Abstract
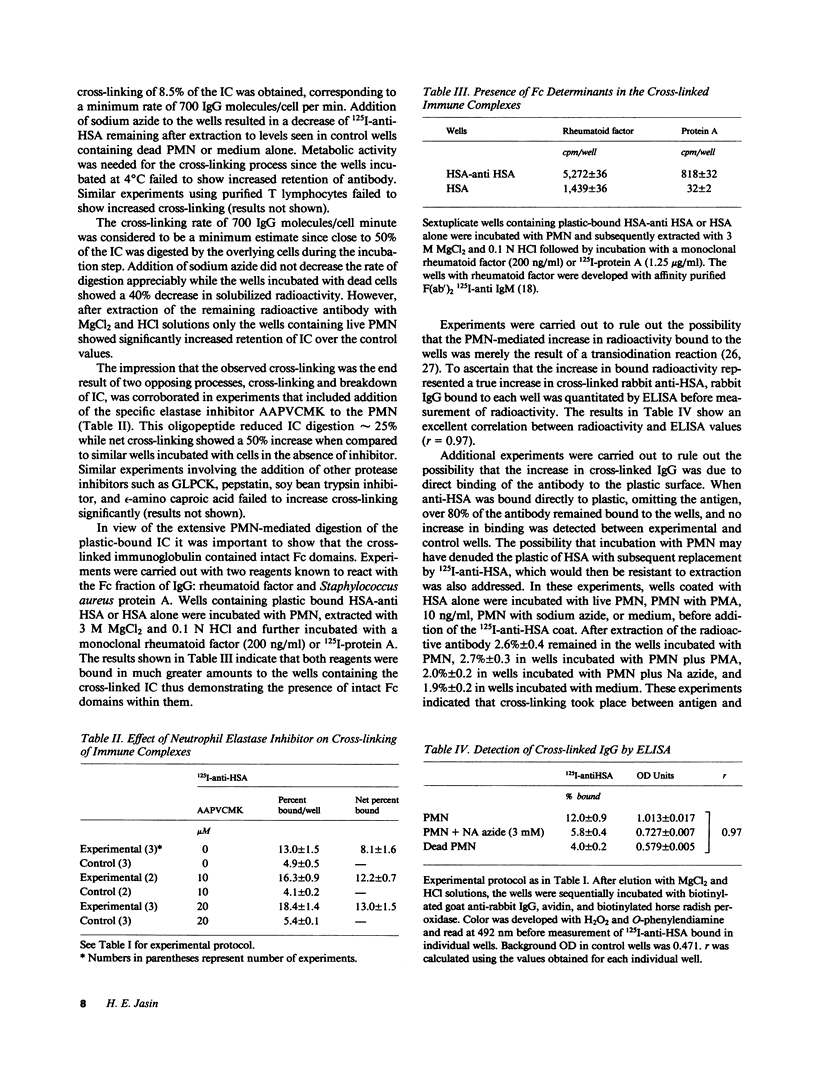
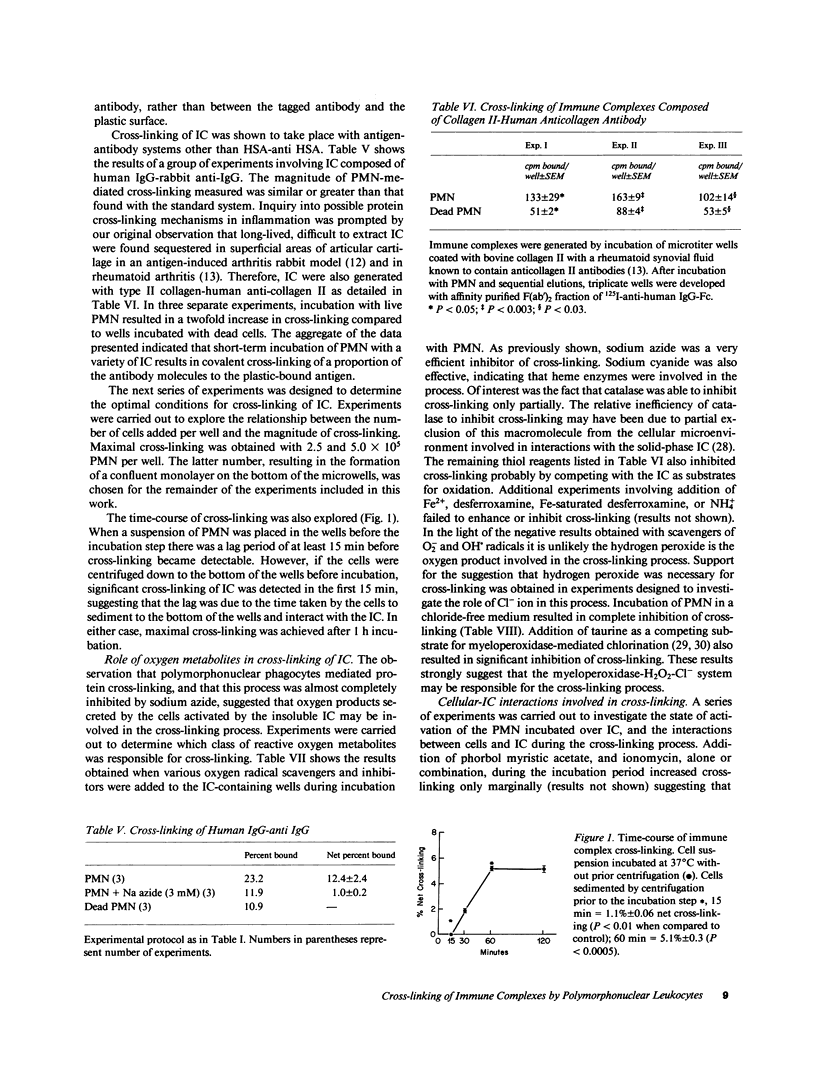
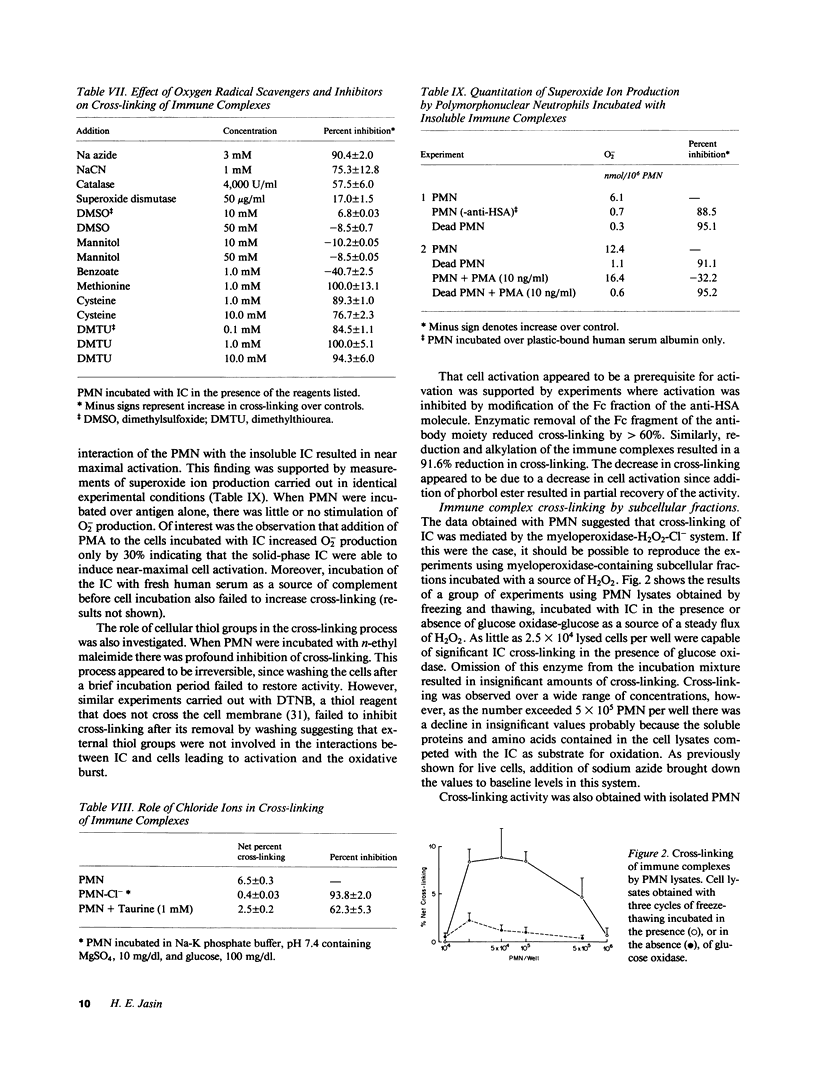
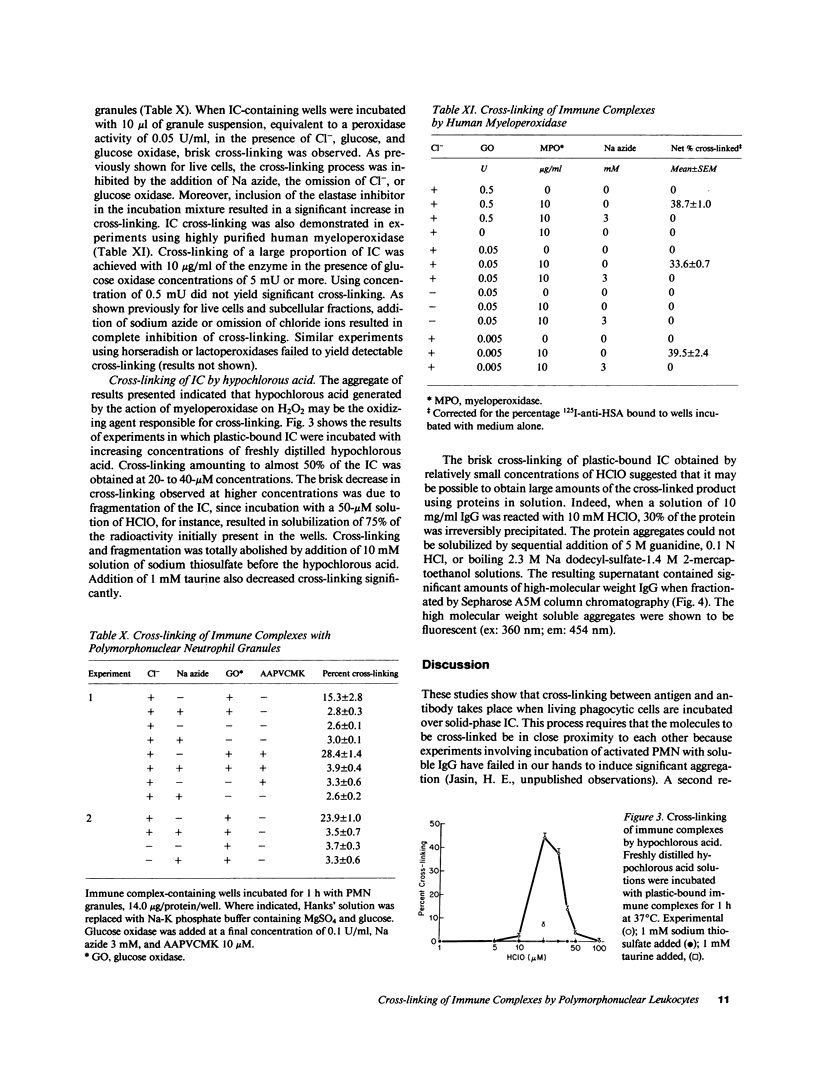
Incubation of human serum albumin-anti-human serum albumin immune complexes bound to a plastic surface, with human polymorphonuclear leukocytes for 1 h at 37 degrees C resulted in covalent cross-linking of 8.5% +/- 0.5 of the complexes, corresponding to a minimum rate of 700 antibody molecules per cell per minute. Similar results were obtained with IgG-anti-IgG and type II collagen-anticollagen II human antibodies. Cross-linking was defined as the antibody remaining attached to plastic-bound antigen after extraction with 3 M MgCl2 and 0.1 N HCl solutions. The effects of addition of oxygen radical scavengers, heme-enzyme inhibitors, and omission of Cl- indicated that the cross-linking process was mediated by the myeloperoxidase-H2O2-Cl- system. Cross-linking was also obtained with cell lysates, polymorphonuclear granules, and purified human myeloperoxidase in the presence of a steady flux of H2O2 provided by glucose oxidase-glucose. Cross-linking by the cell-free systems was also abolished by sodium azide or omission of chloride ions. Cross-linked immune complexes were also generated by incubation with 20 to 50 microM solutions of freshly distilled hypochlorous acid. Addition of 10 mM hypochlorous acid to soluble IgG resulted in the formation of protein precipitates insoluble in 5 M guanidine, 0.1 N HCl, or boiling 2.3 M sodium dodecyl sulfate-1.4 M 2-mercaptoethanol. The remaining soluble IgG contained fluorescent high molecular aggregates (ex: 360 nm; em: 454 nm). Oxidative cross-linking of antigen-antibody molecules, and of immune complexes to connective tissue macromolecules may play a pathogenic role in acute and chronic inflammatory processes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adeniyi-Jones S. K., Karnovsky M. L. Oxidative decarboxylation of free and peptide-linked amino acids in phagocytizing guinea pig granulocytes. J Clin Invest. 1981 Aug;68(2):365–373. doi: 10.1172/JCI110264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrich J. M., McCarthy C. A., Hurst J. K. Biological reactivity of hypochlorous acid: implications for microbicidal mechanisms of leukocyte myeloperoxidase. Proc Natl Acad Sci U S A. 1981 Jan;78(1):210–214. doi: 10.1073/pnas.78.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amit A. G., Mariuzza R. A., Phillips S. E., Poljak R. J. Three-dimensional structure of an antigen-antibody complex at 2.8 A resolution. Science. 1986 Aug 15;233(4765):747–753. doi: 10.1126/science.2426778. [DOI] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Bailey A. J., Ranta M. H., Nicholls A. C., Partridge S. M., Elsden D. F. Isolation of alpha-amino adipic acid from mature dermal collagen and elastin. Evidence for an oxidative pathway in the maturation of collagen and elastin. Biochem Biophys Res Commun. 1977 Oct 24;78(4):1403–1410. doi: 10.1016/0006-291x(77)91448-6. [DOI] [PubMed] [Google Scholar]
- Barnett Foster D. E., Dorrington K. J., Painter R. H. Structure and function of immunoglobulin domains. VII. Studies on the structural requirements of human immunoglobulin G for granulocyte binding. J Immunol. 1978 Jun;120(6):1952–1956. [PubMed] [Google Scholar]
- COHEN S., PORTER R. B. STRUCTURE AND BIOLOGICAL ACTIVITY OF IMMUNOGLOBULINS. Adv Immunol. 1964;27:287–349. doi: 10.1016/s0065-2776(08)60710-5. [DOI] [PubMed] [Google Scholar]
- Campbell E. J., Senior R. M., McDonald J. A., Cox D. L. Proteolysis by neutrophils. Relative importance of cell-substrate contact and oxidative inactivation of proteinase inhibitors in vitro. J Clin Invest. 1982 Oct;70(4):845–852. doi: 10.1172/JCI110681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodirker W. B., Bock G. N., Vaughan J. H. Isolation of human PMN leukocytes and granules: observations on early blood diluion and on heparin. J Lab Clin Med. 1968 Jan;71(1):9–19. [PubMed] [Google Scholar]
- Clark S., Harrison L. C. Disulfide exchange between insulin and its receptor. A possible post-binding step in insulin action. J Biol Chem. 1983 Oct 10;258(19):11434–11437. [PubMed] [Google Scholar]
- Cooke T. D., Hurd E. R., Ziff M., Jasin H. E. The pathogenesis of chronic inflammation in experimental antigen-induced arthritis. II. Preferential localization of antigen-antibody complexes to collagenous tissues. J Exp Med. 1972 Feb 1;135(2):323–338. doi: 10.1084/jem.135.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahinden C. A., Fehr J., Hugli T. E. Role of cell surface contact in the kinetics of superoxide production by granulocytes. J Clin Invest. 1983 Jul;72(1):113–121. doi: 10.1172/JCI110948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerder C. A., Klebanoff S. J., Shapiro B. M. Hydrogen peroxide production, chemiluminescence, and the respiratory burst of fertilization: interrelated events in early sea urchin development. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3183–3187. doi: 10.1073/pnas.75.7.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerder C. A., Shapiro B. M. Release of ovoperoxidase from sea urchin eggs hardens the fertilization membrane with tyrosine crosslinks. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4214–4218. doi: 10.1073/pnas.74.10.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote C. S., Goyne T. E., Lehrer R. I. Assessment of chlorination by human neutrophils. Nature. 1983 Feb 24;301(5902):715–716. doi: 10.1038/301715a0. [DOI] [PubMed] [Google Scholar]
- Henson P. M. The immunologic release of constituents from neutrophil leukocytes. I. The role of antibody and complement on nonphagocytosable surfaces or phagocytosable particles. J Immunol. 1971 Dec;107(6):1535–1546. [PubMed] [Google Scholar]
- Jasin H. E. Autoantibody specificities of immune complexes sequestered in articular cartilage of patients with rheumatoid arthritis and osteoarthritis. Arthritis Rheum. 1985 Mar;28(3):241–248. doi: 10.1002/art.1780280302. [DOI] [PubMed] [Google Scholar]
- Jasin H. E. Generation of IgG aggregates by the myeloperoxidase-hydrogen peroxide system. J Immunol. 1983 Apr;130(4):1918–1923. [PubMed] [Google Scholar]
- Keightley R. G., Cooper M. D., Lawton A. R. The T cell dependence of B cell differentiation induced by pokeweed mitogen. J Immunol. 1976 Nov;117(5 Pt 1):1538–1544. [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967 Dec 1;126(6):1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunec J., Blake D. R., McCleary S. J., Brailsford S., Bacon P. A. Self-perpetuating mechanisms of immunoglobulin G aggregation in rheumatoid inflammation. J Clin Invest. 1985 Dec;76(6):2084–2090. doi: 10.1172/JCI112212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARSTON H. R. Cobalt, copper and molybdenum in the nutrition of animals and plants. Physiol Rev. 1952 Jan;32(1):66–121. doi: 10.1152/physrev.1952.32.1.66. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem. 1968 Nov 10;243(21):5753–5760. [PubMed] [Google Scholar]
- Olsen N., Ziff M., Jasin H. E. In vitro synthesis of immunoglobulins and IgM-rheumatoid factor by blood mononuclear cells of patients with rheumatoid arthritis. Rheumatol Int. 1982;2(2):59–66. doi: 10.1007/BF00541247. [DOI] [PubMed] [Google Scholar]
- Paul B. B., Jacobs A. A., Strauss R. R., Sbarra A. J. Role of the Phagocyte in Host-Parasite Interactions XXIV. Aldehyde Generation by the Myeloperoxidase-H(2)O(2)-Chloride Antimicrobial System: a Possible In Vivo Mechanism of Action. Infect Immun. 1970 Oct;2(4):414–418. doi: 10.1128/iai.2.4.414-418.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I., Tuderman L., Guzman N. A. The biosynthesis of collagen and its disorders (first of two parts). N Engl J Med. 1979 Jul 5;301(1):13–23. doi: 10.1056/NEJM197907053010104. [DOI] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Stossel T. P. Myeloperoxidase-mediated iodination by granulocytes. Intracellular site of operation and some regulating factors. J Clin Invest. 1974 May;53(5):1207–1215. doi: 10.1172/JCI107667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauberman N., Fortier N. L., Joshi W., Piotrowski J., Snyder L. M. Spectrin-haemoglobin crosslinkages associated with in vitro oxidant hypersensitivity in pathologic and artificially dehydrated red cells. Br J Haematol. 1983 May;54(1):15–28. doi: 10.1111/j.1365-2141.1983.tb02063.x. [DOI] [PubMed] [Google Scholar]
- Selvaraj R. J., Paul B. B., Strauss R. R., Jacobs A. A., Sbarra A. J. Oxidative peptide cleavage and decarboxylation by the MPO-H2O2-Cl- antimicrobial system. Infect Immun. 1974 Feb;9(2):255–260. doi: 10.1128/iai.9.2.255-260.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R. C. Biosynthesis of collagen crosslinks: increased activity of purified lysyl oxidase with reconstituted collagen fibrils. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4826–4830. doi: 10.1073/pnas.71.12.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R. C., Fu J. C. Collagen cross-linking. Purification and substrate specificity of lysyl oxidase. J Biol Chem. 1976 Sep 25;251(18):5779–5785. [PubMed] [Google Scholar]
- Stahmann M. A., Spencer A. K. Cross linking of proteins in vitro by peroxidase. Biopolymers. 1977 Jun;16(6):1307–1318. doi: 10.1002/bip.1977.360160611. [DOI] [PubMed] [Google Scholar]
- Stahmann M. A., Spencer A. K. Deamination of protein lysyl epsilon-amino groups by peroxidase in vitro. Biopolymers. 1977 Jun;16(6):1299–1306. doi: 10.1002/bip.1977.360160610. [DOI] [PubMed] [Google Scholar]
- Strauss R. R., Paul B. B., Jacobs A. A., Sbarra A. J. Role of the Phagocyte in Host-Parasite Interactions XXVII. Myeloperoxidase-H(2)O(2)-Cl-Mediated Aldehyde Formation and Its Relationship to Antimicrobial Activity. Infect Immun. 1971 Apr;3(4):595–602. doi: 10.1128/iai.3.4.595-602.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X. L., Olsen N., Ziff M., Jasin H. E. Human IgG aggregates induce selective stimulation of IgM rheumatoid factor synthesis by rheumatoid blood mononuclear cells. Arthritis Rheum. 1984 May;27(5):502–508. doi: 10.1002/art.1780270504. [DOI] [PubMed] [Google Scholar]
- Test S. T., Lampert M. B., Ossanna P. J., Thoene J. G., Weiss S. J. Generation of nitrogen-chlorine oxidants by human phagocytes. J Clin Invest. 1984 Oct;74(4):1341–1349. doi: 10.1172/JCI111544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Dixon F. J. The biology and detection of immune complexes. Adv Immunol. 1979;28:89–220. doi: 10.1016/s0065-2776(08)60800-7. [DOI] [PubMed] [Google Scholar]
- Thomas E. L. Myeloperoxidase, hydrogen peroxide, chloride antimicrobial system: nitrogen-chlorine derivatives of bacterial components in bactericidal action against Escherichia coli. Infect Immun. 1979 Feb;23(2):522–531. doi: 10.1128/iai.23.2.522-531.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Klein R., Slivka A., Wei M. Chlorination of taurine by human neutrophils. Evidence for hypochlorous acid generation. J Clin Invest. 1982 Sep;70(3):598–607. doi: 10.1172/JCI110652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann G., Smolen J. E., Korchak H. M. Release of inflammatory mediators from stimulated neutrophils. N Engl J Med. 1980 Jul 3;303(1):27–34. doi: 10.1056/NEJM198007033030109. [DOI] [PubMed] [Google Scholar]
- Zgliczyński J. M., Stelmaszyńska T., Domański J., Ostrowski W. Chloramines as intermediates of oxidation reaction of amino acids by myeloperoxidase. Biochim Biophys Acta. 1971 Jun 16;235(3):419–424. doi: 10.1016/0005-2744(71)90281-6. [DOI] [PubMed] [Google Scholar]
- Zgliczyński J. M., Stelmaszyńska T., Ostrowiski W., Naskalski J., Sznajd J. Myeloperoxidase of human leukaemic leucocytes. Oxidation of amino acids in the presence of hydrogen peroxide. Eur J Biochem. 1968 May;4(4):540–547. doi: 10.1111/j.1432-1033.1968.tb00246.x. [DOI] [PubMed] [Google Scholar]


