Abstract
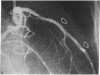
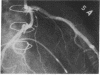
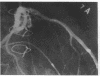
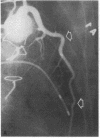
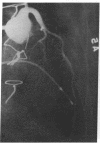
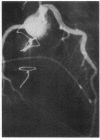
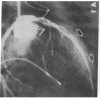
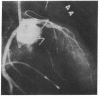
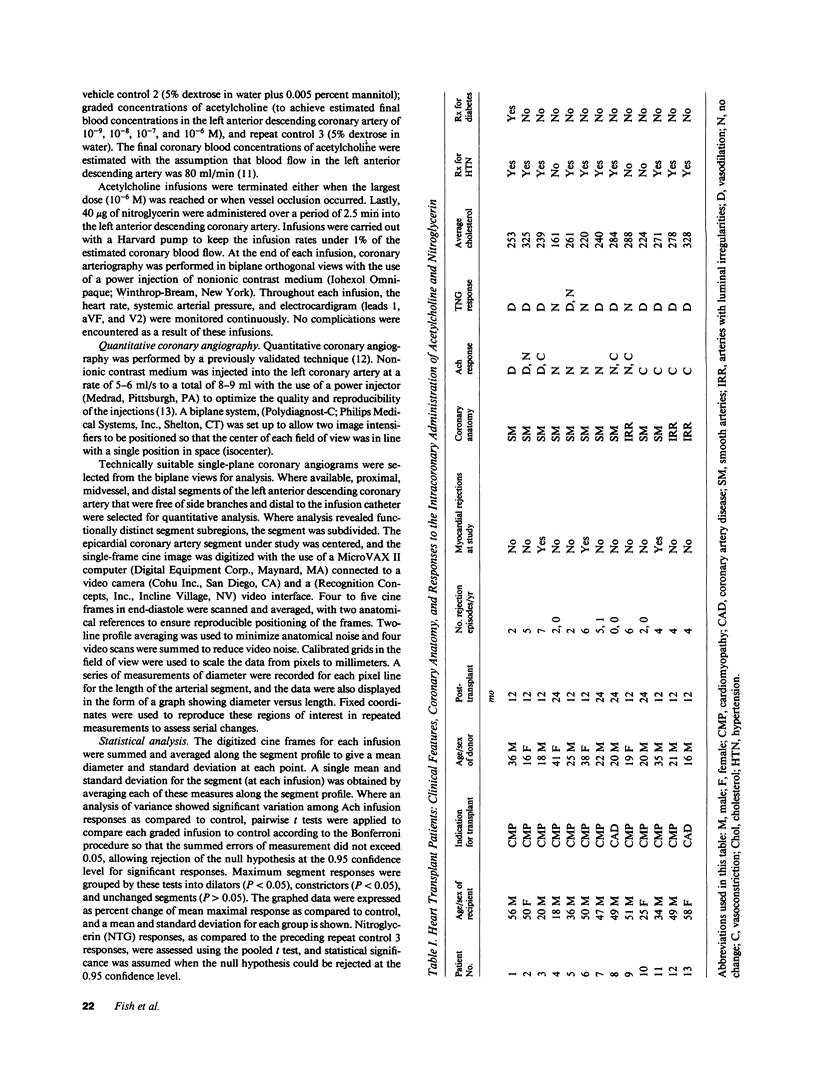
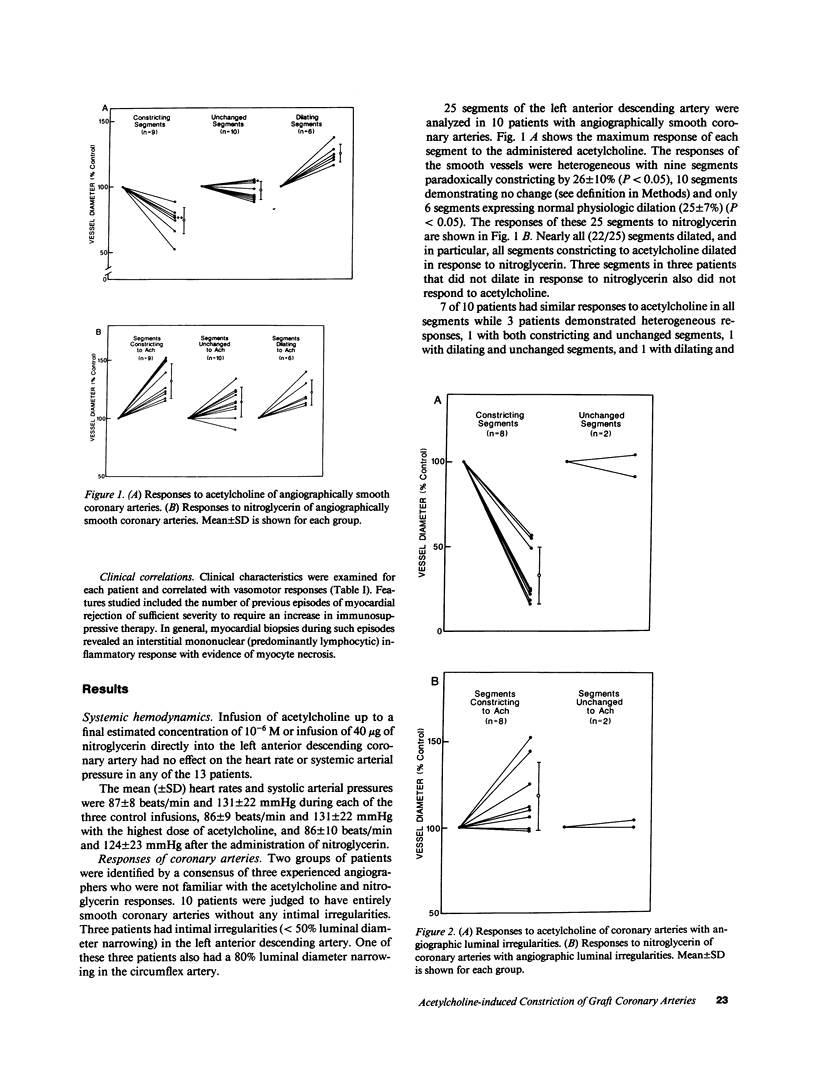
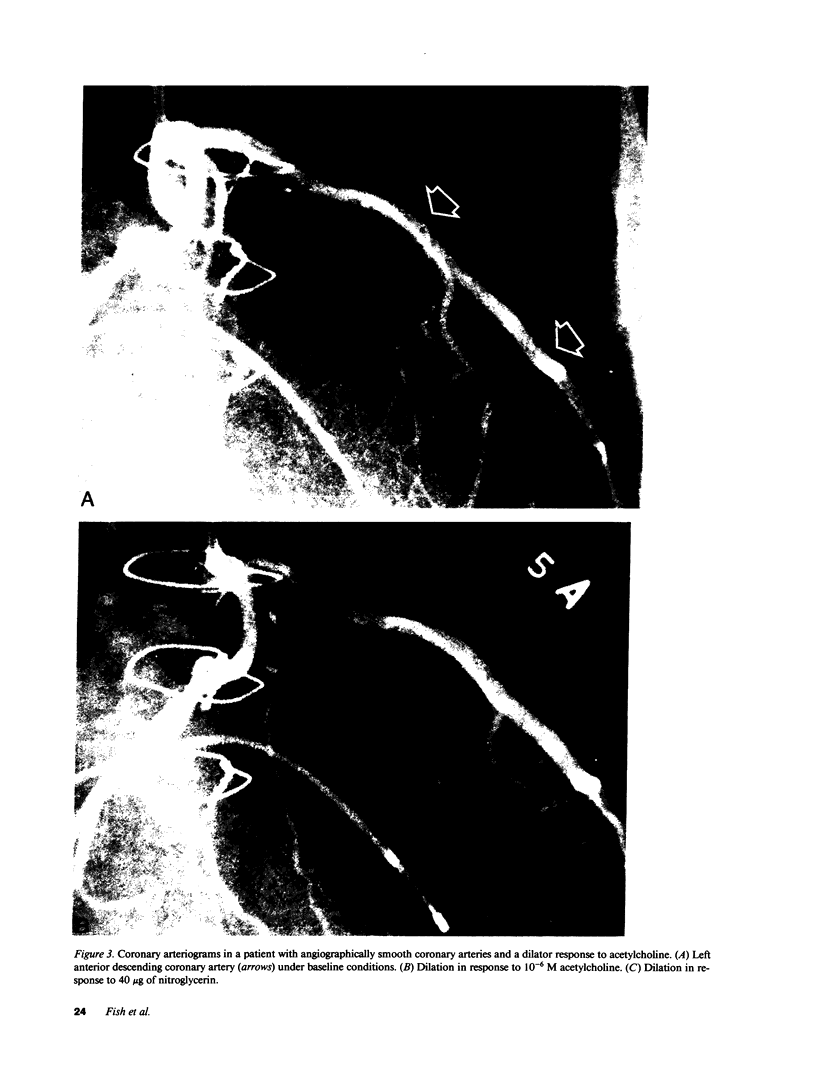
Accelerated coronary atherosclerosis is a major cause of graft failure after heart transplantation. Graft atherosclerosis is typically diffuse and difficult to detect even with coronary arteriography. Recently, acetylcholine was shown to dilate blood vessels by releasing a vasorelaxant substance from the endothelium (endothelium-derived relaxing factor). We have demonstrated paradoxical vasoconstriction induced by acetylcholine both early and late in the course of coronary atherosclerosis in patients, suggesting an association of endothelial dysfunction and atherosclerosis. In this report, we tested the hypothesis that coronary arteries of heart transplant patients can show endothelial dysfunction before or in the early stages of angiographically evident coronary atherosclerosis. Acetylcholine was infused into the left anterior descending artery of 13 heart transplant patients at 12 (n = 9) and 24 (n = 4) mo after transplantation. Vascular responses were evaluated by quantitative angiography. Among patients with angiographically smooth coronary arteries, relatively few (6/25) arterial segments had preserved vasodilator responses, while the majority failed to dilate (10/25) or paradoxically constricted (9/25). Angiographically irregular coronary arteries were present in three patients, in whom 8/10 segments showed marked paradoxical constriction and the remaining 2/10 failed to dilate. Only 1 of 13 patients retained appropriate dilation to acetylcholine in all segments. Nitroglycerin, which acts directly on vascular smooth muscle, dilated nearly all segments. No clinical features of the patients, including myocardial rejection appeared to correlate with the impaired functional response of vessels. Thus impaired response to acetylcholine is a common early finding in heart transplant patients and emphasizes the potential importance of endothelial dysfunction in the development of atherosclerosis.
Full text
PDF
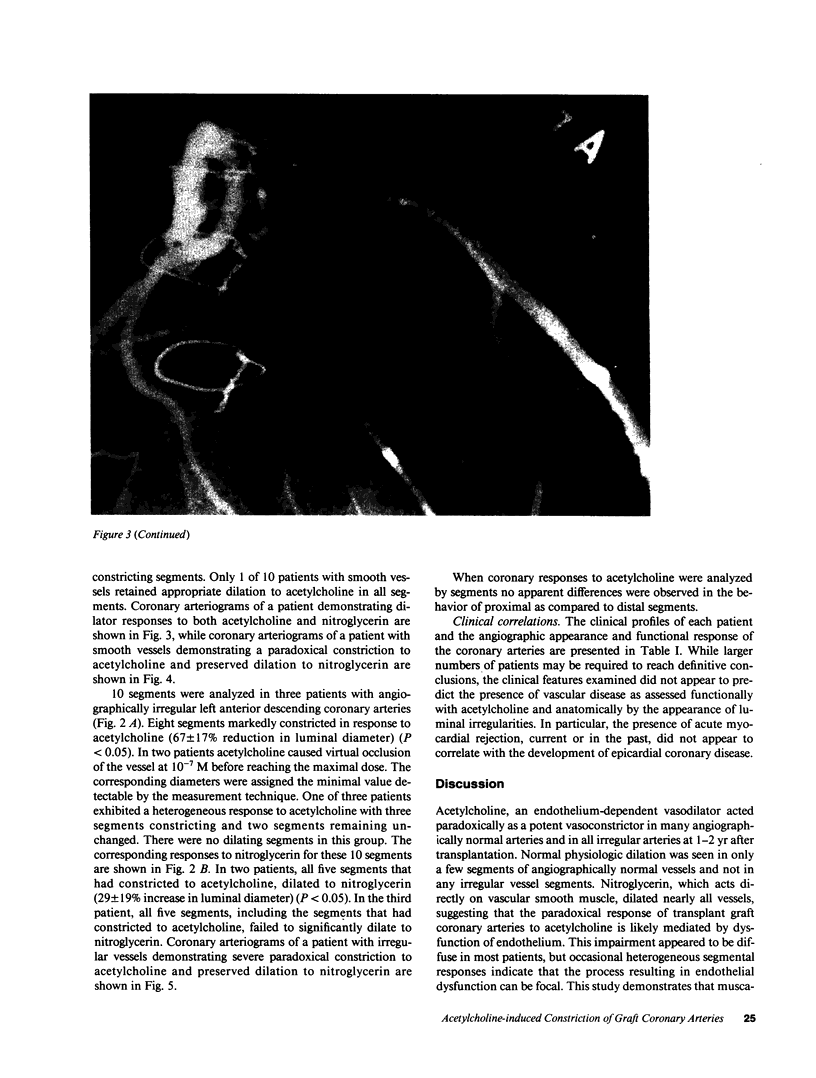
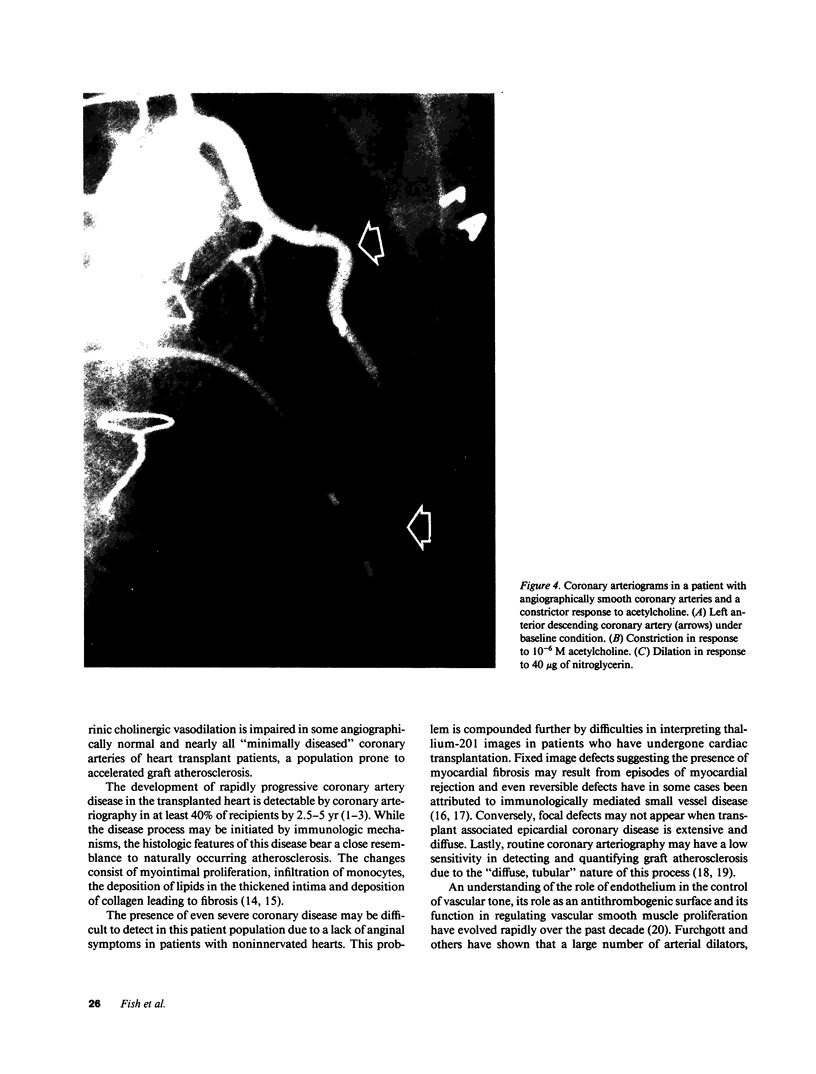





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevilacqua M. P., Pober J. S., Majeau G. R., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 (IL-1) induces biosynthesis and cell surface expression of procoagulant activity in human vascular endothelial cells. J Exp Med. 1984 Aug 1;160(2):618–623. doi: 10.1084/jem.160.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossaller C., Habib G. B., Yamamoto H., Williams C., Wells S., Henry P. D. Impaired muscarinic endothelium-dependent relaxation and cyclic guanosine 5'-monophosphate formation in atherosclerotic human coronary artery and rabbit aorta. J Clin Invest. 1987 Jan;79(1):170–174. doi: 10.1172/JCI112779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasile L., Zerbe T., Rabin B., Clarke J., Abrams A., Cerilli J. Identification of the antibody to vascular endothelial cells in patients undergoing cardiac transplantation. Transplantation. 1985 Dec;40(6):672–675. doi: 10.1097/00007890-198512000-00020. [DOI] [PubMed] [Google Scholar]
- Davies P. F. Vascular cell interactions with special reference to the pathogenesis of atherosclerosis. Lab Invest. 1986 Jul;55(1):5–24. [PubMed] [Google Scholar]
- DiCorleto P. E., de la Motte C. A. Characterization of the adhesion of the human monocytic cell line U937 to cultured endothelial cells. J Clin Invest. 1985 Apr;75(4):1153–1161. doi: 10.1172/JCI111810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Ganz P., Davies P. F., Leopold J. A., Gimbrone M. A., Jr, Alexander R. W. Short- and long-term interactions of endothelium and vascular smooth muscle in coculture: effects on cyclic GMP production. Proc Natl Acad Sci U S A. 1986 May;83(10):3552–3556. doi: 10.1073/pnas.83.10.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz W., Tamura K., Marcus H. S., Donoso R., Yoshida S., Swan H. J. Measurement of coronary sinus blood flow by continuous thermodilution in man. Circulation. 1971 Aug;44(2):181–195. doi: 10.1161/01.cir.44.2.181. [DOI] [PubMed] [Google Scholar]
- Gardiner G. A., Jr, Meyerovitz M. F., Boxt L. M., Harrington D. P., Taus R. H., Kandarpa K., Ganz P., Selwyn A. P. Selective coronary angiography using a power injector. AJR Am J Roentgenol. 1986 Apr;146(4):831–833. doi: 10.2214/ajr.146.4.831. [DOI] [PubMed] [Google Scholar]
- Kosek J. C., Bieber C., Lower R. R. Heart graft arteriosclerosis. Transplant Proc. 1971 Mar;3(1):512–514. [PubMed] [Google Scholar]
- Ludmer P. L., Selwyn A. P., Shook T. L., Wayne R. R., Mudge G. H., Alexander R. W., Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986 Oct 23;315(17):1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- McKillop J. H., Billingham M., Schroeder J. S., McDougall I. R. Correlation of an abnormal rest 201T1 myocardial image: pathological findings in cardiac transplant recipients. Eur J Nucl Med. 1982;7(6):243–247. doi: 10.1007/BF00251474. [DOI] [PubMed] [Google Scholar]
- McKillop J. H., Goris M. L. Thallium-201 myocardial imaging in patients with previous cardiac transplantation. Clin Radiol. 1981 Jul;32(4):447–449. doi: 10.1016/s0009-9260(81)80298-x. [DOI] [PubMed] [Google Scholar]
- Nitkin R. S., Hunt S. A., Schroeder J. S. Accelerated atherosclerosis in a cardiac transplant patient. J Am Coll Cardiol. 1985 Jul;6(1):243–245. doi: 10.1016/s0735-1097(85)80283-7. [DOI] [PubMed] [Google Scholar]
- Schroeder J. S., Hunt S. A. Cardiac transplantation: where are we? N Engl J Med. 1986 Oct 9;315(15):961–963. doi: 10.1056/NEJM198610093151511. [DOI] [PubMed] [Google Scholar]
- Spears J. R., Sandor T., Als A. V., Malagold M., Markis J. E., Grossman W., Serur J. R., Paulin S. Computerized image analysis for quantitative measurement of vessel diameter from cineangiograms. Circulation. 1983 Aug;68(2):453–461. doi: 10.1161/01.cir.68.2.453. [DOI] [PubMed] [Google Scholar]
- Uys C. J., Rose A. G. Cardiac transplantation: aspects of the pathology. Pathol Annu. 1982;17(Pt 2):147–178. [PubMed] [Google Scholar]
- Vanhoutte P. M., Rimele T. J. Role of the endothelium in the control of vascular smooth muscle function. J Physiol (Paris) 1982;78(7):681–686. [PubMed] [Google Scholar]
- Wohlgelernter D., Stevenson L. W., Brunken R. Reversal of ischemic myocardial dysfunction by PTCA in a cardiac transplant patient. Am Heart J. 1986 Oct;112(4):837–839. doi: 10.1016/0002-8703(86)90482-5. [DOI] [PubMed] [Google Scholar]