scholar]
- 59.Ivens ABF, Nash DR, Poulsen M, Boomsma JJ. 2009. Caste-specific symbiont policing by workers of Acromyrmex fungus-growing ants. Behav. Ecol. 20, 378–384. ( 10.1093/beheco/arn150) [DOI] [Google Scholar]
- 60.Cueva C, et al. 2010. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 161, 372–382. ( 10.1016/j.resmic.2010.04.006) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.