Abstract
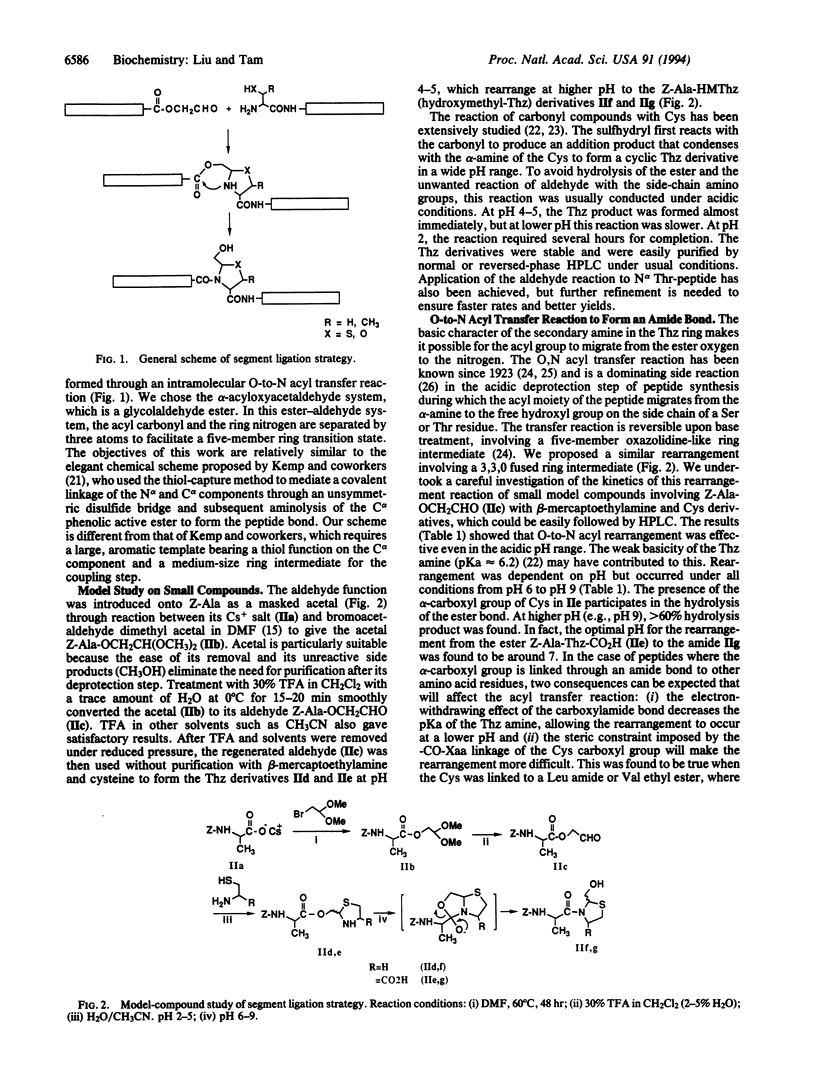
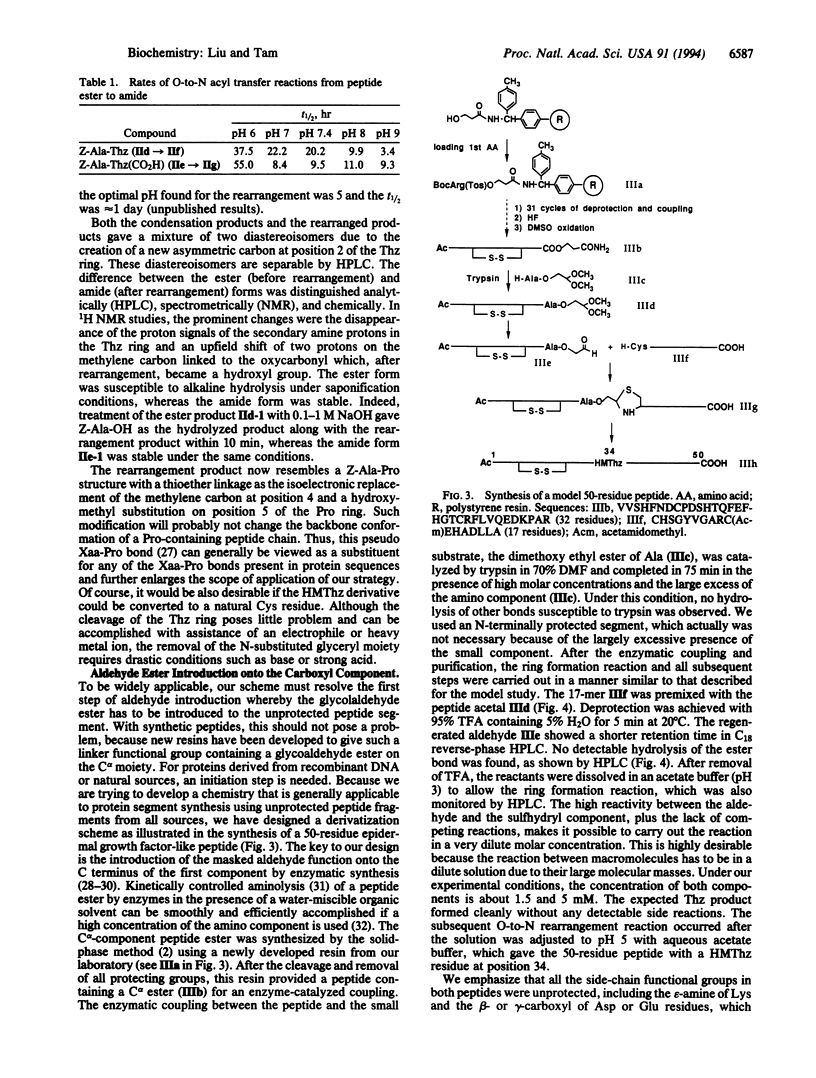
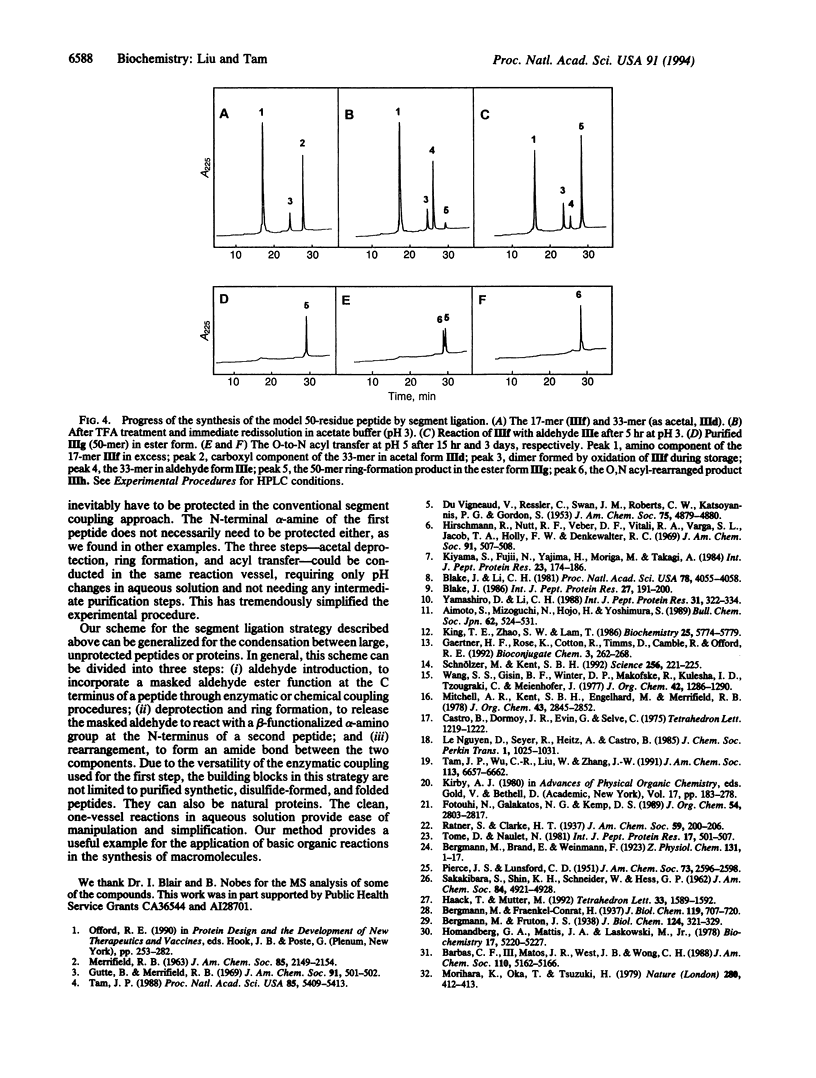
We describe the concept and the verification of a chemical ligation approach to the synthesis of proteins using peptide segments with no protecting groups and no activation of the C-terminal alpha-carboxyl group. This approach consists of three steps: (i) aldehyde introduction, in which a masked glycolaldehyde ester is linked to the carboxyl terminus of an unprotected peptide by reverse proteolysis; (ii) ring formation, in which the unmasked aldehyde reacts with the N-terminal alpha-amino group of the second unprotected peptide containing either a cysteine or a threonine residue to form a thiazolidine or oxazolidine ring at an acidic pH; and (iii) rearrangement in which O-acyl ester linkage is transferred to N-acyl amide linkage to form a peptide bond with a pseudoproline structure at higher pH. The feasibility of this scheme was verified by a model study on small compounds and its potential was demonstrated by the synthesis of a 50-residue epidermal growth factor-like peptide containing a preformed disulfide bond.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake J., Li C. H. New segment-coupling method for peptide synthesis in aqueous solution: application to synthesis of human [Gly17]-beta-endorphin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4055–4058. doi: 10.1073/pnas.78.7.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake J. Total synthesis of S-carbamoylmethyl bovine apocytochrome c by segment coupling. Int J Pept Protein Res. 1986 Feb;27(2):191–200. doi: 10.1111/j.1399-3011.1986.tb01810.x. [DOI] [PubMed] [Google Scholar]
- Gaertner H. F., Rose K., Cotton R., Timms D., Camble R., Offord R. E. Construction of protein analogues by site-specific condensation of unprotected fragments. Bioconjug Chem. 1992 May-Jun;3(3):262–268. doi: 10.1021/bc00015a010. [DOI] [PubMed] [Google Scholar]
- Gutte B., Merrifield R. B. The total synthesis of an enzyme with ribonuclease A activity. J Am Chem Soc. 1969 Jan 15;91(2):501–502. doi: 10.1021/ja01030a050. [DOI] [PubMed] [Google Scholar]
- Hirschmann R., Nutt R. F., Veber D. F., Vitali R. A., Varga S. L., Jacob T. A., Holly F. W., Denkewalter R. G. Studies on the total synthesis of an enzyme. V. The preparation of enzymatically active material. J Am Chem Soc. 1969 Jan 15;91(2):507–508. doi: 10.1021/ja01030a055. [DOI] [PubMed] [Google Scholar]
- Homandberg G. A., Mattis J. A., Laskowski M., Jr Synthesis of peptide bonds by proteinases. Addition of organic cosolvents shifts peptide bond equilibria toward synthesis. Biochemistry. 1978 Nov 28;17(24):5220–5227. doi: 10.1021/bi00617a023. [DOI] [PubMed] [Google Scholar]
- King T. P., Zhao S. W., Lam T. Preparation of protein conjugates via intermolecular hydrazone linkage. Biochemistry. 1986 Sep 23;25(19):5774–5779. doi: 10.1021/bi00367a064. [DOI] [PubMed] [Google Scholar]
- Kiyama S., Fujii N., Yajima H., Moriga M., Takagi A. Studies on peptides. CXIII. Synthesis of the heptacosapeptide amide corresponding to the entire amino acid sequence of chicken secretin. Int J Pept Protein Res. 1984 Feb;23(2):174–186. [PubMed] [Google Scholar]
- Morihara K., Oka T., Tsuzuki H. Semi-synthesis of human insulin by trypsin-catalysed replacement of Ala-B30 by Thr in porcine insulin. Nature. 1979 Aug 2;280(5721):412–413. doi: 10.1038/280412a0. [DOI] [PubMed] [Google Scholar]
- Schnölzer M., Kent S. B. Constructing proteins by dovetailing unprotected synthetic peptides: backbone-engineered HIV protease. Science. 1992 Apr 10;256(5054):221–225. doi: 10.1126/science.1566069. [DOI] [PubMed] [Google Scholar]
- Tam J. P. Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigenic peptide system. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5409–5413. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tome D., Naulet N. Carbon 13 nuclear magnetic resonance studies on formaldehyde reactions with polyfunctional amino-acids. Int J Pept Protein Res. 1981 Apr;17(4):501–507. doi: 10.1111/j.1399-3011.1981.tb02020.x. [DOI] [PubMed] [Google Scholar]
- Wang S-S, Gisin B. F., Winter D. P., Makofske R., Kulesha I. D., Tzougraki C., Meienhofer J. Facile synthesis of amino acid and peptide esters under mild conditions via cesium salts. J Org Chem. 1977 Apr 15;42(8):1286–1290. doi: 10.1021/jo00428a004. [DOI] [PubMed] [Google Scholar]
- Yamashiro D., Li C. H. New segment synthesis of alpha-inhibin-92 by the acyl disulfide method. Int J Pept Protein Res. 1988 Mar;31(3):322–334. doi: 10.1111/j.1399-3011.1988.tb00040.x. [DOI] [PubMed] [Google Scholar]



