Abstract
Odours of vertebrates often contain information about the major histocompatibility complex (MHC), and are used in kin recognition, mate choice or female investment in pregnancy. It is, however, still unclear whether MHC-linked signals can also affect male reproductive strategies. We used horses (Equus caballus) to study this question under experimental conditions. Twelve stallions were individually exposed either to an unfamiliar MHC-similar mare and then to an unfamiliar MHC-dissimilar mare, or vice versa. Each exposure lasted over a period of four weeks. Peripheral blood testosterone levels were determined weekly. Three ejaculates each were collected in the week after exposure to both mares (i.e. in the ninth week) to determine mean sperm number and sperm velocity. We found high testosterone levels when stallions were kept close to MHC-dissimilar mares and significantly lower ones when kept close to MHC-similar mares. Mean sperm number per ejaculate (but not sperm velocity) was positively correlated to mean testosterone levels and also affected by the order of presentation of mares: sperm numbers were higher if MHC-dissimilar mares were presented last than if MHC-similar mares were presented last. We conclude that MHC-linked signals influence testosterone secretion and semen characteristics, two indicators of male reproductive strategies.
Keywords: life history, male reproductive strategy, equine leucocyte antigen, major histocompatibility complex-linked odours, kin recognition, male–male interactions
1. Introduction
The major histocompatibility complex (MHC) is a group of highly polymorphic genes that play a critical role in the immune system of vertebrates [1]. The MHC also has important functions in the social signalling of various mammals, birds, reptiles, amphibians and fish (recent examples include [2–13]). Ruff et al. [14] therefore suggested that MHC social signalling may be the basis of a vertebrate-wide chemosensory communication system. MHC-linked signals are often used in individual recognition or as a signal of genetic relatedness, for example, to facilitate cooperative behaviour among kin, or they are used in mate choice as indicators of genetic compatibility (including inbreeding avoidance) or genetic quality [15]. Both males and females have been found to express MHC-linked odour preferences [16–18]. However, little is known about potential further effects of MHC-linked signals on male reproductive strategies. We chose the horse (Equus caballus) as experimental model to test whether female MHC types can affect male reproductive strategies. We concentrated on blood testosterone levels and semen characteristics as potential indicators of male strategies.
Testosterone is a steroid hormone that has numerous functions in various contexts, including the regulation of time and energy put into competitive and sexual behaviour such as mate seeking, male–male competition, dominance, mating effort, paternal behaviour and the degree of polygyny [19,20], with testosterone secretion typically showing high inter- and within-species plasticity [21]. Several studies in mice and other mammals have found that exposure of males to females or their scent induces a ‘testosterone surge’ that activates a variety of courtship and mating behaviours [22–24]. Wingfield et al. [25] predicted that patterns of testosterone secretion are useful proxies of male reproductive strategies (see also [26]).
Sperm production is costly [27]. Therefore, ejaculate characteristics are predicted to be plastic traits that change in response to the social context, including the perceived quality of females [28,29]. Arguably the most important ejaculate characteristic that is typically discussed in this context is sperm number [29], but sperm velocity could potentially be plastic too [30,31]. Both traits are predicted to be physiologically expensive and positively linked to fertilization success [32–35].
Here, we sequentially exposed stallions each to an MHC-similar and an MHC-dissimilar mare (or vice versa) over a total period of eight weeks, so that each mare would ovulate at least once during each type of exposure. As potential correlates of male reproductive strategies, we determined blood testosterone levels during each type of exposure and collected ejaculates in the ninth week to study semen characteristics. We predicted that if MHC sharing between mares and stallions plays a role, male testosterone levels should be high when paired with a MHC-dissimilar mare (because stallions are expected to invest in attractiveness and mate guarding) and low when paired with a MHC-similar mare [36]. We also tested whether stallions invested more in ejaculates (i.e. ejaculate more and/or faster sperm) after exposure to MHC-dissimilar mares than after exposure to MHC-similar mares.
2. Material and methods
(a). Exposure to stallions and mares
Twelve clinically healthy and sexually experienced stallions (Franches-Montagnes horses, 7–20 years old, with proved fertility) and six healthy mares (three Franches-Montagnes and three Warmblood horses, 9–14 years old) were used. Seven experimental stables were available for the study: six identical stables consisting of 2 × 2 boxes (12 m2 per box) and a corridor (2.9 m wide) in between, with solid wood up to a height of 1.3 m and a metal grille above as separation between the boxes and towards the corridor. This allowed visual, olfactory and limited tactile contact between the animals. The seventh stable consisted of 2 × 4 boxes but was otherwise identical to the others. All boxes were bedded with straw.
The stallions were randomly allocated to two groups of six stallions each. The experiment was carried out in a crossed design during two periods (figure 1). In period 1, six stallions were housed together with one mare in one of the six separate stables each, with the stallion and the mare in adjacent boxes. (The other six stallions were distributed to six of the eight boxes of the large stable without any contact to mares in the course of a parallel study on the effects of perceived risks of sperm competition [37].) Half of the stallions were housed with an MHC-similar mare (i.e. sharing at least one MHC antigen with the respective stallion) for four weeks and then with a MHC-dissimilar mare (i.e. sharing no MHC antigens) for the remaining four weeks. The other stallions received the reverse treatment (i.e. first paired with an MHC-dissimilar and then with an MHC-similar mare). No contact with other stallions or mares was allowed for these stallions over the entire eight weeks. After a transitory week during which ejaculates were collected (see below), all 12 stallions were switched from one group type to the other and the experiments were repeated (period 2) so that, by the end of the study, all 12 stallions had each been exposed to an MHC-similar and an MHC-dissimilar mare, and the design was fully balanced with respect to the order of presenting MHC-similar or MHC-dissimilar mares.
Figure 1.
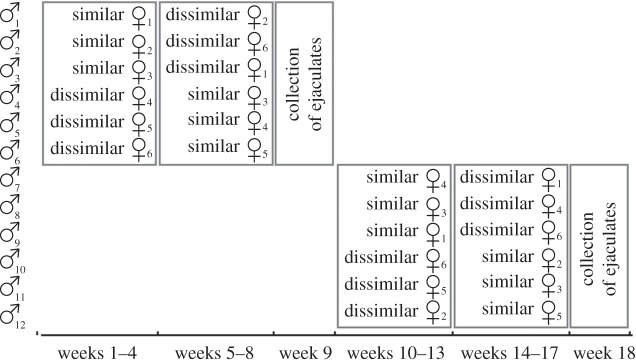
Schematic of the experiment. Six stallions were distributed to six separate stables and sequentially exposed over four weeks each to an MHC-similar mare followed by an MHC-dissimilar mare or vice versa (balanced and full-factorial within-subject design). Three ejaculates per stallion were collected during the ninth week. Then the stables were emptied and cleaned, and the experiment repeated with six new stallions.
All stimulus mares turned out to be cycling normally throughout the experiment and were at least once (range 1–2) in oestrus during each exposure to a stallion. The mares and the stallions were not familiar to each other (i.e. it was unlikely that they had met before the experiment).
(b). Major histocompatibility complex and testosterone analyses
Equine leucocyte antigen (ELA) class I and class II were determined serologically in microcytotoxicity tests with alloantisera detecting 18 internationally recognized (A1, A2, A3, A4, A5, A6, A7, A8, A9, A10, W11, A14, A15, A16, A17, A18, A19 and A20) and five locally defined (Be22, Be25, Be27, Be28 and Be108) ELA-A (MHC class I) specificities. The ELA-C allele W21 and MHC class II alleles DW13, DW22, DW23, DBe200 and DBeVIII were tested in a classical two-step microcytotoxicity test in Terasaki plates according to Lazary et al. [38]. Briefly, peripheral blood lymphocytes of horses were obtained from heparinized blood samples by Ficoll density gradient centrifugation. The cells were washed twice in PBS, re-suspended in RPMI and then diluted to 2 × 106 cells ml−1. Two microlitres of the cell suspension was added to each well on the Terasaki typing plates. The wells contained defined antisera against the different ELA specificities. After 20 min incubation at room temperature, 2 μl rabbit complement was added to each well. Incubation for 1 h at room temperature followed. Visualization of the reaction was performed by adding 5 μl eosin to each well, followed by 5 μl formaldehyde for fixation. The reaction was analysed using an inverted microscope. A positive reaction led to killing of more than 50% of target cells. Stallions and mares were classified into pairs sharing or not sharing at least one ELA. The number of shared ELA varied from one to four within MHC-similar pairings.
During the entire experiment, blood samples (EDTA) were collected from the stallions once per week for testosterone analysis. This was done every Wednesday between 10 and 10.30 via jugular venipuncture. The samples were immediately centrifuged (4000g for 10 min) and the plasma frozen (−80°C for less than three months) until analysis. Testosterone was determined via electrochemiluminescence immunoassay (Elecsys 2010, Roche Diagnostics, Basel, Switzerland) as described and validated by Janett et al. [39] (inter- and intra-assay coefficients of variation were 2.2% and 1.4%, respectively).
(c). Semen characteristics
We concentrated on two semen characteristics: sperm number per ejaculate and sperm velocity. If there is plasticity in these characteristics, sperm number per ejaculate may be quickly adjusted while plasticity in sperm velocity (i.e. changes in sperm morphology and/or ATP content [31,35,40]), is likely to require more time to be implemented. In horses, spermiogenesis takes 57 days [41]. In order not to miss any potential treatment effects, we only collected ejaculates during the week that followed each eight-week block of exposure to mares (figure 1). Semen was collected three times (Monday, Wednesday and Friday) to record total sperm number per ejaculate and average sperm velocity. Semen collection was done in a separate room using an artificial vagina (Avenches model, Switzerland) and a phantom, with always the same ovariectomized teaser mare standing in a box in front of the phantom (i.e. following a standard procedure that all stallions had frequently experienced before). This teaser mare had not been included in the other treatments. Her MHC type was also determined in order to test for possible effects on ejaculate characteristics. Extra-gonadal sperm reserves [42,43] were minimized in daily semen collections (Monday–Friday) the week before each (i.e. during the eighth week of each experimental period).
Immediately after semen collection, total sperm number was calculated from ejaculate volume and sperm concentration as determined in a nucleocounter (SP-100, ChemoMetec, Allerød, Denmark). Sperm velocity was assessed in 10 µl raw semen diluted in 20 µl INRA 96TM (IMV, L'Aîgle, France) and with a computer-assisted sperm analyser (HTM-IVOS, v. 12, Beverly, MA, USA) using a 20 μm standard count analysis chamber (Art. Nr. SC 20–01-C, Leja, Nieuw-Vennep, The Netherlands). Sperm velocity (µm s−1) was determined as straight-line velocity (VSL, i.e. the mean distance between the sperm heads' first detected positions to their last), as curvilinear velocity (VCL, i.e. the curvilinear path the sperm heads took) and as average-path velocity (VAP, i.e. the smoothed paths the sperm heads took during an observational period).
(d). Statistics and ethical note
Treatment effects on testosterone blood levels were tested as repeated measures in a MANOVA (Huynh-Feldt corrected), with the order of presenting MHC-dissimilar or -similar mares included as fixed factors to account for potential order effects. General linear models (GLMs) were used to test whether semen characteristics could be predicted by the order of presenting MHC-dissimilar or -similar mares and the sharing of MHC antigens with the teaser mare during semen collection. The interaction between these two main effects was never significant and therefore excluded from the final models (i.e. the final models were based on type II sums of squares). Mean testosterone blood levels had to be added to the latter GLM because Burger et al. [37] found it to be correlated to mean sperm number, i.e. its effect on semen characteristics had to be controlled for in order to study possible MHC effects. Correlations were analysed with Kendall's rank correlation coefficient τ .
The animals had ad libitum access to water and were fed three times per day with hay, oats, barley, corn and pellets supplemented with minerals. The stallions were regularly and individually exercised, and had daily access for about 1 h to a paddock without any direct contact to other stallions or mares. Mares were turned out daily in groups for 3 h in paddocks without any stallion contact. All horses had been dewormed before the experiments and absence of intestinal parasites could be confirmed using the McMaster method with a detection limit of 50 EpG [44] on faeces samples.
3. Results
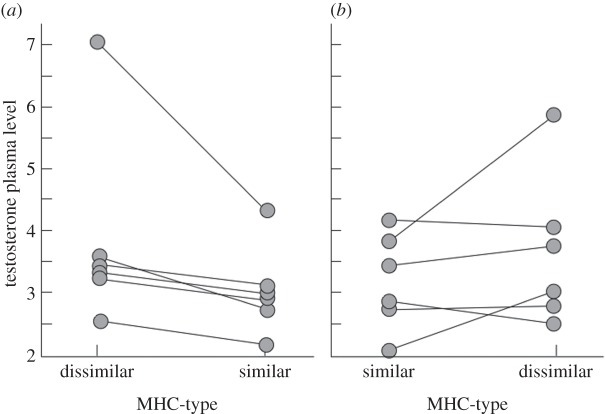
Average peripheral plasma testosterone levels were significantly correlated across treatments (i.e. some stallions had consistently higher testosterone levels than others; figure 2). Nevertheless, the experimental treatment affected average peripheral plasma testosterone levels. In 10 of 12 stallions, average testosterone levels were lower when exposed to an MHC-similar mare than to an MHC-dissimilar mare (Wilcoxon signed-rank test: p = 0.016; figure 3 and table 1). Peripheral testosterone levels were on average 19.2% lower (95% CI = 4.2–34.2) when stallions were exposed to MHC-similar mares as compared exposure with MHC dissimilar ones (figure 3). The order of presenting MHC-similar and MHC-dissimilar mares showed no significant effect on mean testosterone levels (table 1).
Figure 2.
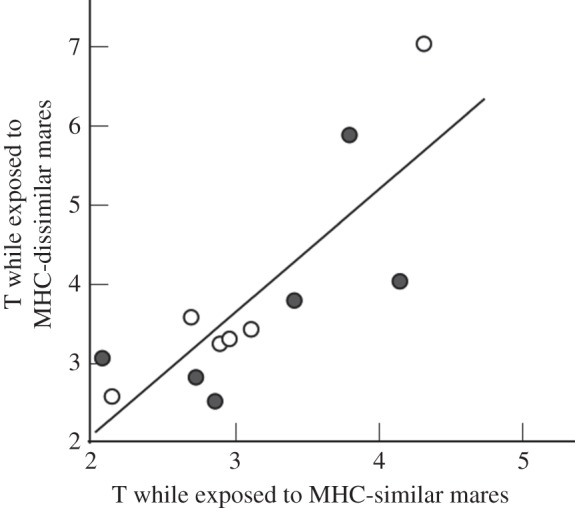
Correlation between average peripheral plasma testosterone (T) levels (in nmol l−1) while exposed to MHC-similar and to MHC-dissimilar mares (Kendall's τ = 0.67, p = 0.003). Stallions were either first exposed to an MHC-dissimilar mare and then to MHC-similar ones (open circle) or vice versa (filled circle). The line gives the regression over all treatment groups.
Figure 3.
Treatment effects on testosterone. Average peripheral plasma testosterone levels (nmol l−1) of (a) the six stallions that were first exposed to MHC-dissimilar and then to MHC-similar mares, and (b) the six stallions that were first exposed to MHC-similar and then to MHC-dissimilar mares.
Table 1.
MANOVA on mean plasma testosterone levels in stallions exposed to an MHC-similar and an MHC-dissimilar mare.
| F | d.f. | p | |
|---|---|---|---|
| MHC sharing (within subjects) | 5.46 | 1, 10 | 0.04 |
| order of presentation (between subjects) | <0.1 | 1, 10 | 0.98 |
| MHC sharing × order of presentation | 0.36 | 1, 10 | 0.56 |
Mean sperm number after eight weeks of exposure to mares was affected by the order of presenting the MHC-dissimilar and the MHC-similar mare: stallions that had first been exposed to MHC-similar and then to MHC-dissimilar mares showed higher sperm numbers at the end of the study than stallions that had last been exposed to MHC-similar mares (figure 4 and table 2). The three velocity measures were all correlated (comparing means: VSL versus VCL: τ = 0.58; VSL versus VAP: τ = 0.52; VCL versus VAP: τ = 0.45; p always <0.05). None of these velocity measures seem to be affected by the order of presentation (electronic supplementary material, table S1) and was neither correlated with mean sperm number (0.00 < τ < 0.30, p always >0.05) nor with mean testosterone levels in either of two treatment groups (−0.03 < τ < 0.42, p always >0.05). Sharing MHC antigens with the teaser mare during semen collection did not lead to any significant effects on sperm numbers or sperm velocity (table 2; electronic supplementary material, table S1).
Figure 4.
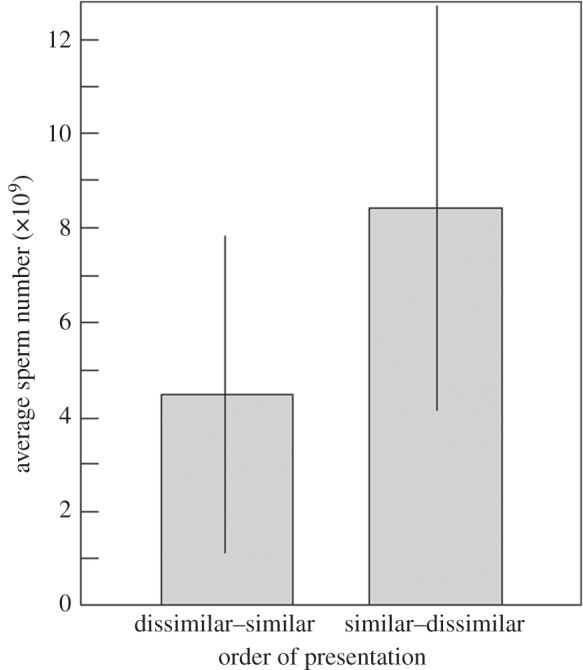
Average sperm number per ejaculate (means of six stallions each ±95% CI) in relation to the order of exposure to MHC-similar and MHC-dissimilar mares. See table 2 for statistics.
Table 2.
GLM on mean sperm number per ejaculate after eight weeks of exposure to mares, in response to mean plasma testosterone levels during these eight weeks, the order presentation of the MHC-similar and the MHC-dissimilar mare, and whether the teaser mare was MHC-similar or -dissimilar to the stallion (full model: F3,8 = 6.17, p = 0.018).
| estimate (s.e.) | t | p | |
|---|---|---|---|
| intercept | −2929.3 (2882.1) | −1.02 | 0.34 |
| order of presentation (MHC sharing) | −1801.2 (781.4) | −2.31 | 0.05 |
| mean testosterone level | 2592.6 (807.9) | 3.21 | 0.01 |
| MHC of teaser mare | −994.2 (904.3) | −1.10 | 0.30 |
4. Discussion
Male and female reproductive strategies of mammals are generally expected to differ because of the differences in potential reproductive rates [45,46]. Females are therefore predicted to be, on average, choosier than males. This might explain why previous research on MHC-linked mate preferences has focused on females [14]. Here, we concentrated on males and tested whether MHC sharing also affects male reproductive strategies. We used three possible indicators of such strategies, namely testosterone blood levels, average sperm numbers per ejaculate and average sperm velocity. We found male testosterone levels to be dependent on the sharing of MHC antigens between mares and stallions. Exposure to MHC-dissimilar mares provoked higher testosterone levels than exposure to MHC-similar mares. We found no significant MHC effects on sperm velocity, but average sperm numbers per ejaculate were correlated to both average testosterone blood levels (as shown in [37]) and the order of presenting MHC-dissimilar and -similar mares. Stallions first exposed to MHC-similar and then to MHC-dissimilar mares ejaculated higher numbers of sperm than stallions last exposed to MHC-similar mares. We conclude from our observations that (i) horses can reveal their MHC type (i.e. communication between horses can be based on MHC-linked signals, most likely to be MHC-linked odours), and horses can be added to the increasing list of species that show some form of MHC-based social signalling [14]; (ii) as in other species, the receiver's own MHC influences the interpretation of MHC-linked signals in horses, either because receivers have learned MHC-linked signals during ontogeny as in mice [47,48], or because the MHC or closely linked genes such as olfactory receptor genes [49] directly influence odour perception; and (iii) stallions adjust their reproductive strategy either in response to the MHC types of the mares they are exposed to or in response to the behaviour mares display when exposed to an MHC-dissimilar stallion.
We concentrated on blood testosterone levels in reaction to the treatment because numerous studies have found links between this hormone and the behaviour males show towards females [26]. From these previous studies, it could be predicted that if MHC sharing plays a role, male testosterone levels should be high when stallions are paired with MHC-dissimilar mares (because stallions are then expected to invest in attractiveness and mate guarding) and low when paired with an MHC-similar mare [36]. This turned out to be the case. However, the fact that we isolated pairs of stallions and mares over longer periods could have played an important role here, because male testosterone levels are expected to be influenced also by the presence or absence of other stallions [50]. Indeed, when stallions were kept in groups and repeatedly exposed to oestrous and dioestrous mares (in another study that was designed to test for MHC-linked female preferences; D. Burger 2015, unpublished data), average male testosterone levels were found to be elevated with increasing number of MHC-similar mares the stallions had been exposed to. This suggests that mares are, on average, perceived differently by stallions when alone or when housed with other stallions. One possibility is that, in the group situation, MHC-linked signals were mostly used as an indicator of the average level of relatedness between the stallions and the mares. The high testosterone levels could then reflect the stallions' willingness to protect and support kin. Kin recognition is indeed essential for various forms of cooperative behaviours and requires that an individual is able to discriminate between related and unrelated conspecifics, and ideally even to recognize varying degrees of genetic relatedness [51]. Several possible mechanisms for kin recognition have been identified [52], including MHC-linked signals [14]. It is therefore possible that individuals with similar MHC types are perceived as related even if, as in our studies, they are not related and not even familiar to each other. Whatever the causalities, the observation that stallions reacted very differently to the MHC of mares when kept in a group of other stallions (D. Burger 2015, unpublished data) or alone (this study) supports previous findings in other species that reactions to MHC-linked signals can be very context-specific [14,36].
Despite the observed MHC effects on testosterone levels, stallions still showed much consistency in overall testosterone blood levels across exposure to the different types of mares (this study) and across exposure to mares or stallions over longer periods [37] (i.e. there are important between-subject differences in testosterone levels that remain unexplained). By taking weekly blood samples, we concentrated on average testosterone levels over longer periods of time. It would also be interesting to study short-term changes in testosterone levels (e.g. during the first minutes or hours after the introduction of a female [22–24]) and to test whether such short-term changes can influence semen characteristics.
Sperm competition theory predicts differences in semen characteristics to be plastically adjusted to how males perceive female quality (i.e. to reflect male mate preferences) [28,29]. This may be especially the case in polygynous species such as the horse [53]. Indeed, we found sperm number per ejaculate (but not sperm velocity) to be dependent either on the order of presenting MHC-similar and -dissimilar mares or on the MHC sharing to the last mares the stallions were exposed to. Kelly & Jennions [29] argued that of all semen characteristics, sperm number is the trait that is most likely to show phenotypic plasticity because rapid changes may be comparatively easily achieved. Moreover, sperm number in horses is positively correlated with blood testosterone levels in males when exposed to females [37], and testosterone levels can be expected to react quickly to social stimuli. Plasticity in sperm velocity is likely to require more time to be implemented than plasticity in sperm number because changes in velocity may involve changes in sperm morphology and ATP content that would have to be realized at one stage during the 57 days of spermiogenesis [31,35,40] while relative expenditure of available sperm could potentially be determined during ejaculation only. The fact that we found significant treatment effects on sperm number but not on sperm velocity therefore suggests that MHC sharing with the last mares the stallions were exposed to was responsible for the effects we observed. We cannot exclude that longer exposure to the same type of mare would affect sperm velocity. Burger et al. [37] compared longer periods of exposure to stallions or to mares (eight weeks each) and found one of three sperm velocity measures (VCL) to be elevated when stallions were exposed to other stallions. However, very short exposures to a novel ovariectomized mare did not seem to influence strategic ejaculation: the MHC of the teaser mare during semen collection did not produce significant effects on any semen characteristics.
Our study was based on 12 stallions only (i.e. on a comparatively small sample size as compared with other studies on MHC effects). We compensated for this fact by using powerful within-subject comparisons, averaging repeated measures over a period of in total 18 weeks under very standardized conditions and concentrating a priori on only three dependent variables (testosterone blood level, sperm number and sperm velocity). Even if sperm velocity was determined in three ways, these three measures were highly correlated and showed no treatment effect, while testosterone blood level and sperm number did. It seems unlikely that there are many similar experiments on large mammals that remained unpublished because of no significant treatment effects. We therefore argue that despite the low sample size, our study is not specifically prone to type I error.
In conclusion, male testosterone levels and sperm number per ejaculate changed in reaction to the sharing of male and female MHC types. Stallions appeared to invest more in attractiveness and ejaculate quality when exposed to an MHC-dissimilar mare than when exposed to an MHC-similar mare. These changes are likely to reveal male preferences that have evolved to reduce inbreeding (MHC-linked cues being used as marker of kinship), or to increase MHC heterozygosity [36] and hence improve resistance, especially to co-infections [54].
Supplementary Material
Acknowledgements
We thank G. Cosentino and F. Flahaut of the Laboratory Dr. Risch, CH-Liebefeld, for the testosterone analyses, C. F. Frey, J. Janda, S. Lazary, S. Meinecke-Tillmann, C. Meuwly, S. Thomas and the ‘vet team’ of the Swiss Institute of Equine Medicine for discussion and/or assistance, and D. Penn and three reviewers for helpful comments on the manuscript
Ethics statement
Animal experimentation was performed following approval from the local animal ethics committee (Etat de Vaud, Service Vétérinaire, approval no. 2454).
Data accessibility
Data associated with this paper are deposited in the Dryad Digital Repository (doi:10.5061/dryad.b0k20).
Funding statement
We thank ISMEquine Research for financial support.
Authors' contributions
D.B., H.S. and C.W. designed the study, G.D. and E.M. performed the experiments, D.B. and C.W. analysed the data and wrote the manuscript.
References
- 1.Davies DM. 2013. The compatibility gene. London, UK: Allen Lane. [Google Scholar]
- 2.Bos DH, Williams RN, Gopurenko D, Bulut Z, Dewoody JA. 2009. Condition-dependent mate choice and a reproductive disadvantage for MHC-divergent male tiger salamanders. Mol. Ecol. 18, 3307–3315. ( 10.1111/j.1365-294X.2009.04242.x) [DOI] [PubMed] [Google Scholar]
- 3.Miller HC, Moore JA, Nelson NJ, Daugherty CH. 2009. Influence of major histocompatibility complex genotype on mating success in a free-ranging reptile population. Proc. R. Soc. B 276, 1695–1704. ( 10.1098/rspb.2008.1840) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eizaguirre C, Lenz TL, Sommerfeld RD, Harrod C, Kalbe M, Milinski M. 2011. Parasite diversity, patterns of MHC II variation and olfactory based mate choice in diverging three-spined stickleback ecotypes. Evol. Ecol. 25, 605–622. ( 10.1007/s10682-010-9424-z) [DOI] [Google Scholar]
- 5.Setchell JM, Vaglio S, Abbott KM, Moggi-Cecchi J, Boscaro F, Pieraccini G, Knapp LA. 2011. Odour signals major histocompatibility complex genotype in an Old World monkey. Proc. R. Soc. B 278, 274–280. ( 10.1098/rspb.2010.0571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baratti M, et al. 2012. MHC genotype predicts mate choice in the ring-necked pheasant Phasianus colchicus. J. Evol. Biol. 25, 1531–1542. ( 10.1111/j.1420-9101.2012.02534.x) [DOI] [PubMed] [Google Scholar]
- 7.Cutrera AP, Sol Fanjul M, Rita Zenuto R. 2012. Females prefer good genes: MHC-associated mate choice in wild and captive tuco-tucos. Anim. Behav. 83, 847–856. ( 10.1016/j.anbehav.2012.01.006) [DOI] [Google Scholar]
- 8.Evans ML, Dionne M, Miller KM, Bernatchez L. 2012. Mate choice for major histocompatibility complex genetic divergence as a bet-hedging strategy in the Atlantic salmon (Salmo salar). Proc. R. Soc. B 279, 379–386. ( 10.1098/rspb.2011.0909) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juola FA, Dearborn DC. 2012. Sequence-based evidence for major histocompatibility complex-disassortative mating in a colonial seabird. Proc. R. Soc. B 279, 153–162. ( 10.1098/rspb.2011.0562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Farrell B, Benzie JAH, McGinnity P, Carlsson J, de Eyto E, Dillane E, Graham C, Coughlan J, Cross T. 2012. MHC-mediated spatial distribution in brown trout (Salmo trutta) fry. Heredity 108, 403–409. ( 10.1038/hdy.2011.87) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reichard M, Spence R, Bryjova A, Bryja J, Smith C. 2012. Female rose bitterling prefer MHC-dissimilar males: experimental evidence. PLoS ONE 7, e40780 ( 10.1371/journal.pone.0040780) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strandh M, Westerdahl H, Pontarp M, Canback B, Dubois M-P, Miquel C, Taberlet P, Bonadonna F. 2012. Major histocompatibility complex class II compatibility, but not class I, predicts mate choice in a bird with highly developed olfaction. Proc. R. Soc. B 279, 4457–4463. ( 10.1098/rspb.2012.1562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milinski M, Croy I, Hummel T, Boehm T. 2013. Major histocompatibility complex peptide ligands as olfactory cues in human body odour assessment. Proc. R. Soc. B 280, 20122889 ( 10.1098/rspb.2012.2889) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruff JS, Nelson AC, Kubinak JL, Potts WK. 2012. MHC signaling during social communication. Adv. Exp. Med. Biol. 738, 290–313. ( 10.1007/978-1-4614-1680-7_17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts SC, Gosling LM. 2003. Genetic similarity and quality interact in mate choice decisions by female mice. Nat. Genet. 35, 103–106. ( 10.1038/ng1231) [DOI] [PubMed] [Google Scholar]
- 16.Yamazaki K, Boyse EA, Mike V, Thaler HT, Mathieson BJ, Abbott J, Boyse J, Zayas ZA, Thomas L. 1976. Control of mating preference in mice by genes in the major histocompatibility complex. J. Exp. Med. 144, 1324–1335. ( 10.1084/jem.144.5.1324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamazaki K, Yamaguchi M, Baranoski L, Bard J, Boyse EA, Thomas L. 1979. Recognition among mice: evidence from the use of a Y-maze differentially scented by congenic mice of different major histocompatibility types. J. Exp. Med. 150, 755–760. ( 10.1084/jem.150.4.755) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wedekind C, Füri S. 1997. Body odour preferences in men and women: do they aim for specific MHC combinations or simply heterozygosity? Proc. R. Soc. Lond. B 264, 1471–1479. ( 10.1098/rspb.1997.0204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bribiescas RG. 2001. Reproductive ecology and life history of the human male. Yearb. Phys. Anthropol. 44, 148–176. ( 10.1002/ajpa.10025) [DOI] [PubMed] [Google Scholar]
- 20.Ellison PT. 2003. Energetics and reproductive effort. Am. J. Hum. Biol. 15, 342–351. ( 10.1002/ajhb.10152) [DOI] [PubMed] [Google Scholar]
- 21.Moore MC. 1991. Application of organization: activation theory to alternative male reproductive strategies: a review. Horm. Behav. 25, 154–179. ( 10.1016/0018-506X(91)90048-M) [DOI] [PubMed] [Google Scholar]
- 22.Macrides F, Bartke A, Dalterio S. 1975. Strange females increase plasma testosterone levels in male mice. Science 189, 1104–1106. ( 10.1126/science.1162363) [DOI] [PubMed] [Google Scholar]
- 23.Gleason ED, Fuxjager MJ, Oyegbile TO, Marler CA. 2009. Testosterone release and social context: when it occurs and why. Front. Neuroendocrinol. 30, 460–469. ( 10.1016/j.yfrne.2009.04.009) [DOI] [PubMed] [Google Scholar]
- 24.Petrulis A. 2013. Chemosignals, hormones and mammalian reproduction. Horm. Behav. 63, 723–741. ( 10.1016/j.yhbeh.2013.03.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wingfield JC, Hegner RE, Dufty AMJ, Ball GF. 1990. The ‘challenge hypothesis’: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am. Nat. 136, 829–846. ( 10.1086/285134) [DOI] [Google Scholar]
- 26.Hirschenhauser K, Oliveira RF. 2006. Social modulation of androgens in male vertebrates: meta-analyses of the challenge hypothesis. Anim. Behav. 71, 265–277. ( 10.1016/j.anbehav.2005.04.014) [DOI] [Google Scholar]
- 27.Wedell N, Gage MJG, Parker GA. 2002. Sperm competition, male prudence and sperm-limited females. Trends Ecol. Evol. 17, 313–320. ( 10.1016/S0169-5347(02)02533-8) [DOI] [Google Scholar]
- 28.Parker GA, Pizzari T. 2010. Sperm competition and ejaculate economics. Biol. Rev. 85, 897–934. ( 10.1111/j.1469-185X.2010.00140.x) [DOI] [PubMed] [Google Scholar]
- 29.Kelly CD, Jennions MD. 2011. Sexual selection and sperm quantity: meta-analyses of strategic ejaculation. Biol. Rev. 86, 863–884. ( 10.1111/j.1469-185X.2011.00175.x) [DOI] [PubMed] [Google Scholar]
- 30.Fitzpatrick JL, Montgomerie R, Desjardins JK, Stiver KA, Kolm N, Balshine S. 2009. Female promiscuity promotes the evolution of faster sperm in cichlid fishes. Proc. Natl Acad. Sci. USA 106, 1128–1132. ( 10.1073/pnas.0809990106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomez Montoto L, Varea Sanchez M, Tourmente M, Martin-Coello J, Jose Luque-Larena J, Gomendio M, Roldan ERS. 2011. Sperm competition differentially affects swimming velocity and size of spermatozoa from closely related muroid rodents: head first. Reproduction 142, 819–830. ( 10.1530/rep-11-0232) [DOI] [PubMed] [Google Scholar]
- 32.Gage MJG, Macfarlane CP, Yeates S, Ward RG, Searle JB, Parker GA. 2004. Spermatozoal traits and sperm competition in Atlantic salmon: relative sperm velocity is the primary determinant of fertilization success. Curr. Biol. 14, 44–47. ( 10.1016/j.cub.2003.12.028) [DOI] [PubMed] [Google Scholar]
- 33.Pizzari T, Parker GA. 2009. Sperm competition and sperm phenotype. In Sperm biology: an evolutionary perspective (eds Birkhead TR, Hosken DJ, Pitnick SS.), pp. 207–245. Oxford, UK: Academic Press. [Google Scholar]
- 34.Gasparini C, Simmons LW, Beveridge M, Evans JP. 2010. Sperm swimming velocity predicts competitive fertilization success in the green swordtail Xiphophorus helleri. PLoS ONE 5, e0012146 ( 10.1371/journal.pone.0012146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tourmente M, Rowe M, Mar Gonzalez-Barroso M, Rial E, Gomendio M, Roldan ERS. 2013. Postcopulatory sexual selection increases ATP content in rodent spermatozoa. Evolution 67, 1838–1846. ( 10.1111/evo.12079) [DOI] [PubMed] [Google Scholar]
- 36.Penn DJ, Potts WK. 1999. The evolution of mating preferences and major histocompatibility complex genes. Am. Nat. 153, 145–164. ( 10.1086/303166) [DOI] [PubMed] [Google Scholar]
- 37.Burger D, Dolivo G, Wedekind C. Submitted. Ejaculate characteristics dependent on social environment in the horse (Equus caballus). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lazary S, Antczak DF, Bailey E, Bell TK, Bernoco D, Byrns G, McClure JJ. 1988. Joint report of the 5th iternational workshop on lymphocyte alloantigens of the horse, Baton Rouge, Louisiana, 31 October–1 November 1987. Anim. Genet. 19, 447–456. ( 10.1111/j.1365-2052.1988.tb00836) [DOI] [PubMed] [Google Scholar]
- 39.Janett F, Stump R, Burger D, Thun R. 2009. Suppression of testicular function and sexual behaviour by vaccination against GnRH (EquityTM) in the adult stallion. Anim. Reprod. Sci. 115, 88–102. ( 10.1016/j.anireprosci.2008.11.011) [DOI] [PubMed] [Google Scholar]
- 40.Tourmente M, Gomendio M, Roldan ERS. 2011. Sperm competition and the evolution of sperm design in mammals. BMC Evol. Biol. 11 ( 10.1186/1471-2148-11-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson L, Blanchard TL, Varner DD, Scrutchfield WL. 1997. Factors affecting spermatogenesis in the stallion. Theriogenology 48, 1199–1216. ( 10.1016/s0093-691x(97)00353-1) [DOI] [PubMed] [Google Scholar]
- 42.Gebauer MR, Pickett BW, Swierstr EE. 1974. Reproductive physiology of the stallion. 3. Extra-gonadal transit time and sperm reserves. J. Anim. Sci. 39, 737–742. ( 10.2134/jas1974.394737x) [DOI] [PubMed] [Google Scholar]
- 43.Berndtson WE. 1977. Methods for quantifying mammalian spermatogenesis: review. J. Anim. Sci. 44, 818–833. ( 10.2134/jas1977) [DOI] [PubMed] [Google Scholar]
- 44.Bauer C. 2006. Untersuchungsmethoden. In Veterinär-medizinische Parasitologie (ed. Schnieder T.), pp. 89–94. Stuttgart, Germany: Parey. [Google Scholar]
- 45.Trivers RL. 1972. Parental investment and sexual selection. In Sexual selection and the descent of man 1871–1971 (ed. Campbell B.), pp. 136–179. Chicago, IL: Aldine. [Google Scholar]
- 46.Cluttonbrock TH, Parker GA. 1992. Potential reproductive rates and the operation of sexual selection. Q. Rev. Biol. 67, 437–456. ( 10.1086/417793) [DOI] [Google Scholar]
- 47.Yamazaki K, Beauchamp GK, Kupniewski D, Bard J, Thomas L, Boyse EA. 1988. Familial imprinting determines H-2 selective mating preferences. Science 240, 1331–1332. ( 10.1126/science.3375818) [DOI] [PubMed] [Google Scholar]
- 48.Penn D, Potts W. 1998. MHC-disassortative mating preferences reversed by cross fostering. Proc. R. Soc. Lond. B 265, 1299–1306. ( 10.1098/rspb.1998.0433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ziegler A, Kentenich H, Uchanska-Ziegier B. 2005. Female choice and the MHC. Trends Immunol. 26, 496–502. ( 10.1016/j.it.2005.07.003) [DOI] [PubMed] [Google Scholar]
- 50.McDonnell SM, Murray SC. 1995. Bachelor and harem stallion behavior and endocrinology. Biol. Reprod. Monogr. 1, 577–590. [Google Scholar]
- 51.Hamilton WD. 1964. The genetical evolution of social behaviour. J. Theor. Biol. 7, 1–16. ( 10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- 52.Tang-Martinez Z. 2001. The mechanisms of kin discrimination and the evolution of kin recognitionin vertebrates: a critical re-evaluation. Behav. Proc. 53, 21–40. ( 10.1016/S0376-6357(00)00148-0) [DOI] [PubMed] [Google Scholar]
- 53.Pizzari T, Wedell N. 2013. The polyandry revolution. Phil. Trans. R. Soc. B 368, 20120041 ( 10.1098/rstb.2012.0041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McClelland EE, Penn D, Potts WK. 2003. Major histocompatibility complex heterozygote superiority during coinfection. Infect. Immun. 71, 2079–2086. ( 10.1128/IAI.71.4.2079-2086.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data associated with this paper are deposited in the Dryad Digital Repository (doi:10.5061/dryad.b0k20).