Abstract
Mast cells are classically considered innate immune cells that act as first responders in many microbial infections and have long been appreciated as potent contributors to allergic reactions. However, recent advances in the realm of autoimmunity have made it clear that these cells are also involved in the pathogenic responses that exacerbate disease. In the murine models of multiple sclerosis, rheumatoid arthritis and bullous pemphigoid, both the pathogenic role of mast cells and some of their mechanisms of action are shared. Similar to their role in infection and a subset of allergic responses, mast cells are required for the efficient recruitment of neutrophils to sites of inflammation. Although this mast cell-dependent neutrophil response is protective in infection settings, it is postulated that neutrophils promote local vascular permeability and facilitate the entry of inflammatory cells that enhance tissue destruction at target sites. However, there is still much to learn. There is little information regarding mechanisms of mast cell activation in disease. Nor is it known how many mast cell-derived mediators are relevant and whether interactions with other cells are implicated in these diseases including T cells, B cells and astrocytes. Here we review the current state of knowledge about mast cells in autoimmune disease. We also discuss findings regarding newly discovered mast cell actions and factors that modulate mast cell function. We speculate that much of this new information will ultimately contribute to a greater understanding of the full range of mast cell actions in autoimmunity.
Keywords: Mast cells, Autoimmunity, TNF, Neutrophils, Inflammation
INTRODUCTION: THE MAST CELL, THE “ JACK OF ALL TRADES” IMMUNE CELL
Mast cells are members of the innate immune system that develop from CD34+ hematopoietic precursor cells in the bone marrow and circulate in the blood in an immature form. Only after they have established residency in a particular tissue, do they complete their tissue-specific differentiation and maturation [1]. Mast cells are considered first line defenders against infections because of their prevalence in tissues such as the skin, gut, respiratory tract and urinary tract that form the barriers between self and the environment. They are also found in close association with blood vessels, lymphatic vessels and nerves. These anatomical locations license mast cells to contribute to a multitude of protective and pathologic events including angiogenesis, wound healing and the exacerbation of inflammation (Reviewed in [2,3,4]).
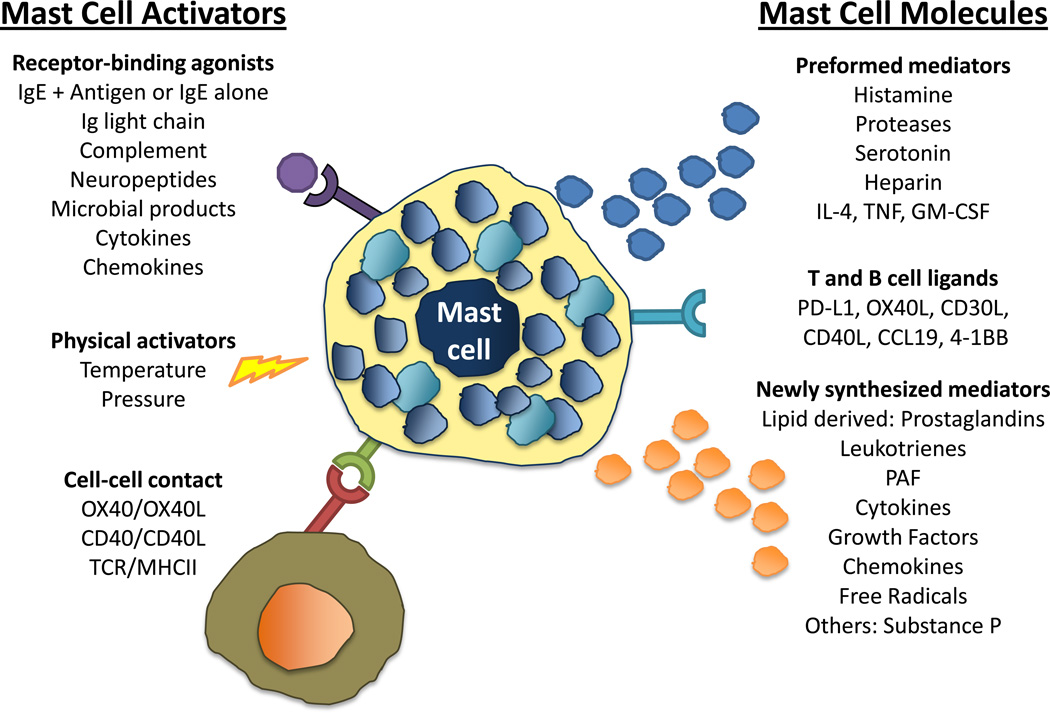
The potential for mast cells to influence a wide range of physiological events also stems from their ability to be activated by both “immune” and “non immune” stimuli. Mast cells are infamous for their role in hypersensitivity reactions where they are activated by cross-linking of the high affinity IgE receptor (FcεRI). Tissue mast cells are the major resident cell population expressing FcεRI and IgE-antigen cross-linking results in the release of several preformed molecules that are stored in mast cell granules including include histamine, serotonin, tryptase, chymase as well as the lipid-derived mediators prostaglandin D2 (PGD2) and leukotriene B4 (LTB4) (Figure 1). These mediators affect many aspects of the early-phase allergic reactions such as vasodilation, local or systemic increases in vascular permeability, constriction of smooth muscle and mucus secretion. Such activation also leads to the release of newly synthesized mediators (Figure 1), which can initiate more severe and prolonged late-phase allergic responses [5].
Figure 1. The mast cell, the “jack of all trades” immune cell.
The multitude of activators and the many modes of mast cell response account for their ability impact a variety of physiological and pathogenic processes. Receptor-binding agonists, physical activators and cell-cell contact can all activate mast cells. Responses to activation are heterogeneous and include the release of preformed mediators stored within granules and the synthesis and release of new products. Activated mast cells can also respond by increasing the expression of ligands that mediate cell interactions with T and B cells.
IgE-independent activation of mast cells is more prevalent in non-allergic responses. Mast cells can be activated by IgG-antigen complexes, pathogen associated molecular patterns (PAMPs), complement, cell-cell contact, cytokines, certain drugs, hormones and physical activators such as temperature and pressure. These stimuli can also result in the release of both preformed and newly synthesized mediators, including cytokines and chemokines, and regulate the expression of ligands on mast cells that allow direct cell-cell interaction with T and B cells [5,6] (Figure 1).
In the last ten years it has become evident that mast cells are critical players in the defense against certain bacterial, parasitic and viral infections. In bacterial infections such as Citrobacter rodentium, E. coli, and Heliobactor felis a major role for mast cells is to produce TNF and leukotrienes that enhance early neutrophil recruitment resulting in the escalation of the host defense [7,8]. In helminth infections of the gut, mast cells aid in resolving infection by producing soluble mediators (e.g. leukotrienes, prostaglandins, histamine Th2-like cytokines and proteases) that promote luminal flow, nerve stimulation, gut contractility and moderate intestinal inflammation leading to parasitic expulsion [9,10]. Most studies of mast cells in viral infections have focused on their role HIV infection. It has been proposed in HIV/AIDS that mast cells play two pathogenic roles. First, mast cells are an inducible reservoir of infectious viral clones [11,12]. Secondly, throughout infection, viral glycoprotein gp120 is shed. Gp120 can then activate mast cells and basophils, via IgE bound to FcεRI, resulting in a Th2 dominated response which down-regulates the protective anti-viral response [13]. Mast cells have also been implicated in autoimmunity. Here we review the most recent data that supports a role for mast cells in various autoimmune diseases. We also discuss new information regarding mast cell activities in health and disease and speculate on how this may provide insights into their role in autoimmunity.
CURRENT MODELS TO STUDY MAST CELLS
Although cell co-culture assays have been useful in revealing the molecular basis of mast cell influence, the relevance of in vitro findings in disease settings is often unclear. The most definitive evidence of mast cell contributions in vivo is derived from studies using two strains of mast cell-deficient mice, (WBxC57BL/6) F1-KitW/KitWv (W/Wv) and C57BL/6KitWsh/KitWsh (W-sash). These mice carry mutations in the c-kit gene, historically termed the white spotting locus (W), that cause reduced tyrosine kinase-dependent c-kit signaling needed for proper mast cell development and survival [4,14,15,16,17]. Both W/Wv and W-sash mutations also result in other phenotypic abnormalities. W/Wv mice are anemic, neutropenic, have impaired melanogenesis and are sterile [16]. While W-sash mice are fertile and not anemic, they have neutrophilia, defects in skin pigmentation, exhibit an anxiety-like phenotype and show a time dependent loss in mast cells with full deficiency achieved only at 10–12 weeks of age [17,18].
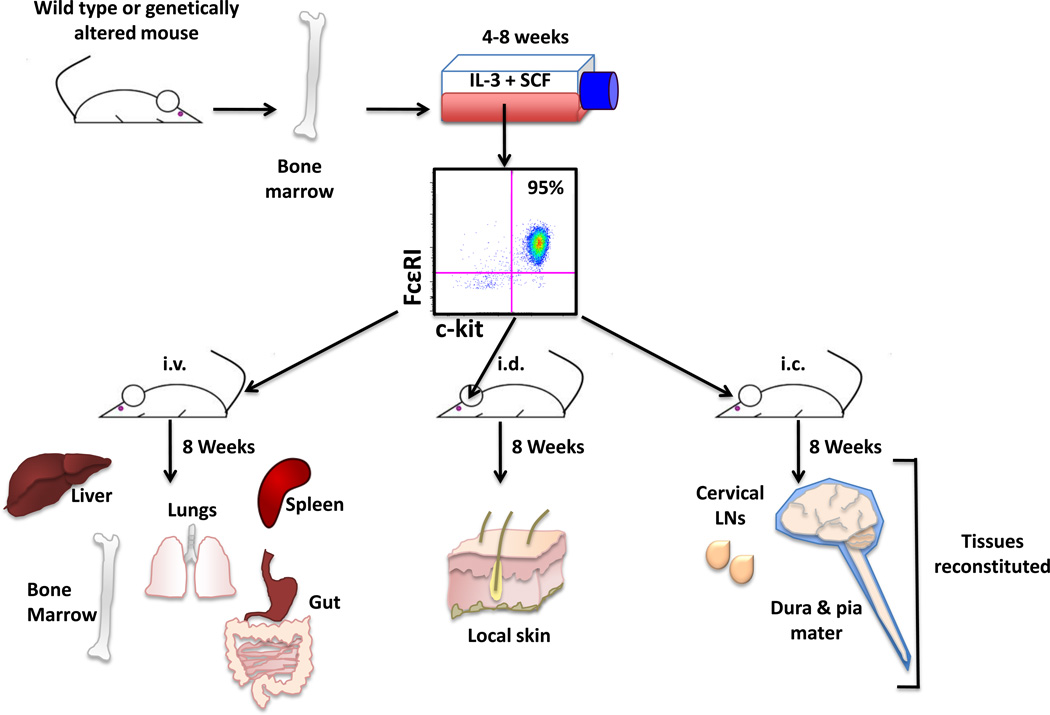
To verify mast cell contributions to a specific phenotype, mast cell populations can be reconstituted in these mice without prior irradiation by systemic or local injections of either whole bone marrow or in vitro bone marrow-derived mast cells (Figure 2). Such “knock in” strategies can also be exploited to determine which local mast cell subpopulation is relevant in disease, a difficult task given mast cells are ubiquitous in many tissues. For example, intravenous (i.v.) reconstitution restores many peripheral tissues but not the parachyma of the CNS [19], intracranial (i.c.) reconstitution restores mast cells to areas of the meninges (dura and pia mater) and cervical lymph nodes [20] and intradermal (i.d.) reconstitution restores mast cells to the local skin [15] (Figure 2). Although these models represent the current standard for the functional analyses of mast cells, the search for better models continues and there is great need for conditional mast cell knock-outs and mast cell-deficient animals on distinct genetic backgrounds.
Figure 2. Systemic and local mast cell reconstitution.
Mast cell “knock-in” strategies allow for the analysis of mast cell-specific contributions to physiological and pathological events. Mast cell populations can be derived from murine bone marrow cultured with IL-3 and SCF. There are multiple reconstitution techniques that result in the restoration of mast cells to select sets of tissues. This technique can be exploited to assess the requirement of mast cell residence within particular anatomical locations for particular phenotypes. Intravenous (i.v.) injection of mast cells results in the reconstitution of many but not all of the tissues where mast cells normally reside and include the liver, spleen, lungs, bone marrow, meninges and gut but not the parenchyma of the CNS. Intradermal (i.d.) injection of mast cells results in restoration of the local skin and intracranial (i.c.) reconstitution populates some areas of the meninges (dura and pia mater) and cervical lymph nodes.
AUTOIMMUNE DISEASE: ANOTHER MAST CELL-DEPENDENT “HYPERSENSITIVE” RESPONSE?
Some features of the immune responses in autoimmune disease are very much like those of traditional allergic responses. The antigen target is not inherently harmful and it is the overzealous immune response that causes the pathology. In addition, similar to allergies, T cells are important in directing and initiating the immune response to the target tissue, but other cells are perhaps equally important in exacerbating the inflammatory damage. There is a growing body of evidence that mast cells are involved in the exacerbation of several autoimmune diseases. Here we summarize the most unequivocal data from both human and mouse autoimmune disease studies.
Mast cells and multiple sclerosis (MS)
MS is a progressive demyelinating disease of the central nervous system (CNS) characterized by the presence of widespread inflammatory lesions in the brain and spinal cord. Symptoms of MS result from the interruption of myelinated tracts in the CNS and include visual disturbances, bowel and bladder incontinence, as well as sensory and motor dysfunction. Cognitive deficits are also common and include memory loss, impaired attention and slowed information processing [21,22]. Experimental autoimmune encephalomyelitis (EAE) is a widely studied rodent model of MS that was first described over 50 years ago [23,24]. As in MS, EAE is characterized by an early breach of the blood-brain barrier (BBB) allowing significant inflammatory cell infiltration into the CNS and targeted destruction of myelin and oligodendrocytes, the myelinproducing cells. The resulting demyelination of axons and often, axonal transection, along with concurring edema, leads to progressive paralysis [25,26]. Encephalitogenic CD4+ T cells specific for myelin antigens are major contributors to the pathogenic autoimmune response in EAE. IFNγ-secreting T helper 1 cells (Th1), IL-17 producing Th17 cells and IL-9 producing Th9 cells are implicated in disease [27]. The role for these cells in MS is still unclear [28].
Mast cells were first observed in the CNS lesions of MS patients over one hundred years ago [29] and data suggesting that mast cells play a role in MS have continued to accumulate. An increase of mast cells is commonly observed at sites of inflammatory demyelination in the brain and spinal cord of MS patients as well as in rodents with EAE [30,31]. Elevated levels of tryptase, a mast cell specific protease, are present in the cerebrospinal fluid of MS patients [32] and microarray analysis of MS lesions show that transcripts encoding tryptase, histamine R1 and FcεRI are significantly increased in chronic disease [33]. In vitro, mast cell proteases degrade myelin protein and myelin directly stimulates mast cell degranulation [34,35,36]. Treatment with inhibitors of mast cell degranulation (proxicromil), a serotonin receptor antagonist (cyproheptadine) or a depletor of vasoactive amines in mast cell granules (reserpine) inhibits EAE [37,38].
The most direct evidence for mast cell action in EAE comes from studies utilizing W/Wv mice in a model of primary progressive (PP) MS [39]. In this model disease is induced by immunization with MOG35–55 in CFA along with pertussis toxin. Mast cell deficiency leads to significantly less clinical disease that is associated with loss of BBB permeability and inflammatory cell infiltration into the parenchyma of the CNS [20,39]. Recent studies have implicated TNF produced by meningeal mast cells in these events. Consistent with the well established ability of mast cells to recruit neutrophils to sites of infection and allergic inflammatory responses, the early disease-associated entry of neutrophils into the meninges and the CNS parenchyma is abrogated in the absence of mast cells or mast cell-derived TNF [20,39].
SJL mice immunized with PLP139–151 in CFA exhibit relapsing remitting (RR) EAE that mimics the most common form of MS [40,41]. Our laboratory has recently generated mast cell-deficient SJL mice to study the role of mast cells in RR disease. As in the PP model of MS, mast cells contribute to severe disease in SJL mice (Submitted for publication [42]). Conventional wisdom has dismissed a role for neutrophils in MS because they are not detected in mature MS plaques. Yet consistent with data in PP EAE, recent studies have demonstrated that neutrophils are required for BBB permeability and clinical disease in RR EAE [43]. Thus, one common role of mast cells in EAE and by extension, MS, may be to recruit and activate neutrophils to facilitate BBB permeability and initiate disease. It is likely that comparison of RR and PP disease in these mast cell-deficient models will allow the identification of other common mechanisms of mast cell action.
Mast cells and Rheumatoid Arthritis (RA)
RA is a chronic inflammatory disease that can involve many tissues, although the primary targets of immune destruction are the synovial joints. In RA the cells of the synovial lining, in particular synovial fibroblasts (SFs), undergo extensive hyperplasia, forming a structure termed the “synovial pannus”, which invades and destroys cartilage and bone. Although RA is considered an autoimmune disease, no specific target autoantigen has been identified. Rheumatoid factor, an immunoglobulin with specificity for the Fc portion of IgG, and antibodies against citrullinated cyclic peptide are characteric in patients with RA [44].
A variety of animal models exist to study RA [44] but three are most commonly utilized. The K/BxN mouse (KRN T cell receptor transgenic mouse on the C57BL/6×NOD background) spontaneously develops an early onset, rapidly progressive arthritis mediated by autoantibodies that bind glucose-6-phosphate isomerase. Serum from K/BxN mice that have developed disease can passively transfer disease to most recipient strains of mice in autoantibody-induced arthritis (AIA). Collagen-induced arthritis (CIA) is elicited by immunization with heterologous type II collagen in CFA. Mast cells were first implicated in the AIA model of RA using two mast cell-deficient mouse strains, SI/SId and W/Wv. These mice exhibited little to no clinical or histological evidence of arthritis compared with control littermates and disease susceptibility after serum transfer was restored upon reconstitution of mast cells [45].
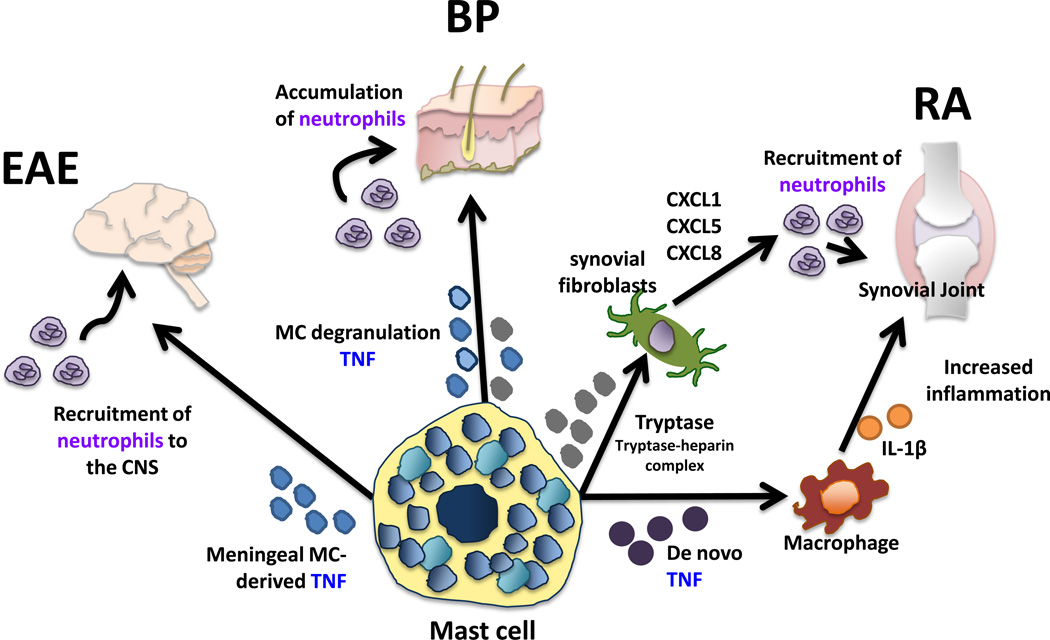
Mast cells are normally present in the synovial compartments of healthy individuals but are found in increased numbers in RA patients. Furthermore, there is evidence that cytokines and proteases produced by mast cells, notably TNF, IL-1b, IL-17 and tryptase, are intimately involved. For example, it has been reported that mast cells are the main source of IL-17 in human RA synovium [46]. Activated mast cells present at these sites also produce TNF de novo that in turn induces IL-1b by neighboring macrophages (Figure 3) [47]. Mast cell-derived tryptase expressed within the synovium can form complexes with heparin and act on RASFs to upregulate neutrophil chemotactic factors in AIA, suggesting an indirect role for mast cells in neutrophil recruitment (Figure 3)[48]. In addition, tryptase-mediated activation of protease-activated receptor 2 (PAR-2) on synovial cells leads to increased vascular permeability and inflammation in the joint and can inhibit Fas-mediated apoptosis of RASFs contributing to fibroblast hyperplasia and joint damage [49,50]. In CIA [51] and AIA [52] RASFs were shown to produce IL-33, which directly activates mast cells to express high levels of proinflammatory cytokines such as (IL-17, IL-1b, IL-6, IL-13, GM-CSF) and chemokines (MCP-1 and MIP-1a)[46]. These molecules recruit inflammatory cells including neutrophils, as well as promote an autoreactive Th17 phenotype [46].
Figure 3. Common pathogenic mechanisms in autoimmune disease: mast cell-derived TNF and the recruitment of neutrophils.
Murine models of MS (EAE), RA and BP have common underlying mechanisms of mast cell influcence on disease. In primary progressive EAE, meningeal mast cell-derived TNF is necessary for severe disease and its expression corresponds with neutrophil recruitment to the CNS. Similarly in BP, the degranulation and release of preformed mediators including TNF from mast cells resident in the skin results in an accumulation of neutrophils and skin blistering. Finally, in RA the de novo synthesis of TNF by mast cell results in IL-1β release of macrophages leading to an increase in inflammatory cell infiltrates including neutrophils in synovial joints. Mast cell-derived tryptase via tryptase-heparin complexes activates synovial fibroblasts to express chemokines that recruit neutrophils to the joint.
Mast cells and Bullous Pemphigoid (BP)
A prominent role for mast cells exists in BP, which is an acquired autoimmune skin disease characterized by the presence of autoantibodies against two hemidesmosomal antigens, BP230 and BP180, and the presence of subepidermal blisters [53]. In mice, passive transfer of autoantibodies directed to the murine BP180 ectodomain triggers a blistering skin disease that is dependent upon complement activation and neutrophil infiltration and closely mimics the human disease [54,55]. Intradermal injection of wild type neonatal mice with IgG antibodies directed against BP180 results in extensive mast cell degranulation in the skin that precedes an accumulation of neutrophils and skin blistering [54]. Conversely, this neutrophil accumulation and the development of skin lesions is significantly diminished in W/Wv mice, but can be restored to wild type levels if mice are treated with the neutrophil chemoattractant IL-8 at the site of antibody treatment. Neutrophil accumulation and skin blistering were also blocked when mice were treated with the mast cell stabilizer cromolyn [53]. Strong evidence also exists for mast cell involvement in human disease. Degranulated mast cells are prominent in the skin of BP patients and significant levels of histamine, several mast cell-derived chemoattractants and elevated levels of tryptase are present in the blister fluid of BP patients [56,57,58,59,60].
Mast cells and Type 1 Diabetes (T1D)
T1D results from an autoimmune attack against the insulin producing β-cells in the islet of langerhans of the pancreas. The resulting failure of glucose homeostasis leads to damage to the blood vessels and nerves [61]. Widely used models to study T1D include the non-obese spontaneously diabetic mouse (NOD), the lymphopenic and spontaneously diabetic BioBreeding (BB) DRlyp/lyp rat and inducible BB DR (+/+) rat. These rodent models of diabetes share some features with the human disease. All exhibit a number of related genetic susceptibility loci and NOD mice have a similar and profound breakdown in immune regulation that results in the expansion of autoreactive CD4+ and CD8+ T cells, and autoantibody-producing B cells, as well as the activation of innate immune cells that act collectively to destroy insulin-producing β-cells [61].
The data that supports mast cell involvement in diabetes is still indirect. Two lines of evidence suggest a role in the spontaneous BB rat model. First, treatment with the mast cell stabilizer cromolyn significantly delayed disease onset and secondly, mast cell gene expression profiling of the pancreatic lymph nodes revealed an activated mast cell population in disease [62]. Data from Louvet et al. showed that while tyrosine kinase inhibitors (c-kit is one of many tyrosine kinase associated receptors) inhibit and reverse T1D in NOD mice, specific c-kit inhibitors were only marginally effective [63]. Preliminary data from our laboratory using mast cell-deficient NODW/Wv mice show a significant decrease in disease incidence. However a specific role for mast cells in disease has yet to be validated because reconstitution at 4–6 weeks of age fails to restore disease susceptibility. These observations suggest that either mast cells act earlier in promoting the insulitis that is a prerequisite for full disease or that another c-kit related mechanism is operational [Quirion et al., manuscript in preparation]. Notably, mast cells have also been implicated in the pathogenesis of non-autoimmune diet-induced obesity and diabetes in mice although how mast cells function in these conditions has not been delineated [64].
Mast cells in other autoimmune diseases
The most convincing evidence of mast cell influence in autoimmunity occurs within the context of the diseases discussed above. However, mast cells are implicated in other autoimmune diseases including Guillain-Barré Syndrome, Graves’ Opthamology, Pemphigus vulgaris, Systemic lupus erythematosus and Sjogren’s syndrome [6]. Data linking mast cells to these conditions comes primarily from observations of mast cell activation during disease or from use of mast cell targeted therapies that result in decreased disease severity [65].
POTENTIAL ROLES OF MAST CELLS IN AUTOIMMUNE DISEASE
The specific mechanisms of mast cell action in autoimmunity are not completely understood. As a “jack of all trades,” mast cells interact with and modulate the function of many cells types, thus, giving them the potential to affect these disease processes in a variety of ways. For example, mast cells have the potential to affect T cell priming through the expression of MHC class II, provide direct costimulation via expression of cell surface receptors and enhance T cell proliferation and activation through the wide variety of cytokines they express. Mast cells also affect dendritic cell maturation, migration, and function, and can indirectly affect T cell function through DC function. (For a more complete review of mast cell interactions with other immune cells see [2,6]). Here we summarize only the most recent reports on the interaction of mast cells with Tregs, Th17 cells, B cells, cells of the CNS, and the relationship of mast cells with other recently recognized molecules of interest. While these studies are not in the context of autoimmune disease, the results may be applicable to our ultimate understanding of this group of diseases.
DIRECT CELL INTERACTIONS WITH MAST CELLS
It has been appreciated for some time that mast cells can cooperate with other cell types such as dendritic cells and T effector cells to enhance activation and migration either directly through cell–cell interactions or via secreted products [2,6]. As discussed below, recent data has emerged that delineates new mast cell interactions with many other cell types that have established and critical roles in autoimmunity diseases.
Mast cells and T regulatory cell (Treg) interactions
Tregs, defined by their expression of CD4, CD25, FoxP3 and the ability to suppress T effector cell responses, are among the major cell types that maintain immune homeostasis [66]. Tregs have a major role in mediating autoreactive T cell tolerance and there is much evidence that aberrant regulatory T cell activity contributes to autoimmunity. As mast cells and Treg cells are often found in close proximity in secondary lymphoid organs and specific sites of tissue inflammation, it is not surprising that their interaction results in effects on the functional capabilities of both cell types.
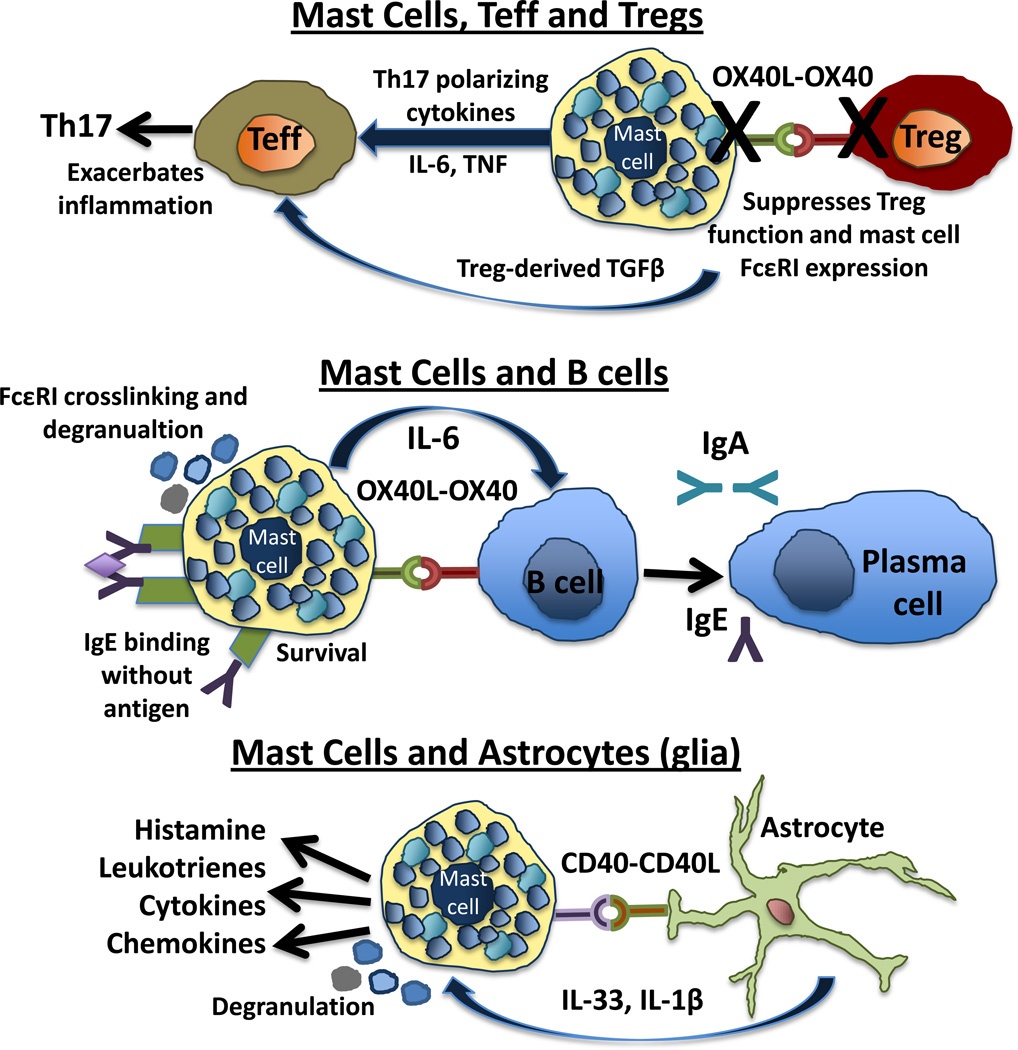
The OX40-OX40L axis is perhaps the best-defined molecular determinant of mast cell-Treg interactions. Mast cells constitutively express OX40L, while OX40 is constitutively expressed on Treg cells. Both in vitro and in vivo studies demonstrate that Tregs are able to down regulate FceRI expression and inhibit FceRI dependent mast cell degranulation [67,68]. Conversely, mast cells can reverse Treg suppression of T effector cells and reduce T effector cell susceptibility to Treg suppression [69](Figure 4).
Figure 4. Direct cell interactions involving mast cells with potential to affect autoimmune disease.
Mast cells, Teffs and Tregs. Mast cells produce cytokines that can promote distinct CD4+ T cell fates. Mast cell-derived IL-6 and TNF can act with Tregderived TGFβ, to skew T effector cells (CD4+ CD25−) to a proinflammatory Th17 phenotype. The direct interaction of OX40L-OX40 on mast cells and Tregs respectively leads to the inhibition of FcεRI expression and degranulation on mast cells and to an inability of Tregs to suppress effector T cell activity.
Mast cells and B cells. Mast cells affect B cell survival, proliferation and differentiation into IgA secreting plasma cells through the production of IL-6 and CD40-CD40L interactions. The production of IgE antibodies impacts both mast cell survival and activation potential. IgG triggering of FcγRII and FcγRIII expressed on mast cells are important in autoimmune diseases such as EAE, BP, RA and systemic lupus.
Mast cells and astrocytes. Astrocytes, a type of microglia, are of particular interest in immune mediated diseases of the CNS. Mast cells and astrocytes have a dynamic relationship as a result of their coincident activation through CD40-CD40L. The resulting mast cell activation results in the release of IL-33 and IL-1β release from glial cells, which further promotes mast cell activation.
Mast cells also supress Treg activity in an OX40L-independent manner. In a model of skin allograft tolerance mast cells promote allograft rejection. Graft recipients are tolerized to alloantigens by a combination of anti-CD154 and i.v. transfusion of allogenic cells prior to skin grafting. In this setting, mast cell degranulation triggered by IgE and antigen leads to a transient loss of Treg function resulting in the breakdown of peripheral tolerance [70]. There is evidence that histamine is responsible for this effect. Blocking of the H1 receptor in Treg cells using the H1 specific antagonist, loratadine, rescues Treg cell suppressor function in the presence of exogenous histamine [71]. Clearly the dynamic and reciprocal relationship between these two cells needs to be further explored in the context of autoimmunity.
Mast cells and Treg-Th17 cell plasticity
Th17 cells are CD4+ T cells that are defined by their expression of the transcription factor RORγt and a variety of hallmark cytokines including IL-17a and IL-17f. Together with IFNγ-producing Th1 cells, they are implicated in the mouse models of MS, RA and T1D [72]. Th17 cells arise from a naïve CD4+ T cell upon antigen activation under the influence of a unique cytokine microenvironment including some combination of TGFβ, IL-6, IL-21, IL-23 and IL-1β in mice and humans. TGFβ alone is essential for the development of Tregs and this differentiation can be inhibited by IL-6 (Figure 4). Multiple lines of evidence support the idea that pro-inflammatory Th17 cells and protective Tregs have a reciprocal developmental relationship. The balance of cytokines in the local environment, which regulate the expression and function of the respective transcription factors RORγt and FoxP3, determine the relative frequency of Th17 and Treg cells [73]. Under certain conditions mast cells express all of the relevant cytokines (IL-6, IL-21, IL-23, and TGFβ) that drive Th17 and Treg cell differentiation and plasticity. In mast cell-T cell co-culture experiments activated BMMC produce IL-6 and TNF, which together with TGFβ-producing Treg cells, can induce IL-17 production in effector T cells (CD4+CD25−). These mast cell-derived cytokines can also directly promote Treg skewing to a Th17 phenotype [69]. This interaction may play an important role in vivo as Tregs, T effector cells and mast cells co-localize at sites of T cell priming as well as within tissues where secondary activation events occur. Such effects of mast cells on T cell differentiation are also observed in certain tumor microenvironments where mast cell derived IL-6 contributes to a proinflammatory Th17-dominant environment leading to concomitant autoimmunity [74](Figure 4).
Mast cells and B cells
Mast cells express a number of B cell-modulating molecules and Ig receptors and antibodies produced by differentiated B cells that act on mast cells suggest an intimate connection between these two cell types. Monomeric IgE bound to FcεRI without antigen promotes the survival and priming of mast cells [75]. In addition to FcεRI, human and murine mast cells express the IgG receptors FcγRII and FcγRIII. When cross-linked with IgG-antigen complexes these receptors are potent inducers of degranulation [76]. FcγRII and FcγRIII are important in autoimmune diseases classified as type II and III hypersensitivity diseases such as BP, RA and SLE and in the type IV hypersensitivity disease EAE [6]. It was demonstrated that low mast cell concentrations are effective in influencing B cell survival and proliferation in vitro, regardless of the state of mast cell activation. Mast cells also promote the differentiation of B cells into CD138+ plasma cells and selective IgA secretion. All of these effects are dependent on mast cell-derived IL-6 and the expression of CD40-CD40L on B cells and mast cells respectively [77](Figure 4).
Mast cells and CNS cells
There are also a number of mast cell interactions with resident CNS cells. The best-described interaction is with the astrocyte, a cell that plays a critical role in MS and EAE [78]. Astrocytes, a subtype of glial cells, are the most abundant cell type in the brain. They affect neuronal function via the release of neurotropic factors, contribute to the metabolism of neurotransmitters, regulate BBB permeability and can present antigen to T cells. Mast cells and astrocytes have a dynamic relationship, facilitated by their co-localization in the thalamus and at perivascular sites. Mast cell-astrocyte co-culture results in the activation of both cell types, an event dependent on CD40-CD40L and the release of mediators including histamine, leukotrienes, cytokines and chemokines [79] (Figure 4). The combination of PAMPs and ATP activate glia to produce IL-33 and IL-1β directly eliciting the production of large amounts of inflammatory cytokines by mast cells [80]. IL-13 is also expressed by mast cells in this inflammatory cascade and activates glia to produce other proinflammatory molecules such as arginase 1, IL-6, MCP-1 and TNF [79,81].
MAST CELLS PRODUCTS AND ACTIVATORS
The appreciation of molecules that are intimately involved in mast cell function is growing and it is of interest that some of these newly identified molecules have established roles in autoimmune disease. Although their expression by mast cells in autoimmunity has not been verified, they are good candidates for further exploration.
Osteopontin
Osteopontin is a protein that is clearly implicated in MS and EAE. It is member of the Sibling (small integrin binding, N-linked glycoproteins) family of proteins. Its early characterization in these diseases was based on its inducible expression on inflamed endothelium in the extracellular matrix of perivascular cuffs [82]. Together with VCAM-1 it is a binding partner of a4b1 integrin, a homing molecule expressed on lymphocytes that are trafficiking to the CNS. However, osteopontin has several additional binding partners and also participates in bone remodeling, wound healing, dystrophic calcification, coronary restenosis and tumor cell metastasis [83,84]. Osteopontin is produced by a variety of immune cells including macrophages, activated T cells, myeloid DCs, plasmacytoid DCs, NKT and mast cells [85,86,87,88,89]. In both MS and EAE, osteopontin is associated with relapses through at least two proposed mechanisms including the induction of proinflammatory T cell activity and the inhibition of autoreactive T cell apoptosis [82].
Mast cell production of osteopontin was first reported by Nagasaka et al. in fetal skin-derived mast cells after ionomycin stimulation or FcεRI cross-linking [89]. They also demonstrated that mice with targeted deletions in the osteopontin gene displayed reduced IgE-mediated passive cutaneous anaphylaxis, perhaps due to its ability to regulate mast cell migration to sites of inflammation. Osteopontin transcripts together with a number of mast cell specific mRNAs are among those whose expression is significantly increased in CNS lesions compared to normal brain tissue [33]. Although there is no direct link between mast cell expression of osteopontin and MS, the presence of mast cells at sites where this protein is highly expressed in inflammatory CNS plaques and its ability to modulate mast cell function is intriguing.
Substance P (SP)
The CNS is generally considered impermeable to immune cell infiltration, yet evidence is accumulating that suggests this is not an accurate depiction. It is now appreciated that there is a dynamic relationship between the CNS and the cells of the peripheral immune system. One mode of crosstalk is mediated through neuropeptides and their receptors [90]. Of relevance to autoimmunity is the neuropeptide SP, a nonomer peptide secreted by nerves, macrophages, esosinophils, lymphocytes and DCs. Unlike most neuropeptides which are anti-inflammatory, SP is pro-inflammatory and may play a role in the induction of immune responses in the CNS [91]. SP acts by binding to the neurokinin-1 receptor, which is widely expressed, and this interaction contributes to other inflammatory diseases such as atopic dermatitis, asthma, sarcoidosis, chronic bronchitis, irritable bowel syndrome and RA [90,92]. SP is also a potent activator of mast cell gene expression including TNF expression [93]. Considering the contribution of mast cell-derived TNF in multiple autoimmune diseases (Figure 3), it is possible that SP is one of the critical activators of mast cells in autoimmunity.
IL-33
IL-33 is a member of the IL-1 family of cytokines that includes IL-1α, IL-1β and IL-18. In contrast to other IL-1 related cytokines, except for IL-1a, IL-33 is primarily localized in the nucleus and it associates with chromatin where it can bind to the surface of nucleosomes and affect chromatin remodeling [94,95]. It is crucial for the induction of Th2 type cytokine-associated immune responses and thus has been extensively studied in the context of allergic disease where it has proinflammatory effects and in helminth infections where it is protective [96]. In models of anaphylaxis IL-33 directly induces mast cell degranulation after IgE sensitization. There appears to be an autocrine inflammatory loop induced by IL-33 in mast cells. IL-33 is produced by murine mast cells and mast cells also constitutively express ST2, a receptor subunit that together with IL-1 receptor accessory protein (IL-1RAcP) makes up the heterodimeric IL-33 receptor. Notably, similar to IL-3 and SCF, IL-33 can also directly induce cytokine and chemokine secretion from mast cells without affecting degranulation [94,95]. The proinflammatory activities of IL-33 and its link to mast cells make it a good candidate for studies in mast cell-dependent autoimmune diseases [96].
CONCLUSIONS
A wealth of new data has increased our appreciation for a significant, disease-exacerbating role for mast cells in autoimmunity. Although there are still many questions to be answered regarding how mast cells are activated, the extent of mast cell involvement in various diseases and their specific mode of action, many of these studies reveal common themes. First, it is clear that mast cells exacerbate, not limit, disease. Mast cells could conceivably exert anti-inflammatory effects on disease via a variety of mediators and potentially alter Treg cell function, but are first and foremost acting as pro-inflammatory cells. Secondly, in at least three models of autoimmune disease (EAE, BP and RA) mast cells promote inflammation and disease severity through a common mechanism involving TNF and/or neutrophil recruitment (Figure 3). Although neutrophils are short-lived cells that have been largely dismissed as major players in many autoimmune diseases, their involvement in multiple disease models is becoming hard to ignore. It is also notable that mast cells are responsible for eliciting neutrophil infiltration that promotes inflammation regardless of whether that outcome is protective (in infections) or pathologic (in contact hypersensitivity and autoimmunity). There is now strong rationale for the serious analysis of mast cell targeted therapies that may alter disease severity and progression in human patients.
Research Highlights.
Mast cells are potent inflammatory cells that are well studied in allergic disease.
Mast cells also exacerbate autoimmune disease in humans as well as in animal models.
Mast cells act to recruit neutrophils to sites of autoimmune destruction in many of these diseases.
Emerging data in mast cell biology offer potential roles for these cells in autoimmunity.
Acknowledgements
The authors thank Dr. Alison Christy for her contribution to the production of the figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Okayama Y, Kawakami T. Development, migration, and survival of mast cells. Immunol Res. 2006;34:97–115. doi: 10.1385/IR:34:2:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rao KN, Brown MA. Mast cells: multifaceted immune cells with diverse roles in health and disease. Ann N Y Acad Sci. 2008;1143:83–104. doi: 10.1196/annals.1443.023. [DOI] [PubMed] [Google Scholar]
- 3.Metz M, Maurer M. Mast cells--key effector cells in immune responses. Trends Immunol. 2007;28:234–241. doi: 10.1016/j.it.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Kneilling M, Rocken M. Mast cells: novel clinical perspectives from recent insights. Exp Dermatol. 2009;18:488–496. doi: 10.1111/j.1600-0625.2009.00860.x. [DOI] [PubMed] [Google Scholar]
- 5.Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008;9:1215–1223. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sayed BA, Christy A, Quirion MR, Brown MA. The master switch: the role of mast cells in autoimmunity and tolerance. Annu Rev Immunol. 2008;26:705–739. doi: 10.1146/annurev.immunol.26.021607.090320. [DOI] [PubMed] [Google Scholar]
- 7.Wei OL, Hilliard A, Kalman D, Sherman M. Mast cells limit systemic bacterial dissemination but not colitis in response to Citrobacter rodentium. Infect Immun. 2005;73:1978–1985. doi: 10.1128/IAI.73.4.1978-1985.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velin D, Bachmann D, Bouzourene H, Michetti P. Mast cells are critical mediators of vaccine-induced Helicobacter clearance in the mouse model. Gastroenterology. 2005;129:142–155. doi: 10.1053/j.gastro.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Lawrence CE, Paterson YY, Wright SH, Knight PA, Miller HR. Mouse mast cell protease-1 is required for the enteropathy induced by gastrointestinal helminth infection in the mouse. Gastroenterology. 2004;127:155–165. doi: 10.1053/j.gastro.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Anthony RM, Rutitzky LI, Urban JF, Jr, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sundstrom JB, Hair GA, Ansari AA, Secor WE, Gilfillan AM, Metcalfe DD, Kirshenbaum AS. IgE-FcepsilonRI interactions determine HIV coreceptor usage and susceptibility to infection during ontogeny of mast cells. J Immunol. 2009;182:6401–6409. doi: 10.4049/jimmunol.0801481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taub DD, Mikovits JA, Nilsson G, Schaffer EM, Key ML, Petrow-Sadowski C, Ruscetti FW. Alterations in mast cell function and survival following in vitro infection with human immunodeficiency viruses-1 through CXCR4. Cell Immunol. 2004;230:65–80. doi: 10.1016/j.cellimm.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Becker Y. HIV-1 induced AIDS is an allergy and the allergen is the Shed gp120--a review, hypothesis, and implications. Virus Genes. 2004;28:319–331. doi: 10.1023/b:viru.0000025778.56507.61. [DOI] [PubMed] [Google Scholar]
- 14.Kitamura Y, Go S, Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978;52:447–452. [PubMed] [Google Scholar]
- 15.Nakano T, Sonoda T, Hayashi C, Yamatodani A, Kanayama Y, Yamamura T, Asai H, Yonezawa T, Kitamura Y, Galli SJ. Fate of bone marrow-derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer into genetically mast celldeficient W/Wv mice. Evidence that cultured mast cells can give rise to both connective tissue type and mucosal mast cells. J Exp Med. 1985;162:1025–1043. doi: 10.1084/jem.162.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galli SJ, Kitamura Y. Genetically mast-cell-deficient W/Wv and Sl/Sld mice. Their value for the analysis of the roles of mast cells in biologic responses in vivo. Am J Pathol. 1987;127:191–198. [PMC free article] [PubMed] [Google Scholar]
- 17.Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–848. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nautiyal KM, Ribeiro AC, Pfaff DW, Silver R. Brain mast cells link the immune system to anxiety-like behavior. Proc Natl Acad Sci U S A. 2008;105:18053–18057. doi: 10.1073/pnas.0809479105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanzola MB, Robbie-Ryan M, Gutekunst CA, Brown MA. Mast cells exert effects outside the central nervous system to influence experimental allergic encephalomyelitis disease course. J Immunol. 2003;171:4385–4391. doi: 10.4049/jimmunol.171.8.4385. [DOI] [PubMed] [Google Scholar]
- 20.Sayed BA, Christy AL, Walker ME, Brown MA. Meningeal mast cells affect early T cell central nervous system infiltration and blood-brain barrier integrity through TNF: a role for neutrophil recruitment? J Immunol. 2010;184:6891–6900. doi: 10.4049/jimmunol.1000126. [DOI] [PubMed] [Google Scholar]
- 21.Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7:1139–1151. doi: 10.1016/S1474-4422(08)70259-X. [DOI] [PubMed] [Google Scholar]
- 22.Lublin FD. Clinical features and diagnosis of multiple sclerosis. Neurol Clin. 2005;23:1–15. doi: 10.1016/j.ncl.2004.09.003. v. [DOI] [PubMed] [Google Scholar]
- 23.Olitsky PK, Yager RH. Experimental disseminated encephalomyelitis in white mice. J Exp Med. 1949;90:213–224. doi: 10.1084/jem.90.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wekerle H. Lessons from multiple sclerosis: models, concepts, observations. Ann Rheum Dis. 2008;67(Suppl 3):iii56–iii60. doi: 10.1136/ard.2008.098020. [DOI] [PubMed] [Google Scholar]
- 25.Kermode AG, Thompson AJ, Tofts P, MacManus DG, Kendall BE, Kingsley DP, Moseley IF, Rudge P, McDonald WI. Breakdown of the blood-brain barrier precedes symptoms and other MRI signs of new lesions in multiple sclerosis. Pathogenetic and clinical implications. Brain. 1990;113(Pt 5):1477–1489. doi: 10.1093/brain/113.5.1477. [DOI] [PubMed] [Google Scholar]
- 26.Steinman L. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell. 1996;85:299–302. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- 27.Jager A, Kuchroo VK. Effector and regulatory T-cell subsets in autoimmunity and tissue inflammation. Scand J Immunol. 2010;72:173–184. doi: 10.1111/j.1365-3083.2010.02432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fletcher JM, Lalor SJ, Sweeney CM, Tubridy N, Mills KH. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2010;162:1–11. doi: 10.1111/j.1365-2249.2010.04143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neuman J. Ueber das Vorkommen der sogneannten"Mastzellen" bei pathologischen Veraenderungen des Gehirns. Arch. Pathol Anat. Physiol. Virchows. 1890;122:378–381. [Google Scholar]
- 30.Bebo BF, Jr, Yong T, Orr EL, Linthicum DS. Hypothesis: a possible role for mast cells and their inflammatory mediators in the pathogenesis of autoimmune encephalomyelitis. J Neurosci Res. 1996;45:340–348. doi: 10.1002/(SICI)1097-4547(19960815)45:4<340::AID-JNR3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 31.Ibrahim MZ, Reder AT, Lawand R, Takash W, Sallouh-Khatib S. The mast cells of the multiple sclerosis brain. J Neuroimmunol. 1996;70:131–138. doi: 10.1016/s0165-5728(96)00102-6. [DOI] [PubMed] [Google Scholar]
- 32.Rozniecki JJ, Hauser SL, Stein M, Lincoln R, Theoharides TC. Elevated mast cell tryptase in cerebrospinal fluid of multiple sclerosis patients. Ann Neurol. 1995;37:63–66. doi: 10.1002/ana.410370112. [DOI] [PubMed] [Google Scholar]
- 33.Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, Klonowski P, Austin A, Lad N, Kaminski N, Galli SJ, Oksenberg JR, Raine CS, Heller R, Steinman L. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 34.Brenner T, Soffer D, Shalit M, Levi-Schaffer F. Mast cells in experimental allergic encephalomyelitis: characterization, distribution in the CNS and in vitro activation by myelin basic protein and neuropeptides. J Neurol Sci. 1994;122:210–213. doi: 10.1016/0022-510x(94)90300-x. [DOI] [PubMed] [Google Scholar]
- 35.Dietsch GN, Hinrichs DJ. Mast cell proteases liberate stable encephalitogenic fragments from intact myelin. Cell Immunol. 1991;135:541–548. doi: 10.1016/0008-8749(91)90297-o. [DOI] [PubMed] [Google Scholar]
- 36.Johnson D, Seeldrayers PA, Weiner HL. The role of mast cells in demyelination. 1. Myelin proteins are degraded by mast cell proteases and myelin basic protein and P2 can stimulate mast cell degranulation. Brain Res. 1988;444:195–198. doi: 10.1016/0006-8993(88)90929-8. [DOI] [PubMed] [Google Scholar]
- 37.Dimitriadou V, Pang X, Theoharides TC. Hydroxyzine inhibits experimental allergic encephalomyelitis (EAE) and associated brain mast cell activation. Int J Immunopharmacol. 2000;22:673–684. doi: 10.1016/s0192-0561(00)00029-1. [DOI] [PubMed] [Google Scholar]
- 38.Dietsch GN, Hinrichs DJ. The role of mast cells in the elicitation of experimental allergic encephalomyelitis. J Immunol. 1989;142:1476–1481. [PubMed] [Google Scholar]
- 39.Secor VH, Secor WE, Gutekunst CA, Brown MA. Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J Exp Med. 2000;191:813–822. doi: 10.1084/jem.191.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trotter JL, Clark HB, Collins KG, Wegeschiede CL, Scarpellini JD. Myelin proteolipid protein induces demyelinating disease in mice. J Neurol Sci. 1987;79:173–188. doi: 10.1016/0022-510x(87)90271-1. [DOI] [PubMed] [Google Scholar]
- 41.Tuohy VK, Lu Z, Sobel RA, Laursen RA, Lees MB. Identification of an encephalitogenic determinant of myelin proteolipid protein for SJL mice. J Immunol. 1989;142:1523–1527. [PubMed] [Google Scholar]
- 42.Sayed B, Walker M, Brown M. Mast cells regulate disease severity in a relasping remitting model of Multiple Sclerosis. Journal of Immunology, Cutting Edge. 2011 doi: 10.4049/jimmunol.1003574. [DOI] [PubMed] [Google Scholar]
- 43.Carlson T, Kroenke M, Rao P, Lane TE, Segal B. The Th17-ELR+ CXC chemokine pathway is essential for the development of central nervous system autoimmune disease. J Exp Med. 2008;205:811–823. doi: 10.1084/jem.20072404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kannan K, Ortmann RA, Kimpel D. Animal models of rheumatoid arthritis and their relevance to human disease. Pathophysiology. 2005;12:167–181. doi: 10.1016/j.pathophys.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 45.Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297:1689–1692. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- 46.Hueber AJ, Asquith DL, Miller AM, Reilly J, Kerr S, Leipe J, Melendez AJ, McInnes IB. Mast cells express IL-17A in rheumatoid arthritis synovium. J Immunol. 2010;184:3336–3340. doi: 10.4049/jimmunol.0903566. [DOI] [PubMed] [Google Scholar]
- 47.Sandler C, Lindstedt KA, Joutsiniemi S, Lappalainen J, Juutilainen T, Kolah J, Kovanen PT, Eklund KK. Selective activation of mast cells in rheumatoid synovial tissue results in production of TNF-alpha, IL-1beta and IL-1Ra. Inflamm Res. 2007;56:230–239. doi: 10.1007/s00011-007-6135-1. [DOI] [PubMed] [Google Scholar]
- 48.Shin K, Nigrovic PA, Crish J, Boilard E, McNeil HP, Larabee KS, Adachi R, Gurish MF, Gobezie R, Stevens RL, Lee DM. Mast cells contribute to autoimmune inflammatory arthritis via their tryptase/heparin complexes. J Immunol. 2009;182:647–656. doi: 10.4049/jimmunol.182.1.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sawamukai N, Yukawa S, Saito K, Nakayamada S, Kambayashi T, Tanaka Y. Mast cell-derived tryptase inhibits apoptosis of human rheumatoid synovial fibroblasts via rho-mediated signaling. Arthritis Rheum. 2010;62:952–959. doi: 10.1002/art.27331. [DOI] [PubMed] [Google Scholar]
- 50.Palmer HS, Kelso EB, Lockhart JC, Sommerhoff CP, Plevin R, Goh FG, Ferrell WR. Protease-activated receptor 2 mediates the proinflammatory effects of synovial mast cells. Arthritis Rheum. 2007;56:3532–3540. doi: 10.1002/art.22936. [DOI] [PubMed] [Google Scholar]
- 51.Xu D, Jiang HR, Kewin P, Li Y, Mu R, Fraser AR, Pitman N, Kurowska-Stolarska M, McKenzie AN, McInnes IB, Liew FY. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc Natl Acad Sci U S A. 2008;105:10913–10918. doi: 10.1073/pnas.0801898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu D, Jiang HR, Li Y, Pushparaj PN, Kurowska-Stolarska M, Leung BP, Mu R, Tay HK, McKenzie AN, McInnes IB, Melendez AJ, Liew FY. IL-33 exacerbates autoantibody-induced arthritis. J Immunol. 2010;184:2620–2626. doi: 10.4049/jimmunol.0902685. [DOI] [PubMed] [Google Scholar]
- 53.Navi D, Saegusa J, Liu FT. Mast cells and immunological skin diseases. Clin Rev Allergy Immunol. 2007;33:144–155. doi: 10.1007/s12016-007-0029-4. [DOI] [PubMed] [Google Scholar]
- 54.Chen R, Ning G, Zhao ML, Fleming MG, Diaz LA, Werb Z, Liu Z. Mast cells play a key role in neutrophil recruitment in experimental bullous pemphigoid. J Clin Invest. 2001;108:1151–1158. doi: 10.1172/JCI11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Z, Diaz LA, Troy JL, Taylor AF, Emery DJ, Fairley JA, Giudice GJ. A passive transfer model of the organ-specific autoimmune disease, bullous pemphigoid, using antibodies generated against the hemidesmosomal antigen, BP180. J Clin Invest. 1993;92:2480–2488. doi: 10.1172/JCI116856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dvorak AM, Mihm MC, Jr, Osage JE, Kwan TH, Austen KF, Wintroub BU. Bullous pemphigoid, an ultrastructural study of the inflammatory response: eosinophil, basophil and mast cell granule changes in multiple biopsies from one patient. J Invest Dermatol. 1982;78:91–101. doi: 10.1111/1523-1747.ep12505711. [DOI] [PubMed] [Google Scholar]
- 57.Baba T, Sonozaki H, Seki K, Uchiyama M, Ikesawa Y, Toriisu M. An eosinophil chemotactic factor present in blister fluids of bullous pemphigoid patients. J Immunol. 1976;116:112–116. [PubMed] [Google Scholar]
- 58.Katayama I, Doi T, Nishioka K. High histamine level in the blister fluid of bullous pemphigoid. Arch Dermatol Res. 1984;276:126–127. doi: 10.1007/BF00511070. [DOI] [PubMed] [Google Scholar]
- 59.D'Auria L, Pietravalle M, Cordiali-Fei P, Ameglio F. Increased tryptase and myeloperoxidase levels in blister fluids of patients with bullous pemphigoid: correlations with cytokines, adhesion molecules and anti-basement membrane zone antibodies. Exp Dermatol. 2000;9:131–137. doi: 10.1034/j.1600-0625.2000.009002131.x. [DOI] [PubMed] [Google Scholar]
- 60.Brockow K, Abeck D, Hermann K, Ring J. Tryptase concentration in skin blister fluid from patients with bullous skin conditions. Arch Dermatol Res. 1996;288:771–773. doi: 10.1007/BF02505295. [DOI] [PubMed] [Google Scholar]
- 61.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464:1293–1300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Geoffrey R, Jia S, Kwitek AE, Woodliff J, Ghosh S, Lernmark A, Wang X, Hessner MJ. Evidence of a functional role for mast cells in the development of type 1 diabetes mellitus in the BioBreeding rat. J Immunol. 2006;177:7275–7286. doi: 10.4049/jimmunol.177.10.7275. [DOI] [PubMed] [Google Scholar]
- 63.Louvet C, Szot GL, Lang J, Lee MR, Martinier N, Bollag G, Zhu S, Weiss A, Bluestone JA. Tyrosine kinase inhibitors reverse type 1 diabetes in nonobese diabetic mice. Proc Natl Acad Sci U S A. 2008;105:18895–18900. doi: 10.1073/pnas.0810246105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu J, Divoux A, Sun J, Zhang J, Clement K, Glickman JN, Sukhova GK, Wolters PJ, Du J, Gorgun CZ, Doria A, Libby P, Blumberg RS, Kahn BB, Hotamisligil GS, Shi GP. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pilartz M, Jess T, Indefrei D, Schroder JM. Adoptive transfer-experimental allergic neuritis in newborn Lewis rats results in inflammatory infiltrates, mast cell activation, and increased Ia expression with only minor nerve fiber degeneration. Acta Neuropathol. 2002;104:513–524. doi: 10.1007/s00401-002-0586-9. [DOI] [PubMed] [Google Scholar]
- 66.Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10:849–859. doi: 10.1038/nri2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gri G, Piconese S, Frossi B, Manfroi V, Merluzzi S, Tripodo C, Viola A, Odom S, Rivera J, Colombo MP, Pucillo CE. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity. 2008;29:771–781. doi: 10.1016/j.immuni.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kashyap M, Thornton AM, Norton SK, Barnstein B, Macey M, Brenzovich J, Shevach E, Leonard WJ, Ryan JJ. Cutting edge: CD4 T cell-mast cell interactions alter IgE receptor expression and signaling. J Immunol. 2008;180:2039–2043. doi: 10.4049/jimmunol.180.4.2039. [DOI] [PubMed] [Google Scholar]
- 69.Piconese S, Gri G, Tripodo C, Musio S, Gorzanelli A, Frossi B, Pedotti R, Pucillo CE, Colombo MP. Mast cells counteract regulatory T-cell suppression through interleukin-6 and OX40/OX40L axis toward Th17-cell differentiation. Blood. 2009;114:2639–2648. doi: 10.1182/blood-2009-05-220004. [DOI] [PubMed] [Google Scholar]
- 70.de Vries VC, Wasiuk A, Bennett KA, Benson MJ, Elgueta R, Waldschmidt TJ, Noelle RJ. Mast cell degranulation breaks peripheral tolerance. Am J Transplant. 2009;9:2270–2280. doi: 10.1111/j.1600-6143.2009.02755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Forward NA, Furlong SJ, Yang Y, Lin TJ, Hoskin DW. Mast cells down-regulate CD4+CD25+ T regulatory cell suppressor function via histamine H1 receptor interaction. J Immunol. 2009;183:3014–3022. doi: 10.4049/jimmunol.0802509. [DOI] [PubMed] [Google Scholar]
- 72.Hemdan NY, Birkenmeier G, Wichmann G, Abu El-Saad AM, Krieger T, Conrad K, Sack U. Interleukin-17-producing T helper cells in autoimmunity. Autoimmun Rev. 2010;9:785–792. doi: 10.1016/j.autrev.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 73.Zhu J, Paul WE. Heterogeneity and plasticity of T helper cells. Cell Res. 2010;20:4–12. doi: 10.1038/cr.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tripodo C, Gri G, Piccaluga PP, Frossi B, Guarnotta C, Piconese S, Franco G, Vetri V, Pucillo CE, Florena AM, Colombo MP, Pileri SA. Mast cells and Th17 cells contribute to the lymphomaassociated pro-inflammatory microenvironment of angioimmunoblastic T-cell lymphoma. Am J Pathol. 2010;177:792–802. doi: 10.2353/ajpath.2010.091286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kawakami T, Kitaura J, Xiao W, Kawakami Y. IgE regulation of mast cell survival and function. Novartis Found Symp. 2005;271:100–107. discussion 108–114, 145–151. [PubMed] [Google Scholar]
- 76.Malbec O, Daeron M. The mast cell IgG receptors and their roles in tissue inflammation. Immunol Rev. 2007;217:206–221. doi: 10.1111/j.1600-065X.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 77.Merluzzi S, Frossi B, Gri G, Parusso S, Tripodo C, Pucillo C. Mast cells enhance proliferation of B lymphocytes and drive their differentiation toward IgA-secreting plasma cells. Blood. 2010;115:2810–2817. doi: 10.1182/blood-2009-10-250126. [DOI] [PubMed] [Google Scholar]
- 78.Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J Neurol Sci. 2002;202:13–23. doi: 10.1016/s0022-510x(02)00207-1. [DOI] [PubMed] [Google Scholar]
- 79.Kim DY, Jeoung D, Ro JY. Signaling pathways in the activation of mast cells cocultured with astrocytes and colocalization of both cells in experimental allergic encephalomyelitis. J Immunol. 2010;185:273–283. doi: 10.4049/jimmunol.1000991. [DOI] [PubMed] [Google Scholar]
- 80.Bulanova E, Bulfone-Paus S. P2 receptor-mediated signaling in mast cell biology. Purinergic Signal. 2010;6:3–17. doi: 10.1007/s11302-009-9173-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hudson CA, Christophi GP, Gruber RC, Wilmore JR, Lawrence DA, Massa PT. Induction of IL-33 expression and activity in central nervous system glia. J Leukoc Biol. 2008;84:631–643. doi: 10.1189/jlb.1207830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Steinman L. A molecular trio in relapse and remission in multiple sclerosis. Nat Rev Immunol. 2009;9:440–447. doi: 10.1038/nri2548. [DOI] [PubMed] [Google Scholar]
- 83.Rangaswami H, Bulbule A, Kundu GC. Osteopontin: role in cell signaling and cancer progression. Trends Cell Biol. 2006;16:79–87. doi: 10.1016/j.tcb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 84.Denhardt DT, Burger EH, Kazanecki C, Krishna S, Semeins CM, Klein-Nulend J. Osteopontin-deficient bone cells are defective in their ability to produce NO in response to pulsatile fluid flow. Biochem Biophys Res Commun. 2001;288:448–453. doi: 10.1006/bbrc.2001.5780. [DOI] [PubMed] [Google Scholar]
- 85.O'Regan AW, Hayden JM, Berman JS. Osteopontin augments CD3-mediated interferon-gamma and CD40 ligand expression by T cells, which results in IL-12 production from peripheral blood mononuclear cells. J Leukoc Biol. 2000;68:495–502. [PubMed] [Google Scholar]
- 86.Renkl AC, Wussler J, Ahrens T, Thoma K, Kon S, Uede T, Martin SF, Simon JC, Weiss JM. Osteopontin functionally activates dendritic cells and induces their differentiation toward a Th1-polarizing phenotype. Blood. 2005;106:946–955. doi: 10.1182/blood-2004-08-3228. [DOI] [PubMed] [Google Scholar]
- 87.Shinohara ML, Lu L, Bu J, Werneck MB, Kobayashi KS, Glimcher LH, Cantor H. Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells. Nat Immunol. 2006;7:498–506. doi: 10.1038/ni1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Diao H, Kon S, Iwabuchi K, Kimura C, Morimoto J, Ito D, Segawa T, Maeda M, Hamuro J, Nakayama T, Taniguchi M, Yagita H, Van Kaer L, Onoe K, Denhardt D, Rittling S, Uede T. Osteopontin as a mediator of NKT cell function in T cell-mediated liver diseases. Immunity. 2004;21:539–550. doi: 10.1016/j.immuni.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 89.Nagasaka A, Matsue H, Matsushima H, Aoki R, Nakamura Y, Kambe N, Kon S, Uede T, Shimada S. Osteopontin is produced by mast cells and affects IgE-mediated degranulation and migration of mast cells. Eur J Immunol. 2008;38:489–499. doi: 10.1002/eji.200737057. [DOI] [PubMed] [Google Scholar]
- 90.O'Connor TM, O'Connell J, O'Brien DI, Goode T, Bredin CP, Shanahan F. The role of substance P in inflammatory disease. J Cell Physiol. 2004;201:167–180. doi: 10.1002/jcp.20061. [DOI] [PubMed] [Google Scholar]
- 91.Reinke E, Fabry Z. Breaking or making immunological privilege in the central nervous system: the regulation of immunity by neuropeptides. Immunol Lett. 2006;104:102–109. doi: 10.1016/j.imlet.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 92.Theoharides TC, Donelan JM, Papadopoulou N, Cao J, Kempuraj D, Conti P. Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol Sci. 2004;25:563–568. doi: 10.1016/j.tips.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 93.Ansel JC, Brown JR, Payan DG, Brown MA. Substance P selectively activates TNF-alpha gene expression in murine mast cells. J Immunol. 1993;150:4478–4485. [PubMed] [Google Scholar]
- 94.Oboki K, Ohno T, Kajiwara N, Saito H, Nakae S. IL-33 and IL-33 receptors in host defense and diseases. Allergol Int. 2010;59:143–160. doi: 10.2332/allergolint.10-RAI-0186. [DOI] [PubMed] [Google Scholar]
- 95.Roussel L, Erard M, Cayrol C, Girard JP. Molecular mimicry between IL-33 and KSHV for attachment to chromatin through the H2A-H2B acidic pocket. EMBO Rep. 2008;9:1006–1012. doi: 10.1038/embor.2008.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 2010;10:103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]