Abstract
Many male animals have evolved exaggerated traits that they use in combat with rival males to gain access to females and secure their reproductive success. But some male animals invest in nuptial gifts that gains them access to females. Both these reproductive strategies are costly in that resources are needed to produce the weapon or nuptial gift. In closely related species where both weapons and nuptial gifts are present, little is known about the potential evolutionary trade-off faced by males that have these traits. In this study, we use dobsonflies (order Megaloptera, family Corydalidae, subfamily Corydalinae) to examine the presence and absence of enlarged male weapons versus nuptial gifts within and among species. Many dobsonfly species are sexually dimorphic, and males possess extremely enlarged mandibles that they use in battles, whereas in other species, males produce large nuptial gifts that increase female fecundity. In our study, we show that male accessory gland size strongly correlates with nuptial gift size and that when male weapons are large, nuptial gifts are small and vice versa. We mapped weapons and nuptial gifts onto a phylogeny we constructed of 57 species of dobsonflies. Our among-species comparison shows that large nuptial gift production evolved in many species of dobsonfly but is absent from those with exaggerated weapons. This pattern supports the potential explanation that the trade-off in resource allocation between weapons and nuptial gifts is important in driving the diversity of male mating strategies seen in the dobsonflies, whereas reduced male–male competition in the species producing large spermatophores could be an alternative explanation on their loss of male weapons. Our results shed new light on the evolutionary interplay of multiple sexually selected traits in animals.
Keywords: allometry, resource allocation, sexual dimorphism, sexual selection, nuptial gift
1. Introduction
Male weapons are known to be important in male–male competition over access to mates. From the exaggerated antlers of deer to the enlarged mandibles of stag beetles, these condition-dependent traits grow largest in the best conditioned or highest quality males [1]. In mating systems where male weapons have evolved, the degree of exaggeration is assumed to be costly to the individual to produce such that investment of resources into the exaggerated trait comes at the expense of the growth of other morphological structures [2,3]. For example, the relative sizes of weapons in rhinoceros beetles (horns) and stag beetles (mandibles) are negatively correlated with the relative size of wings [4,5] such that allocation of resources to weapons reduces the growth of wings. Another example is from the flour beetle Gnatocerus cornutus which has sexually dimorphic enlarged mandibles. Artificial selection for increased relative mandible size in males reduced relative wing size, whereas artificial selection for decreased relative mandible size resulted in increased relative wing size [6,7]. Importantly, these morphological trade-offs also resulted in functional costs as those males with the largest mandibles, but smallest wings had the lowest dispersal frequencies [7].
Sperm competition theory predicts that there should be evolutionary trade-offs between energetically expensive traits and testes [8]. Recent studies have indicated that the enlargement of weapons, such as horns, mandibles or the head, reduces the relative sizes of the genitalia and testes or the number of sperm transferred to the female at mating [3,7–10]. But, what about species that have multiple mating strategies including premating competition and female choice?
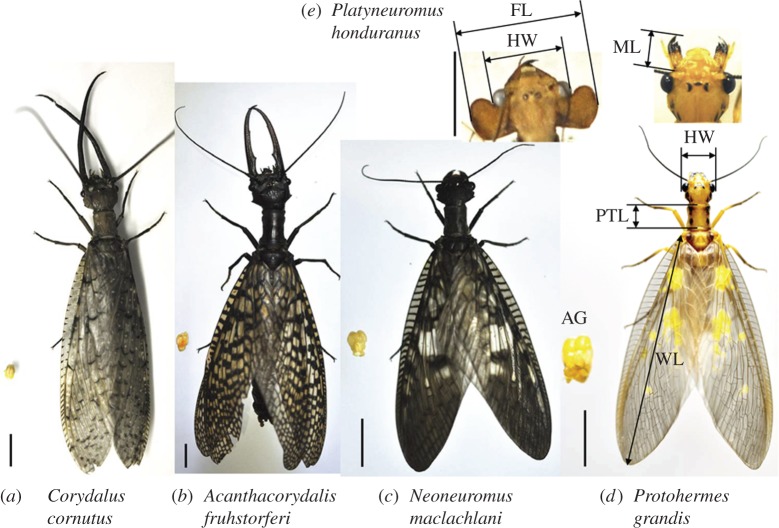
Dobsonflies (Corydalidae: Corydalinae) belong to the holometabolous insect order Megaloptera. Adults of many species of dobsonfly are very large with wingspans frequently over 150 mm, but probably their most conspicuous feature are their sexually dimorphic enlarged mandibles [11,12]. Darwin [13] noted that male dobsonflies in the genus Corydalus (figure 1a) have enlarged mandibles much like stag beetles as a weapon for attacking rival males before mating, which we also directly observed (electronic supplementary material, movie S1). The enlarged head flanges in males of the genus Platyneuromus (figure 1e) are another sexually dimorphic trait that is predicted to act as a male weapon although no observation on how flanges are used by fighting males is available. Thus, dobsonfly species exhibit a diversity of phenotypes from those that possess exaggerated mandibles such as males in the genera Corydalus and Acanthacorydalis (figure 1b) to males in the genus Platyneuromus which have enlarged flanges on their head (figure 1e) but possess normally sized mandibles (figure 1c,d) to those that are not sexually dimorphic for any trait [11].
Figure 1.
Male dobsonflies with large weapons have small accessory glands. (a) Corydalus cornutus, (b) Acanthacorydalis fruhstorferi, (c) Neoneuromus maclachlani, (d) Protohermes grandis and (e) Platyneuromus honduranus (only the male head is shown). Note that the species with elongated mandibles have smaller accessory glands. AG, accessory glands; FL, flange length; HW, head width; ML, mandible length; PTL, prothorax length; WL, forewing length. Scale bar, 10 mm.
Not only do male dobsonflies have exaggerated, sexually selected traits, in some species, they also produce nuptial gifts [14]. Nuptial gifts in the form of spermatophores are common among the Megaloptera with many species attaching a sperm packet to the female genitalia which account for approximately 0.5–4% of the male's total body weight [15,16]. However, Protohermes grandis which lacks enlarged mandibles produces a large, gelatinous spermatophore that they attach to the female genitalia during copulation [14]. These spermatophores are costly to produce as they comprise 10–20% of the male's total body weight [17]. These nuptial gifts, eaten by the female after mating, contribute valuable resources to her and are widespread among Japanese Protohermes species [17]. Females and males benefit from this gift, as female Pr. grandis exhibit increased lifetime fecundity with multiple mating, suggesting that female consumption of nuptial gifts positively influences reproductive output [18].
Dobsonflies are uniquely suited to test the developmental allocation trade-off between weapons and nuptial gifts because they appear to have experienced significant selection for investment into both traits. To the best of our knowledge, this is a characteristic that is unique to dobsonflies. As a first step towards understanding how these traits arise and are maintained, we looked for patterns of male investment in exaggerated mandibles and nuptial gifts across 57 species of dobsonfly in a phylogenetic framework. First, we experimentally confirmed nuptial gift giving in two species that exhibit extreme differences in nuptial gift size by fluorescently tracing the path of male spermatophores into female ovaries. We then conducted a comparative analysis to examine relative weapon size and spermatophore size to determine whether or not the resource allocation trade-off predicts the evolutionary diversification of enlarged mandibles and nuptial gift giving in these insects. Finally, we mapped enlarged male mandibles and nuptial gifts onto our 57 species dobsonfly phylogeny and show a pattern that investment is either into weapons or nuptial gifts but never both.
2. Results
(a). Large nuptial gifts contribute to egg production in dobsonflies
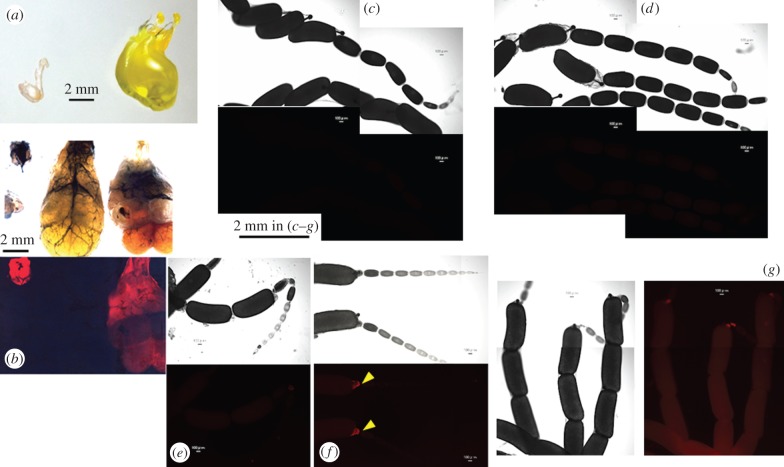
Rhodamine B, a fluorescent thiol-reactive dye that forms a stable covalent bond with proteins, has been used to determine the ultimate fate of components of spermatophores [19]. In our study, rhodamine B was fed to males, and its passage during mating to females was compared between Pr. grandis and Parachauliodes continentalis, two species of Corydalidae. Male Pr. grandis (subfamily Corydalinae) possessed large internal reproductive organs consisting of several accessory glands and a pair of seminal vesicles (figures 1d and 2b right) and transferred a large spermatophore with a yellowish gelatinous substrate to the mated female (figure 2a, right). The spermatophore was eaten by the female after mating (electronic supplementary material, figure S4f). By contrast, Pa. continentalis (subfamily Chauliodinae) had small internal reproductive organs (figure 2b, left) and transferred only a small sperm package to the mated female (figure 2a, left). Females of both species had immature eggs at emergence, which subsequently developed into mature eggs. The rhodamine-fed unmated females incorporated the dye into the developing eggs in the ovarioles (electronic supplementary material, figure S1); if fed with rhodamine later, after emergence, the dye was allocated more to the second row of eggs (electronic supplementary material, figure S1c,f). This independently confirmed that females use nutrients from their diet towards egg development.
Figure 2.
Variation in nuptial gift size (spermatophores) is observed to correspond to male accessory gland size, and this variation extends to the male contribution to egg production in Parachauliodes continentalis and Protohermes grandis. (a) A small spermatophore produced by male Pa. continentalis (left) and a large spermatophore with yellowish gelatinous substrate produced by male Pr. grandis (right). (b) Light (top) and fluorescence (bottom) photographs of male internal reproductive organs excluding the testes of rhodamine-free and rhodamine-fed Pa. continentalis (left two) and Pr. grandis (right two). Red fluorescence in rhodamine-fed males of each species. (c,d) Ovarioles of female Pa. continentalis mated once with rhodamine-free (control) males (c) and those mated once or three times with rhodamine-fed males (d). In both cases, no fluorescent signals were detected. (e–g) Ovarioles of female Pr. grandis mated once with rhodamine-free (control) males (e) and those mated once or three times with rhodamine-fed males (f,g). In (f), fluorescent signals were detected at the end of a row of mature eggs (indicated by arrowheads). In (g), the fluorescent signals were also detected in each egg of the row of mature eggs.
No significant fluorescent signals were detected in females of Pr. grandis (n = 3) mated once with rhodamine-free (control) males (figure 2e). However, females singly (n = 4) and multiply (n = 2) mated with rhodamine-fed males showed fluorescent signals at the end of the first row of eggs in the ovarioles (figure 2f). This part of the ovariole includes nurse cells involved in the transportation of nutrients for egg development [20]. Stronger fluorescence was obtained in the other two females multiply mated with rhodamine-fed males; a weak but significant red signal was seen in each egg of the first row as well as at the end of the row of mature eggs (figure 2g), suggesting that the male nutritional gift is more significant when females have mated multiple times. By contrast, the small spermatophores that male Pa. continentalis transferred to females were not detected in females singly (n = 4) or multiply (n = 3) mated with rhodamine-fed males (figure 2c,d).
(b). Accessory gland size corresponds with the size of the nuptial gift
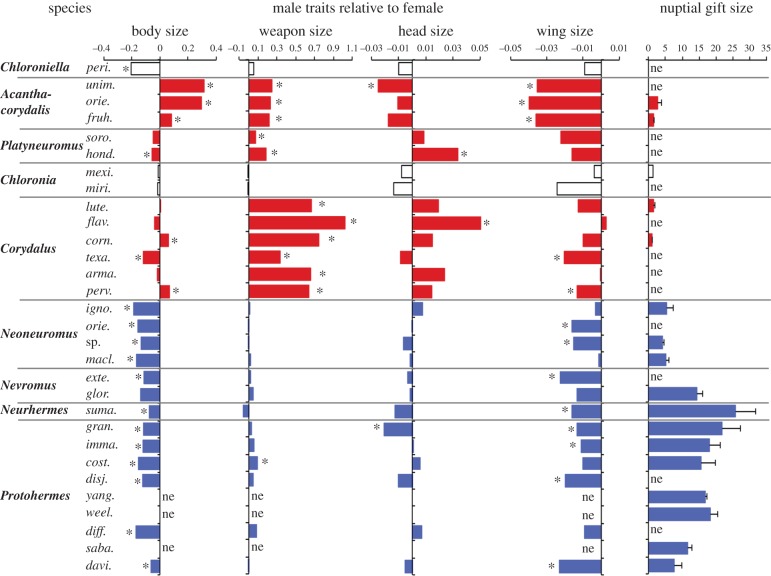
We measured the size of male internal reproductive organs relative to overall body size to predict nuptial gift size. We found that a fully charged accessory gland, indicated as a percentage of body weight, was very small in dobsonfly species of the genera Acanthacorydalis, Corydalus and Chloronia, whereas it was larger in species of the genera Neoneuromus, Nevromus, Neurhermes and Protohermes (figures 1 and 3). In order to estimate the average spermatophore size, the male internal reproductive organ size was measured for unmated (UM), just mated (JM) and recovering males (M), although the data were limited (electronic supplementary material, figure S3). The expected spermatophore size is thus large in species with large accessory glands (the differences between the values of UM and JM at the first copulation, and between the values of M and JM at the remating). To more fully explore this relationship, we observed spermatophores attached just after copulation of eight species that we could collect and maintain in the laboratory. We found that Nevromus gloriosoi, Neurhermes sumatrensis, Protohermes sabahensis, Protohermes costalis, Protohermes immaculatus and Pr. grandis had relatively large spermatophores (electronic supplementary material, figure S4) which corresponded to their large accessory glands relative to overall body size (electronic supplementary material, figure S3). In Corydalus cornutus and Corydalus luteus; however, only a small sperm package without a gelatinous mass was attached to the female genitalia, and it could hardly be seen from the outside even just after mating (electronic supplementary material, figure S4). We were not able to examine the accessory glands of Chloroniella (South Africa) or Platyneuromus (Central America) species because of their extremely narrow distribution in areas where we were unable to collect live insects. Our sample sizes were extremely low owing to the difficult nature of collecting mating pairs and observing matings in the laboratory; therefore, we report no data in this section besides the observational data that we show. Nevertheless, we speculate that because all appendages of the male genitalia of Chloroniella have been measured and found to be smaller and shorter than those of other genera [21], males of this genus probably transfer small spermatophores. In Platyneuromus, males are predicted to transfer a small spermatophore based on the simple male genital structure that has been reported [22]. In fact, the spermatophore has been reported to be invisible on the female abdominal terminalia just after mating in Platyneuromus soror providing further evidence of little to no nuptial gift giving in this genus [23]. Our data on male accessory gland size and relative spermatophore size indicate that nuptial gift donation is greater in Neoneuromus, Nevromus, Neurhermes and Protohermes than in the other genera (figure 3).
Figure 3.
Allometric relationships of traits in dobsonflies. Body, weapon, head and wing sizes were calculated as the relative difference between the male and female means: (male mean—female mean)/female mean. For weapon, head, and wing sizes, the adjusted means were calculated from the male and female regressions (see also the electronic supplementary material, figure S2, dataset S1). Asterisks indicated for the body size bars indicate a significant difference between the male and female means by Student's t-test (p < 0.05), and asterisks for all other traits indicate no overlaps in 95% confidence intervals between male and female adjusted means. Nuptial gift size is shown as the per cent accessory gland weight/body weight ratio (bars, s.d.) of the males, excluding those that had just mated (see the electronic supplementary material, figure S3, dataset S1). peri, peringueyi; unim, unimaculata; orie, orientalis; fruh, fruhstorferi; soro, soror; hond, honduranus; mexi, mexicana; miri, mirifica; lute, luteus; flav, flavicornis; corn, cornutus; texa, texanus; arma, armatus; perv, peruvianus; igno, ignobilis; sp, species; macl, maclachlani; exte, exterior; glor, gloriosoi; suma, sumatorensis; gran, grandis; imma, immaculatus; cost, costalis; disj, disjunctus; yang, yangi; weel, weelei; diff, differentialis; saba, sabahensis; and davi, davidi; ne indicates not examined.
(c). Dobsonfly weapons are disproportionately larger than other traits and have steep allometry slopes
Male mandibles/head flanges exhibited positive allometric slopes (1.62–4.24, significantly greater than 1 at p = 0.01 level) in two of three species of Acanthacorydalis and all species of Platyneuromus and Corydalus, but scaled proportionately in all other genera examined at p = 0.01 (dataset S1, electronic supplementary material, figure S2). In females, mandible length was isometric in all genera (dataset S1, electronic supplementary material, figure S2). Male mandibles/head flanges were thus relatively larger than female mandibles/head flanges at p = 0.05 level in Acanthacorydalis, Platyneuromus and Corydalus, but one exception was Pr. cosalis (figure 3). In these three genera with exaggerated traits, the adjusted mean of male mandible or head flange size was always significantly longer than in females (figure 3). In observations of mating with combinations of two males and one female, male Acanthacorydalis orientalis, C. cornutus and C. luteus used their mandibles to attack rival males, but never the female (electronic supplementary material, movie S1). Although the enlarged flanges in male Platyneuromus have not been examined with regard to their function, the observed positive allometries suggest that these organs are important for sexual selection.
(d). Body parts scale allometrically with body size in dobsonflies
Sexual dimorphism for body size was found in Acanthacorydalis and some Corydalus species (figure 3) in which males were significantly larger than females. Males in the other genera in this study were either similar in size or smaller than females of their own species.
Head width was a strong predictor of body size in both sexes, because the slope of the allometric regression to the prothorax length did not differ from 1 at p = 0.01 level in all species, excluding one exceptional C. cornutus in which a positive allometry suggests that male head size of this species is correlated with mandible size (dataset S1, electronic supplementary material, figure S2). In most species, the adjusted means of head width were similar between the sexes, except in Platyneuromus honduranus and Corydalus flavicornis where males had significantly larger heads than females and Acanthacorydalis unimaculata and Pr. grandis which had significantly smaller heads (figure 3).
Forewing length in many species of dobsonflies is sexually dimorphic. We found that forewing length exhibited a negative allometry slope in the three species of Acanthacorydalis, Pr. grandis and Pr. immaculatus (dataset S1, electronic supplementary material, figure S2). Forewing length was also shorter in males compared with females in most species of Acanthacorydalis, Corydalus, Neoneuromus, Nevromus, Neurhermes and Protohermes (figure 3). However, forewing length was not significantly different between the sexes in Chloroniella, Platyneuromus and Chloronia (figure 3). The difference in forewing size was significantly correlated with species that possessed either enlarged mandibles (Acanthacorydalis and Corydalus) or large nuptial gifts (Neoneuromus, Nevromus, Neurhermes and Protohermes) such that males that possessed either of these two traits has significantly shorter forewings than females (figure 3 and electronic supplementary material, figure S2).
(e). Evolution of male weapons and nuptial gifts
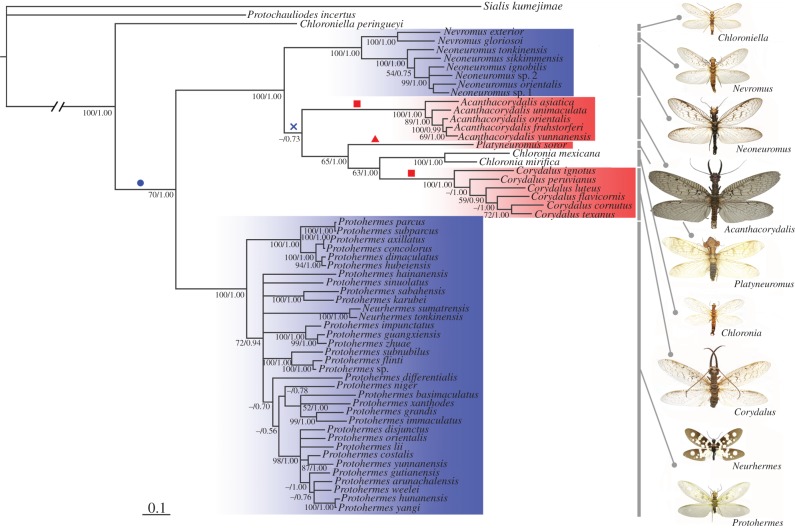
Parsimony and Bayesian phylogenetic analyses based on a combined dataset of 16S rRNA + COI + ND2 sequence data (for a total of 1862 bp) plus 71 morphological data revealed fundamentally consistent topologies, and the 50% majority-rule consensus tree from the Bayesian inference is presented in figure 4, with enlarged male weapons or nuptial gifts mapped onto the relevant lineages. The South African endemic genus Chloroniella, which represents the basal most dobsonfly in our study, has no weapons and is predicted to produce a small spermatophore. In the Asian genera Neurhermes and Protohermes (the Protohermes lineage), males are smaller than females and have no weapons as in Chloroniella, but produce a large spermatophore as a nuptial gift. The remaining genera consist of three lineages, the Neoneuromus lineage (Nevromus and Neoneuromus, endemic to Asia), the Acanthacorydalis lineage (Acanthacorydalis only, endemic to Asia) and the Corydalus lineage (Platyneuromus, Chloronia and Corydalus, endemic to the Americas). First, males of the Neoneuromus lineage have no weapons and have reduced body size, but they produce a relatively large nuptial gift. The Acanthacorydalis males show sexual dimorphism in body size and weapon size, but not in head size and their nuptial gift is small. In the Corydalus lineage, body size differences are small between the sexes. However, Platyneuromus males have exaggerated flanges and Corydalus males have exaggerated mandibles. The ancestral state reconstruction analyses we performed indicate that large male nuptial gifts were gained by most dobsonfly genera except for Chloroniella, but this trait was probably lost in the Acanthacorydalis and Corydalus lineages (figure 4 and the electronic supplementary material, figure S5a). In the latter lineages, enlarged male weapons have evolved in Acanthacorydalis (large mandibles), Platyneuromus (large head flanges) and Corydalus (large mandibles), but not in Chloronia (figure 4). Enlarged male mandibles were gained independently in Acanthacorydalis and Corydalus (electronic supplementary material, figure S5b) and enlargement of the head flanges was unique to Platyneuromus.
Figure 4.
Phylogeny of 57 dobsonflies (family Corydalidae, subfamily Corydalinae) based on the combined molecular + morphological data inferred from Bayesian analysis. This topology is congruent with the parsimony topology. Bootstrap supports ≥50 and Bayesian posterior probabilities ≥0.50 are given at each corresponding node. Besides Chloroniella, four major lineages are recovered, comprising the Protohermes lineage (Protohermes and Neurhermes), the Neoneuromus lineage (Nevromus and Neoneuromus), the Acanthacorydalis lineage (Acanthacorydalis only) and the Corydalus lineage (Platyneuromus, Chloronia and Corydalus). Lineages marked red exhibit male weapons and lineages marked blue produce large nuptial gifts. Evolutionary gains of large nuptial gifts (blue circle) and male enlarged mandibles (red square) across lineages, as well as secondary loss of large nuptial gifts (blue cross), are shown as supported by ancestral state reconstruction (electronic supplementary material, figure S5). Enlargement in male head flanges (red triangle) is unique to Platyneuromus. The outgroup taxa used here are the fishfly Protochauliodes incertus (family Corydalidae, subfamily Chauliodinae) and the alderfly Sialis kumejimae (family Sialidae) of the same Order Megaloptera.
3. Discussion
The phylogeny of the worldwide dobsonfly genera that we constructed combining molecular + morphological data is congruent with the latest morphological phylogeny [24], in which the South African endemic genus Chloroniella was assigned as a sister group with all the other dobsonfly genera. We also found that monophyly of the Protohermes lineage, the Neoneuromus lineage and the Corydalus lineage was well supported here as in the morphological phylogeny. However, Acanthacorydalis was placed as a sister lineage to the Corydalus lineage. Within the Corydalus lineage, Corydalus was assigned as a sister group of Chloronia, as in all former morphological phylogenies of Corydalinae [11,25], although Corydalus and Platyneuromus formed a weakly supported monophyletic clade in the morphological phylogeny of this subfamily reconstructed by Contreras-Ramos [24]. Within the Protohermes lineage, Protohermes was paraphyletic with Neurhermes, reducing support for its generic status and instead attributing it as a synonym of Protohermes.
The outgroup taxa of dobsonflies are alderflies (Sialidae) and fishflies (Corydalidae: Chauliodinae). In all species of alderflies and fishflies in which the mating behaviours have been examined to date, males transfer a spermatophore consisting of only the sperm package to the female and their internal reproductive organs are very small [15,16]. As confirmed in the fishfly Pa. continentalis in this study, their small sperm packages seem to contribute little nutrition to the mated female, even though the package is partially consumed by the female after mating. Thus, transfer of a small sperm package is the most parsimonious ancestral state, with the production and transfer of a large nutritious spermatophore as a derived character state, which is also supported by our ancestral state reconstruction analysis (electronic supplementary material, figure S5a). A large nutritional spermatophore is present in the Protohermes and Neoneuromus lineages, but is absent in the Acanthacorydalis and Corydalus lineages.
Sexual dimorphism in mandible and head shape are also derived trait states based on this phylogeny, as none of the outgroup taxa possess these [12,26]. Our phylogeny supports the independent evolution of male weapons in the dobsonfly lineage at least three times—in Acanthacorydalis, Corydalus and Platyneuromus. Alternatively, two instances of weapon development may have occurred in the Acanthacorydalis and Corydalus lineages (electronic supplementary material, figure S5b); however, Platyneuromus developed head flanges, whereas Corydalus developed mandibles, suggesting independent acquisition of such enlargement of male traits. The well-developed male mandible shapes differ between Acanthacorydalis and Corydalus. Acanthacorydalis males have mandibles with small teeth, but Corydalus males have cylindrical mandibles that lack teeth [12,25]. These weapons of male Acanthacorydalis, Corydalus and Platyneuromus are suggested to be sexually selected traits via male–male combat for mates. Indeed, the allometry between male body size and weapon size is positive (significantly greater than 1), and the adjusted means of male mandible or head flange sizes were always significantly larger than those of females.
In this study, we show that males in different dobsonfly lineages exhibit differences in the relative sizes of two different sexually selected traits—weapons used in male–male competition and nuptial gifts important for male reproductive success. The dobsonfly genera with enlarged weapons (i.e. Acanthacorydalis, Corydalus and Platyneuromus) tended to have relatively small nuptial gifts, whereas those without any sexual dimorphism in mandible shape (i.e. Neoneuromus, Nevromus, Neurhermes and Protohermes) tended to have relatively large nuptial gifts (figure 3). These observations support the hypothesis that competition in resource allocation exists between these two traits in dobsonfly species. In Chloroniella and Chloronia, males have no enlarged weapons and nuptial gifts. The former is the sister genus to all other dobsonfly genera and possesses neither weapons nor nuptial gifts which is the ancestral state reconstructed for dobsonflies. However, Chloronia belongs to the Corydalus lineage and may have secondarily lost large nuptial gifts and did not evolve weapons. To the best of our knowledge, no dobsonfly species has been described that exhibits both exaggerated weapons and large nuptial gifts. Our study relies on field collected individuals and not laboratory studies of resource allocation; therefore, the association between these two traits is based on correlations and phylogenetic patterns and not on experimental manipulation of individuals. Finally, it is important to note that our study has relatively limited phylogenetic power, because we have evidence of relatively few gains and losses, despite the fact that we have analysed all of the genera and most of the species of this insect group that are described in the world.
Nuptial gift giving by male insects' trade-off with other sexually selected traits involved in acquiring matings, such as mate calling. In several bush cricket species, the male feeds his mate with a large gelatinous mass known as a spermatophylax that attaches to the sperm package, whereas others produce only the sperm package [27]. The effects of costly spermatophore production on other male traits associated with mate acquisition have been examined in relation to the energetically expensive calling behaviour of the bush crickets. Interspecific comparisons of these two costly male traits indicated that a trade-off exists between spermatophore size and call frequency (measured as carrier frequency) [28]. There is also evidence of a trade-off in the spermatophore and mate signalling in fireflies. Firefly males transfer a spermatophore to the mated female, which is then digested and used for somatic maintenance and egg development in females [29], and functions as a nuptial gift. In the firefly Photinus greeni, a trade-off has been proposed for males to allocate energy between nuptial gift investment and bioluminescent courtship signalling to attract females [30].
Another interesting trade-off that we found was in male wing size. In insects, body size and trait growth results from physiological mechanisms that link trait growth to nutrition. Thus, male dobsonflies have a general tendency towards shorter wings than conspecific females, but significant shortening of wing size occurred in males of the groups with enlarged weapons or nuptial gifts. These observations suggest a trade-off in male dobsonflies between sexual (weapons and nuptial gifts) and non-sexual (wing) traits. Wing shortening according to weapon development has often been reported in male beetles with elongated horns and mandibles [4–7]. Traits of beetles with nutrition-sensitive patterns of growth have steeper allometric slopes than traits with nutrition-insensitive patterns of growth [1,31,32]. Developmental trade-offs between growing insect structures have been shown to result from competition for stored nutrients that are associated with nutrition. In dobsonflies, during development of adult structures, weapons may compete for resources with gift-producing glands.
The data we present support the hypothesis that there is an evolutionary trade-off between investment in acquiring mates and investment in offspring production in dobsonflies. We found that male dobsonfly species develop enlarged male weapons or are able to produce large, costly nuptial gifts but do not make both. Moreover, our results show that males in species with large weapons or large nuptial gifts have smaller wings than males in species that do not have these energetically expensive sexually selected traits (figure 3). This suggests that it is difficult for males to invest their resources into weapons and spermatophores simultaneously. The fact that both male mating strategies are energetically costly and neither are present together within a single species supports an evolutionary trade-off between these traits.
However, in nuptial gift-giving insects, the production of large nutritious gifts by males increases the choosiness of males for mates and insures that the female he mates with produces more offspring of better quality [33–36]. Thus, females become the competitive sex, and there is a reduction of male sexual competition as the operational sex ratio shifts from male-biased to female-biased. In dobsonflies, male mating frequency decreases in species which have large spermatophores [17], and remating by females is high during their lifetimes [18]. Therefore, an alternative explanation may also predict a reduction in the development of male weapons because of a reduction of sexual selection, particularly male–male competition in the species producing large spermatophores.
In summary, sexually selected traits not only exhibit evolutionary trade-offs with non-sexually selected traits such as wings, but also with costly behavioural traits and morphological traits associated with mate acquisition. Our results show that in dobsonflies, some lineages evolved large weapons, whereas others evolved large nuptial gifts with either no weapons, or small weapons relative to body size. Our data support the hypothesis that individuals invest in either weapons or nuptial gifts but not both, whereas the loss of male weapons in nuptial gift-giving dobsonflies could be due to relaxed selection on male–male competition. Our results from the world's dobsonflies suggest that the evolution of costly sexually selected traits such as male weapons and nuptial gifts probably results from the interaction between nutritional ecology and life-history evolution and results in the remarkable diversity in shape and form in animals. Megaloptera represent an important new system to study the interplay among multiple sexually selected traits. It will be important to examine whether or not the lack of male weaponry in the nuptial gift-giving dobsonflies is due to limited resources, relaxed sexual selection or to other ecological factors in future studies, especially direct empirical studies on resource allocation of weapons and nuptial gifts.
4. Material and methods
(a). Specimens
Most specimens for morphological measurement and molecular phylogenetic analyses have been deposited in the Entomological Museum of China Agricultural University, Beijing, China (CAU) and Hayashi Collection in Tokyo Metropolitan University, Tokyo, Japan (HC). In addition, a few specimens for the morphological measurement were deposited in several other museum collections that are listed in the electronic supplementary material.
(b). Rearing and mating
The larval dobsonflies were collected with hand-nets from stony streams. Methods for rearing can be found in the electronic supplementary material. Matings were conducted by placing a male and a female prior to the dark period in individual cages (20 × 30 × 20 cm3) constructed of nylon netting. At 1 h intervals from darkness to the next light, insects were checked for their mating status under a dim red light. After copulation, individuals were returned to their respective rearing vessels. Individuals of both sexes were allowed to copulate only once. The numbers of mating bouts observed in the laboratory were three in C. luteus and two in C. cornutus in combinations of two male and one female, and two in Nev. gloriosoi, 11 in Neu. sumatrensis, 30 in Pr. grandis, 18 in Pr. immaculatus, 18 in Pr. costalis and two in Pr. sabahensis as pair combinations. We also video-recorded the mating behaviour of two males and one female A. orientalis in the field (n = 1). No specific permits were required for the insects collected for this study in China, Japan, Malaysia, Mexico and USA. The field studies were conducted in accordance with local legislation and did not involve any endangered or protected species.
(c). Rhodamine B treatment
Rhodamine B was traced from the male to the mated female between two species of Megaloptera with contrasting spermatophore shape, Pa. continentalis (Corydalidae: Chauliodinae) with a small spermatophore and Pr. grandis (Corydalidae: Corydalinae) with a large spermatophore. Adults were raised from field-collected final-instar larvae. Collecting information and measurements of adults of these two species can be found in the electronic supplementary material. Adults were given a sugar solution daily after emergence. Among them, several males were given a mix (1 : 5 by volume) of 0.00417 M rhodamine B solution (Wako Pure Chemical Industries, Tokyo, Japan) and sugar solution for two consecutive days, but thereafter, were given only the sugar solution daily. More than 3 days after the last rhodamine-containing feed, the rhodamine-fed males were used for mating trials. Two, four and three females of Pa. continentalis and three, four and four females of Pr. grandis were mated singly with rhodamine-free (control) males, singly with rhodamine-fed males, and multiply with rhodamine-fed males (three times with different males at 1 to 4 day intervals), respectively. Four days after the last mating, the females were anaesthetized with ether and dissected to examine their ovarioles under a fluorescence microscope (BIOREVO BZ-9000; Keyence Corporation, Osaka, Japan). The male internal reproductive organs dissected out after anaesthetization were observed under a fluorescence stereomicroscope (MZ FLIII; Leica, Wetzlar, Germany) with a green filter (excitation maximum 540 nm, emission maximum 625 nm). Unmated females given the rhodamine diet once in the early or late stage after emergence were also examined to determine the incorporation of nutrients from the diet into the developing eggs.
(d). Accessory gland size
Male body weight (BW, mg) was measured to the nearest 0.01 mg with a microbalance (Mettler AT201; Greifensee, Zürich, Switzerland) after they had been killed with ether. The internal reproductive organs were then dissected out and weighed immediately (AG, mg). When dissected out, testes and fine-tubed vas deferens were always cut from the remaining part (clump of accessory glands) of internal reproductive organs. The ratio of AG/BW (%) was calculated for each male. The ratios of AG/BW estimated based on the dry and fresh weight did not differ significantly [17]. Therefore, in this study, the ratio was based on fresh-weight measurements.
(e). Allometric relationships
Morphological measurements were made for a total of 1819 adult specimens. The head width, mandible (or flange) length, prothorax length and forewing length were measured using slide callipers to the nearest 0.05 mm (figure 1). Allometric relationships were quantified from log-transformed data regressions of each trait on the prothorax length as a body size indicator. When the slope of the regression line was greater than 1, 1 or less than 1, the trait was taken to show positive allometry, isometry and negative allometry, respectively, against body size [37]. The slopes were calculated by major axis regression for males and females, respectively, because the standard least-squares method tends to produce underestimations [38]. The adjusted means were calculated directly from the regression lines when the slopes of males and females differed at p = 0.05 on ANCOVA, but estimated from the regressions with the common slopes when they did not differ significantly. The 95% confidence intervals of each adjusted mean were calculated by the bootstrap method with 1000 replicates.
(f). Phylogenetic analysis
Fifty-seven dobsonfly species, representing nearly half of the world's species, were selected for the present analysis, including representatives from all nine genera of Corydalinae worldwide (electronic supplementary material, table S1). We chose three mitochondrial genes, i.e. large ribosomal RNA gene (16S rRNA) and two protein-coding genes, cytochrome oxidase subunit I (COI) and NADH dehydrogenase subunit 2 (ND2), as the molecular markers. Protocols of DNA extraction and PCR can be found in the electronic supplementary material.
The sequences of the three markers were aligned using ClustalX [39] with default settings, whereas the alignment of 16S rRNA sequences was manually checked to improve the accuracy based on the published secondary structure for 16S rRNA of the fishfly species Neochauliodes punctatolosus (Megaloptera: Corydalidae: Chauliodinae) [40], and the protein-coding gene sequences were aligned according to the translated amino acid codons. In addition, we coded 71 morphological characters obtained from the latest study on the morphological phylogeny of Corydalinae [24]: appendix 5. The morphological data were combined with the aligned DNA sequence data, being the dataset for the subsequent phylogenetic analyses (electronic supplementary material, dataset S2). Parsimony analyses were conducted using PAUP* 4.0 [41]. Bayesian analyses were performed using MrBayes 3.1.2 [42]. Detailed settings of tree reconstruction can be found in the electronic supplementary material.
(g). Ancestral state reconstruction
To test the evolution of the two sexually selected male traits across our phylogeny of dobsonfly genera, we performed an ancestral state reconstruction by using the program RASP2.0 beta [43]. We tested sexually dimorphic mandibles and nuptial gifts separately tested as independent characters, and the presence/absence of these two characters was used as the character state for each terminal taxon. The fully resolved intergeneric phylogeny of Corydalinae with binary topology was used for character mapping. A Bayesian binary Markov chain Monte Carlo analysis was run with the default settings. Proportional probabilities of each ancestral state were indicated at corresponding nodes.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Atilano Contreras-Ramos, Oliver S. Flint Jr., Mamoru Owada, Akiko Saito, Ros Urban, Margie Cochrane, Gexia Qiao and Zhu Li for providing specimens, José Asael Nájera Carpio, Jun Abe and Yoshitaka Kamimura for their help in the field, Tamotsu Kusano for his help with allometric analyses, Chengquan Cao for video-recording, and Allen J. Moore, Douglas J. Emlen, and two anonymous referees for helpful comments on the manuscript.
Data accessibility
Our sequence data are available in the NCBI with the GenBank accessions listed in the electronic supplementary material, table S1. Other dataset are available in doi:10.5061/dryad.4bd12.
Funding statement
The work was supported partly by the National Natural Science Foundation of China (nos. 31320103902, 31322051, 31000973 to X.L. and D.Y.), the grant-in-aid for Scientific Research (C) from the Japan Society for the Promotion of Science (JSPS; no. 21570099 to F.H.), the National Key Basic Research Programme of China (973 Programme; no. 2013CB127600 to X.L.), the Foundation for the Author of National Excellent Doctoral Dissertation of PR China (no. 201178 to X.L.), and the grant-in-aid for fellows of JSPS relating to the JSPS Postdoctoral Fellowship for Foreign Researchers (no. 20-08417 to X.L.)
Authors' contributions
X.L. and F.H. designed the study, carried out the experiments and data analysis, and drafted the manuscript; D.Y. and L.L. coordinated the study and helped draft the manuscript. All authors gave final approval for publication.
References
- 1.Warren IA, Gotoh H, Dworkin IM, Emlen DJ, Lavine LC. 2013. A general mechanism for conditional expression of exaggerated sexually-selected traits. Bioessays 35, 889–899. ( 10.1002/bies.201300031) [DOI] [PubMed] [Google Scholar]
- 2.Stearns SC. 1989. Trade-offs in life history evolution. Funct. Ecol. 3, 259–268. ( 10.2307/2389364) [DOI] [Google Scholar]
- 3.Parzer HF, Moczek AP. 2008. Rapid antagonistic coevolution between primary and secondary sexual characters in horned beetles. Evolution 62, 2423–2428. ( 10.1111/j.1558-5646.2008.00448.x) [DOI] [PubMed] [Google Scholar]
- 4.Kawano K. 1995. Horn and wing allometry and male dimorphism in giant rhinoceros beetles (Coleoptera: Scarabaeidae) of tropical Asia and America. Ann. Entomol. Soc. Am. 88, 92–99. ( 10.1093/aesa/88.1.92) [DOI] [Google Scholar]
- 5.Kawano K. 1997. Cost of evolving exaggerated mandibles in stag beetles (Coleoptera: Lucanidae). Ann. Entomol. Soc. Am. 90, 453–461. ( 10.1093/aesa/90.4.453) [DOI] [Google Scholar]
- 6.Okada K, Miyatake T. 2009. Genetic correlations between weapons, body shape and fighting behaviour in the horned beetle Gnatocerus cornutus . Anim. Behav. 77, 1057–1065. ( 10.1016/j.anbehav.2009.01.008) [DOI] [Google Scholar]
- 7.Yamane T, Okada K, Nakayama S, Miyatake T. 2011. Dispersal and ejaculatory strategies associated with exaggeration of weapon in an armed beetle. Proc. R. Soc. B 277, 1705–1710. ( 10.1098/rspb.2009.2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simmons LW, Emlen DJ. 2006. Evolutionary trade-off between weapons and testes. Proc. Natl Acad. Sci. USA 103, 16 346–16 351. ( 10.1073/pnas.0603474103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moczek AP, Nijhout HF. 2004. Trade-offs during the development of primary and secondary sexual traits in a horned beetle . Am. Nat. 163, 184–191. ( 10.1086/381741) [DOI] [PubMed] [Google Scholar]
- 10.Kelly CD. 2008. Sperm investment in relation to weapon size in a male trimorphic insect? Behav. Ecol. 19, 1018–1024. ( 10.1093/beheco/arn058) [DOI] [Google Scholar]
- 11.Glorioso MJ. 1981. Systematics of the dobsonfly subfamily Corydalinae (Megaloptera: Corydalidae). Syst. Entomol. 6, 253–290. ( 10.1111/j.1365-3113.1981.tb00440.x) [DOI] [Google Scholar]
- 12.Yang D, Liu X. 2010. Fauna Sinica, Insecta vol. 51, Megaloptera. Beijing, China: Science Press. [Google Scholar]
- 13.Darwin C. 1871. The descent of man, and selection in relation to sex. London, UK: John Murray. [Google Scholar]
- 14.Hayashi F. 1992. Large spermatophore production and consumption in dobsonflies Protohermes (Megaloptera: Corydalidae). Jpn J. Entmol. 60, 59–66. [Google Scholar]
- 15.Hayashi F. 1999a. Rapid evacuation of spermatophore contents and male post-mating behavior in alderflies (Megaloptera: Sialidae). Entomol. Sci. 2, 49–56. [Google Scholar]
- 16.Hayashi F. 1999. Ejaculate production schedule and the degree of protandry in fishflies (Megaloptera: Corydalidae). Funct. Ecol. 13, 178–189. ( 10.1046/j.1365-2435.1999.00289.x) [DOI] [Google Scholar]
- 17.Hayashi F. 1993. Male mating costs in two insect species (Protohermes, Megaloptera) that produce large spermatophores. Anim. Behav. 45, 343–349. ( 10.1006/anbe.1993.1039) [DOI] [Google Scholar]
- 18.Hayashi F. 1998. Multiple mating and lifetime reproductive output in female dobsonflies that receive nuptical gifts. Ecol. Res. 13, 283–289. ( 10.1046/j.1440-1703.1998.00272.x) [DOI] [Google Scholar]
- 19.van der Reijden ED, Monchamp JD, Lewis SM. 1997. The formation, transfer, and fate of spermatophores in Photinus fireflies (Coleoptera: Lampyridae). Can. J. Zool. 75, 1202–1207. ( 10.1139/z97-143) [DOI] [Google Scholar]
- 20.Garbiec A, Kubrakiewicz J. 2012. Differentiation of follicular cells in polytrophic ovaries of Neuroptera (Insecta: Holometabola). Arthropod Struct. Dev. 41, 165–176. ( 10.1016/j.asd.2011.12.003) [DOI] [PubMed] [Google Scholar]
- 21.Penny ND. 1993. The phylogenetic position of Chloroniella peringueyi (Megaloptera: Corydalidae) and its zoogeographic significance. Entomol. News 104, 17–30. [Google Scholar]
- 22.Glorioso MJ, Flint OS., Jr 1984. A review of the genus Platyneuromus (Insecta: Neuroptera: Corydalidae). Proc. Biol. Soc. Wash. 97, 601–614. [Google Scholar]
- 23.Contreras-Ramos A. 1999. Mating behavior of Platyneuromus (Megaloptera: Corydalidae), with life history notes on dobsonflies from Mexico and Costa Rica. Entomol. News 110, 125–135. [Google Scholar]
- 24.Contreras-Ramos A. 2011. Phylogenetic review of dobsonflies of the subfamily Corydalinae and the genus Corydalus Latreille (Megaloptera: Corydalidae). Zootaxa 2862, 1–38. [Google Scholar]
- 25.Contreras-Ramos A. 1998. Systematics of the dobsonfly genus Corydalus Latreille (Megaloptera: Corydalidae). Lanham, MD: Thomas Say Monographs, Entomological Society of America. [Google Scholar]
- 26.New TR, Theischinger G. 1993. Megaloptera (Alderflies, Dobsonflies). Handbuch der Zoologie (Berlin) 4, 1–97. [Google Scholar]
- 27.Gwynne DT. 2001. Katydids and bush-crickets: reproductive behavior and evolution of the Tettigoniidae. New York, NY: Cornell University Press. [Google Scholar]
- 28.Del Castillo RC, Gwynne DT. 2007. Increase in song frequency decreases spermatophore size: correlative evidence of a macroevolutionary trade-off in katydids (Orthoptera: Tettigoniidae). Evol. Biol. 20, 1028–1036. ( 10.1111/j.1420-9101.2006.01298.x) [DOI] [PubMed] [Google Scholar]
- 29.Lewis SM, Cratsley CK. 2008. Flash signal evolution, mate choice, and predation in fireflies. Annu. Rev. Entomol. 53, 293–321. ( 10.1146/annurev.ento.53.103106.093346) [DOI] [PubMed] [Google Scholar]
- 30.South A, Lewis SM. 2012. Flash signal evolution, mate choice and predation in fireflies. Proc. R. Soc. B 279, 3201–3208. ( 10.1098/rspb.2012.0370) [DOI] [PubMed] [Google Scholar]
- 31.Emlen DJ, Warren I, Johns A, Dworkin I, Lavine LC. 2012. A mechanism of extreme growth and reliable signaling in sexually selected ornaments and weapons. Science 337, 860–864. ( 10.1126/science.1224286) [DOI] [PubMed] [Google Scholar]
- 32.Shingleton AW, Frankino WA. 2012. New perspectives on the evolution of exaggerated traits. Bioessays 35, 100–107. ( 10.1002/bies.201200139) [DOI] [PubMed] [Google Scholar]
- 33.Gwynne DT, Simmons LW. 1990. Experimental reversal of courtship roles in an insect. Nature 346, 172–174. ( 10.1038/346172a0) [DOI] [Google Scholar]
- 34.Lehmann GUC. 2012. Weighing costs and benefits of mating in bushcrickets (Insecta: Orthoptera: Tettiogniidae), with an emphasis on nuptial gifts, protandry and mate density. Front. Zool. 9, 19 ( 10.1186/1742-9994-9-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kvarnemo C, Simmons LW. 2013. Polyandry as a mediator of sexual selection before and after mating. Phil. Trans. R. Soc. B 368, 20120042 ( 10.1098/rstb.2012.0042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lüpold S, Tomkins JL, Simmons LW, Fitzpatrick JL. 2014. Female monopolization mediates the relationship between pre- and postcopulatory sexual traits. Nature Commun. 5, 3184 ( 10.1038/ncomms4184) [DOI] [PubMed] [Google Scholar]
- 37.Harvey PH, Pagel MD. 1991. The comparative method in evolutionary biology. Oxford, UK: Oxford University Press. [Google Scholar]
- 38.McArdle BH. 1988. The structural relationship: regression in biology. Can. J. Zool. 66, 2329–2339. ( 10.1139/z88-348) [DOI] [Google Scholar]
- 39.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25, 4876–4882. ( 10.1093/nar/25.24.4876) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang YY, Liu XY, Winterton SL, Yang D. 2012. The first mitochondrial genome for the fishfly subfamily Chauliodinae and implications for the higher phylogeny of Megaloptera. PLoS ONE 7, e47302 ( 10.1371/journal.pone.0047302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swofford DL. 2002. PAUP*: phylogenetic analysis using parsimony (*and other methods). Sunderland, MA: Sinauer. [Google Scholar]
- 42.Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574. ( 10.1093/bioinformatics/btg180) [DOI] [PubMed] [Google Scholar]
- 43.Yu Y, Harris AJ, Blair C, He XJ. 2011. RASP (reconstruct ancestral state in phylogenies): a tool for historical biogeography. Mol. Phyl. Evo. 87, 46–49. ( 10.1016/j.ympev.2015.03.008) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Our sequence data are available in the NCBI with the GenBank accessions listed in the electronic supplementary material, table S1. Other dataset are available in doi:10.5061/dryad.4bd12.