Abstract
Purpose:
To evaluate the anterior keratoprosthesis-cornea interface in eyes with Boston type I keratoprosthesis (Kpro).
Methods:
In a prospective non-interventional study, patients with Boston type I Kpro underwent ultra-high resolution optical coherence tomography (UHR-OCT) evaluation. The images were used to measure and describe characteristics of the anterior keratoprosthesis-cornea interface, epithelial interaction at the keratoprosthesis edge and the keratoprosthesis-cornea interface gap.
Results:
Ten patients including 4 male and 6 female subjects with different preoperative diagnoses, i.e. 8 multiple corneal graft failures and 2 immunological ocular surface diseases, were studied. Mean age was 62.1 ± 20.0 (range, 33.0-83.0) years and mean interval between surgery and UHR-OCT evaluation was 15.2 ± 11.09 months. In eight patients, 360° epithelial growth over the peripheral edge of the Kpro was documented. We detected keratoprosthesis-cornea interface gap in three patients. One subject had developed postoperative endophthalmitis 8 months after surgery and the other two cases were among the high risk group according to the preoperative diagnosis. In one patient with severe ocular hypotony, the Kpro edge was inserted into the anterior stroma and covered with epithelium.
Conclusion:
UHR-OCT showed that corneal epithelium covers the Kpro edge and seals the potential space between the Kpro and cornea in 80% of cases. The presence of a gap in the interface and lack of epithelial sealing around the Kpro edge might be associated with endophthalmitis.
Keywords: Anterior Segment OCT, Boston Type I Keratoprosthesis, Ultra High-resolution OCT
INTRODUCTION
In the recent decade, there has been an exponential increase in the number of Boston type I keratoprosthesis (Kpro) surgeries performed in the United States and worldwide.[1] The Boston type I Kpro is considered as a treatment for a variety of corneal and external diseases including repeated graft failure, Stevens–Johnson syndrome, chemical burns, ocular cicatricial pemphigoid, stem cell deficiency, herpetic keratitis, aniridia and congenital corneal opacification. Although this procedure can provide better vision in severely diseased eyes, it might also result in sight-threatening complications.[1,2,3]
The majority of severe complications related to this procedure (such as corneal melting, corneal ulcers and endophthalmitis) are possibly due to lack of complete integration between the Kpro and the surrounding cornea. Unfortunately, routine slit lamp biomicroscopy cannot detect early, minimal changes and defects in the keratoprosthesis-cornea interface.
In the present case series, a custom-built, ultra high resolution, spectral domain Optical Coherence Tomography (UHR-OCT)[4,5,6] with an axial resolution of 2 μm was used to assess the anterior Kpro-cornea interface focusing on corneal epithelial configuration around the Kpro edge and the anterior Kpro-cornea interface in patients with acceptable anatomic results on slit lamp biomicroscopy.
METHODS
This prospective study was conducted at Bascom Palmer Eye Institute, University of Miami, USA. Institutional review board approval was obtained and the study followed the tenets of the declaration of Helsinki.
Ten eyes of 10 patients who had undergone Boston Type I Keratoprosthesis surgery in the past with complete follow up visit records were included. All surgeries were performed by the same surgeon (VLP). The data collected from the charts comprised of demographic characteristics, past ocular history, time of Kpro surgery, size of Kpro, clinical findings prior to and after surgical intervention.
Before UHR-OCT evaluation, a comprehensive ophthalmic examination was done for all patients including uncorrected and best corrected visual acuity measurement, refraction, slit lamp biomicroscopy and fundus examination. Intraocular pressure was estimated by comparing globe tension to the other eye using palpation. All patients underwent digital corneal photography using a camera (BQ900, Haag-Streit, Koeniz, Switzerland) mounted on a slit lamp.
According to preoperative clinical diagnoses and based on previous studies,[2,7] patients were classified to low or high risk groups. Low risk subjects were those with multiple graft failures in non-cicatrizing eyes, whereas Stevens–Johnson syndrome, ocular cicatricial pemphigoid (OCP) and other immunologic ocular surface diseases (which had adequately wet ocular surface to be appropriate for type I Kpro) were considered as high risk cases.
Ultra High-resolution OCT Examination
A novel custom-built UHR OCT prototype was used for this study. The system contains a superluminescent diode light source (Broadlighter, T840-HP; Superlumdiodes Ltd., Moscow, Russia) with a 100-nm bandwidth centered at 840 nm. It was connected to a telecentric light delivery system and was mounted on a slit lamp. Signals were transferred to a computer workstation (IBM IntelliStation Z Pro, Armonk, NY, USA) for processing and image display. The calibrated axial resolution of the system was approximately 2 μm in the cornea. Detailed information has been published before.[4]
For this study, 6 mm-radial UHR-OCT scans centered on the keratoprosthesis axis were captured at 31 frames per scan. In bandage contact lens (BCL) wearing cases, UHR-OCT was done with and without the contact lens. Images were re-calibrated to reflect the actual dimensions. ImageJ software (NIH, USA) was used to measure the keratoprosthesis-cornea interface gap. Three measurements were taken and the average value was calculated.
RESULTS
A total of 10 eyes of ten patients including 4 male and 6 female subjects with mean age of 62.1 ± 20.0 (range, 33.0-83.0) years who had undergone Boston type I keratoprosthesis were studied. Mean interval between surgery and UHR-OCT evaluation was 15.2 ± 11.09 (range, 1-34; median, 11.5) months. Eight patients were classified as low risk (cases of multiple graft failure) and the remaining two subjects had high risk eyes with peripheral ulcerative keratitis (PUK), and Stevens-Johnson syndrome. All patients used bandage contact lenses (BCL), except two.
Data from charts documented that no intra-or postoperative complication had developed after Kpro surgery. Slit lamp examination revealed that all patients had an acceptable anatomical result without leakage, persistent epithelial defects, corneal melting or infection.
Ultra High-resolution OCT Findings
The epithelial layer around the Kpro was perfectly imaged in all cases. Over the periphery of the Kpro, 360 degree epithelial growth was observed in eight patients sparing the Kpro optic [Figures 1 and 2]. Kpro-cornea interface gap was detected in three patients [Figures 3 and 4] of whom two subjects had been classified as high risk based on preoperative diagnosis and the other patient had developed postoperative endophthalmitis. In this particular case, we found that the gap was connected to the ocular surface area due to lack of epithelial coverage over the Kpro edge.
Figure 1.
Case 1; (a), UHR-OCT image shows keratoprosthesis-cornea interface gap (*) and epithelial defect on the cornea 3 days after surgery; (b), Epithelial growth over the Kpro edge (red arrows) on postoperative day 30 in UHR-OCT image, note that the gap has disappeared.
Figure 2.
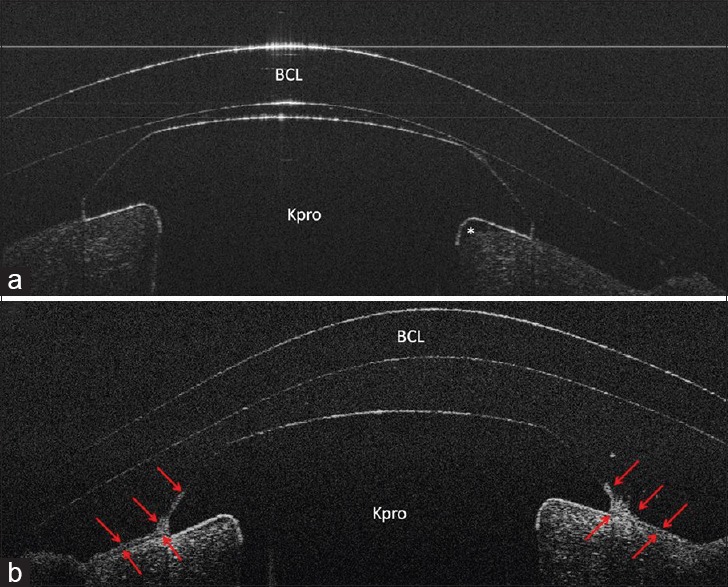
Case 5; (a), Slit lamp photo; (b), UHR-OCT image shows epithelial growth over the Kpro edge (red arrows). Note the epithelial cyst over the Kpro edge on the right (#).
Figure 3.
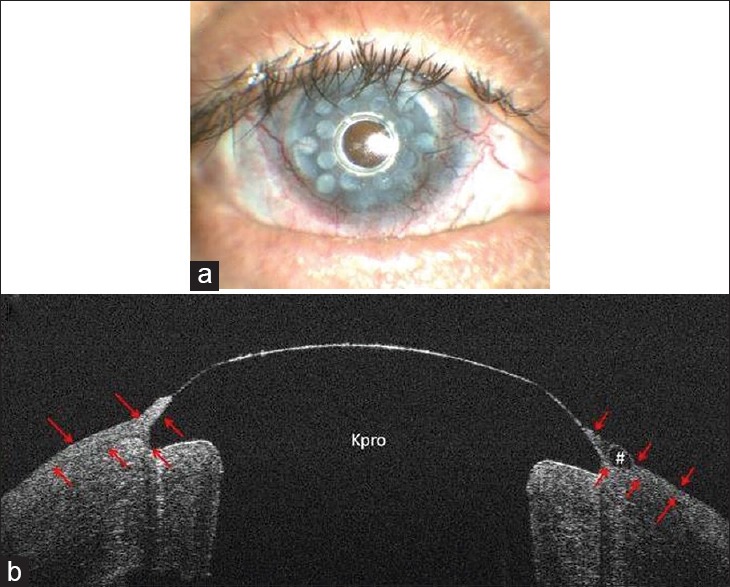
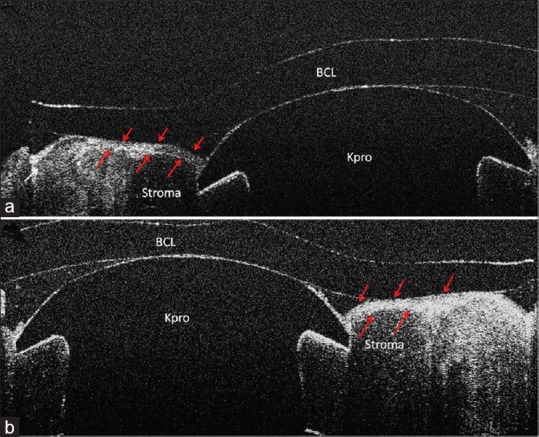
Case 6; (a), UHR-OCT horizontal scan shows keratoprosthesis-cornea interface gap (*) which opens to ocular surface area (white arrow); (b), UHR-OCT vertical scan of the same patient. Note epithelial growth underneath the Kpro edge and over the Kpro on the left and right sides of the image, respectively. The gap only is present on the left side of the image where the epithelial layer has not covered the Kpro edge. Red arrows show the epithelial layer borders; (c), UHR-OCT, in case 8, shows a gap between keratoprosthesis-cornea interface (*). There is no connection between the gap and the ocular surface area, as the epithelium layer covered the Kpro edge and the gap (red arrows).
Figure 4.
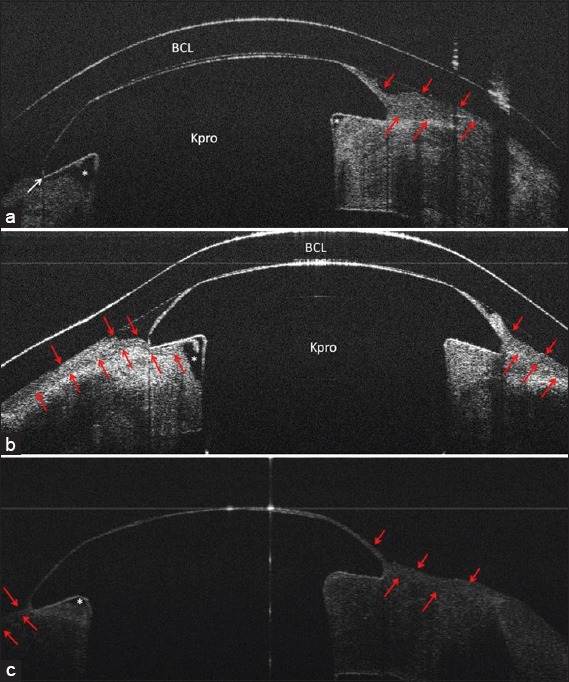
Case 9; (a), Slitlamp photo (b), UHR-OCT image shows lack of epithelial sealing and coverage over the Kpro edge and an associated interface gap. This patient had endophthalmitis at the time of imaging. The red arrows show borders of the epithelium.
In one patient with severe ocular hypotony, UHR-OCT revealed the Kpro to be embedded in the cornea (Kpro edge was in the anterior stroma instead of over it) [Figure 5]. The tear film gap was visualized between the BCL and the cornea-Kpro edge transitional zone in all eyes. BCL had contact with the Kpro in 50% of cases [Figures 1–5]. Table 1 shows the demographics, past ocular history and UHR-OCT findings of all cases. Below, we present more details regarding UHR-OCT characteristics of keratoprosthesis-cornea interface.
Figure 5.
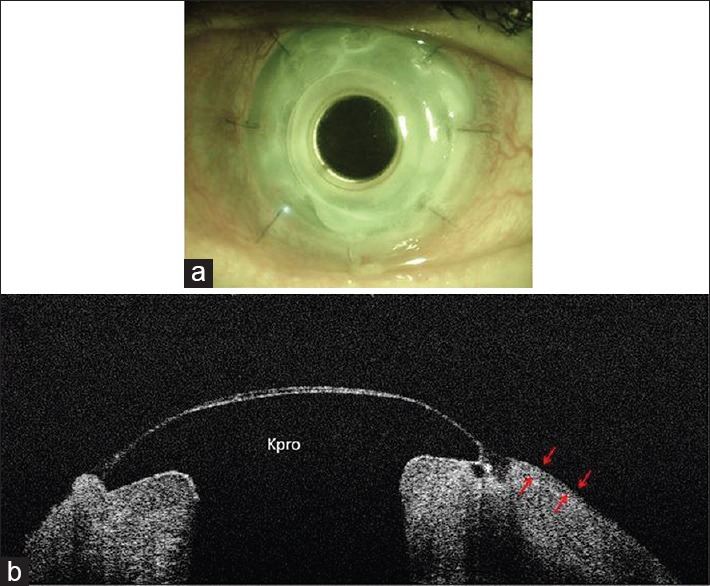
Case 10; (a) UHR-OCT image shows embedded Kpro in the cornea; (b) Kpro edge is completely covered by corneal epithelium (red arrows) and inserted into the anterior stroma.
Table 1.
Demographics, clinical history and the UHR-OCT findings of the patients
Case 1: Epithelial Interaction with Kpro and Interface Gap in the Early Postoperative Period
Case 1 was a 63-year-old man who had undergone Kpro after 2 failed corneal transplants. The background disease was anterior uveitis and glaucoma secondary to sarcoidosis. UHR-OCT imaging was done 3 days after surgery. The image showed corneal epithelial defect around the Kpro and no epithelial growth over it, as expected in the early postoperative phase. A small gap was observed in the keratoprosthesis-cornea interface. The BCL touched on the Kpro. On postoperative day 30, another UHR-OCT evaluation was done at the time which the corneal epithelial defect was healed with growth over the Kpro edge and no gap was observed in any meridian in this visit [Figure 1].
Case 5: Epithelial Growth over the Kpro Edge
A 51-year-old woman with multiple graft failures for treatment of corneal scar and vascularization after LASIK was evaluated with UHR-OCT. Complete and 360-degree epithelial growth over the Kpro edge without interface gap was detected. No epithelial growth underneath the Kpro edge was observed [Figure 2].
Cases 6 and 8: Latent Interface Gap in High Risk Profile Patients
Cases 6 and 8 had immunologic ocular surface diseases and were classified in the high risk group. Slit lamp biomicroscopy showed no sign of gap or epithelial defect in the Kpro-cornea interface in both patients. Case 6, a 70-year-old man with a Kpro implant for treatment of corneal blindness secondary to Stevens–Johnson syndrome, was evaluated 34 months after surgery. We found incomplete (≈174°) epithelial growth over the Kpro edge. In addition, epithelial growth underneath the Kpro was detected by UHR-OCT, in the same area which was not covered by the epithelium. There was also approximately 348 degrees gap between the Kpro and cornea. The gap was around the Kpro's cylindrical optic and opened to the ocular surface through a fistula between the cornea and Kpro edge. Case 8 had Kpro implantation due to PUK. UHR-OCT showed a gap without opening to the ocular surface as the Kpro edge was completely covered by epithelium [Figure 3].
Case 9: Lack of Epithelial Growth over the Kpro and Kpro-cornea Gap in Latent Endophthalmitis
An 89-year old man, classified as a low risk patient, presented with endophthalmitis 8 months after Kpro surgery. UHR-OCT showed an epithelial defect around the Kpro edge with an interface gap. In approximately 60 degrees of the Kpro peripheral edge, the epithelium did not cover the peripheral Kpro edge and the interface gap was connected to the ocular surface [Figure 4]. Culture from the vitreous tap specimen grew Corynebacterium species.
Case 10: Complete Epithelial Covering and Insertion of the Kpro in Anterior Corneal Stroma with Ocular Hypotony
UHR-OCT in a 51-year-old female patient with a history of several retinal detachments and severe ocular hypotony who subsequently underwent Kpro implantation, showed embedded Kpro into anterior corneal stroma with 360 degrees of epithelial growth over the Kpro edge. The epithelial layer had incompletely filled the concave area between Kpro edge and cornea [Figure 5].
DISCUSSION
The Boston type I keratoprosthesis is made of polymethylmethacrylate (PMMA). It is considered as a “biocompatible” but not “biointegrated” Kpro. It has contact with all layers of the cornea and the lack of “biointegration” with corneal epithelium and stroma could potentially hinder the barrier effect against infection and cornea-Kpro structural strength, respectively.[8]
Most complications of Kpro including persistent epithelial defect, corneal melt and corneal ulcer happen around the anterior Kpro-cornea interface. In addition, endophthalmitis could develop secondary to leakage or infection in this interface. The question arises as to whether there are minimal and undetectable changes which might make the area susceptible to these complications. As routine ophthalmic examination has limitations for observing keratoprosthesis-cornea interface, our aim was to evaluate the anterior keratoprosthesis-cornea interface for minimal changes in patients with anatomically acceptable Kpro implant at slit lamp biomicroscopy. In the current study, we used a novel spectral domain OCT (SD-OCT) device with enhanced resolution as compared to conventional OCT.[9,10]
In our series, 8 out of 10 subjects showed epithelial growth over the Kpro edge with sparing of the optic [Table 1]. The epithelial overgrowth on the Kpro was already observed by the ophthalmologist especially when it has covered the optic.[11] Our HR-OCT images showed that the epithelium tends to cover all around the Kpro edge and fill the concavity of the transitional zone in most of the cases (80%). In these cases, a thick epithelial layer masks the irregularities in the transitional zone between the cornea and the Kpro [Figures 1–5]. We believe that sealing and smoothening of this zone by the epithelium could prevent the access of microorganisms to the “non-biointegrated” keratoprosthesis-stroma interface.[12] Epithelial sealing around the Kpro may also facilitate hydration of the corneal stroma to avoid the interface gap. In case 1, the early interface gap resolved one month after surgery. The gap, shortly present after the operation, was associated with a complete epithelial defect around the Kpro edge. The early postoperative data (POD 3) were not included in our statistics. One month after surgery, epithelium covered the Kpro edge completely and the gap disappeared.
In three patients, we observed the interface gap several months after surgery. One of them presented with acute endophthalmitis 8 months after Kpro implantation. In this case, the gap was connected with the ocular surface due to lack of epithelial growth over the Kpro which possibly allowed microorganisms to enter the eye. Garcia et al have published two articles regarding OCT in patients with Kpro implants.[13,14] They have described similar OCT finding in their case series[13] reporting a gap in two eyes. However, the lower axial resolution of their OCT device (AC Cornea OCT prototype; OTI, Canada) did not allow the evaluation of fine changes in the epithelium around the Kpro edge.
In Case 6 (the patient with history of Stevens-Johnson syndrome), the gap was associated with epithelial growth underneath the Kpro edge in approximately half of its circumference. In this area, the Kpro cylinder was exposed to ocular surface environment. The other case with the gap in this study, Case 8, also had a history of PUK, an immunological ocular surface disease, and was classified in the high risk group. In this patient, the gap was completely covered by the epithelium and we did not find any opening between the gap and ocular surface [Figure 3].
The role and importance of the interface gap is still unclear. Interestingly, two out of our three patients with gap, were classified as high risk and one presented with endophthalmitis. According to the findings in the patient with endophthalmitis, we believe that the Kpro gap is an important finding and if it is associated with the lack of epithelial covering around the Kpro, it might allow microorganisms to enter the eye. In addition, in both cases with the lack of epithelial covering of the Kpro edge (cases 6 and 9), we found a gap in the keratoprosthesis-stroma interface. This might show the pivotal role of the epithelium for maintaining hydration and protection of the stroma. However, in the presence of the gap without epithelial defect, the question remains whether the gap could be stable or could change and expand over time. If it expands, it could even reach the anterior chamber or ocular surface as Figure 3 in which the gap already passed half thickness of the cornea. In this case, expansion of the gap might open a fine path between the anterior chamber and ocular surface environment which could be easily overlooked during routine examination and might cause severe complications. Interestingly, in one of our cases with severe ocular hypotony, UHR-OCT images [Figure 5] showed that the Kpro was embedded into the cornea and covered by anterior corneal stroma and epithelium. It is not clear if it will cause more protection and stability for this end-stage atrophic eye.
Although this cross-sectional case series with low sample size had not been designed to find an association between the UHR-OCT findings and postoperative complications, demonstration of the gap and incomplete epithelial growth in a case with endophthalmitis and two high risk patients, seems to be an alarming sign. We suggest a cohort study to evaluate the role of keratoprosthesis-cornea interface gap in the development of complications after Kpro implantation.
In summary, UHR-OCT technology provides cross-sectional high-resolution images of the anterior keratoprosthesis-cornea interface which could not be evaluated by slitlamp biomicroscopy. It showed that the corneal epithelium covers the Kpro edge and seals the potential space between the Kpro and the cornea in most cases. The images also documented the lack of epithelial growth over the Kpro edge and the presence of an interface gap in 20% and 30% of the patients, respectively. Further studies are required to elucidate the importance of these findings.
Footnotes
Source of Support: R01 EY018624-01 (VLP), P30 EY014801, Research to Prevent Blindness.
Conflict of Interest: None declared.
REFERENCES
- 1.Aldave AJ, Kamal KM, Vo RC, Yu F. The Boston type I keratoprosthesis: Improving outcomes and expanding indications. Ophthalmology. 2009;116:640–651. doi: 10.1016/j.ophtha.2008.12.058. [DOI] [PubMed] [Google Scholar]
- 2.Zerbe BL, Belin MW, Ciolino JB. Boston Type 1 Keratoprosthesis Study Group. Results from the multicenter Boston Type 1 Keratoprosthesis Study. Ophthalmology. 2006;113:1779.e1–7. doi: 10.1016/j.ophtha.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Bradley JC, Hernandez EG, Schwab IR, Mannis MJ. Boston type 1 keratoprosthesis: The university of california davis experience. Cornea. 2009;28:321–327. doi: 10.1097/ICO.0b013e31818b8bfa. [DOI] [PubMed] [Google Scholar]
- 4.Shousha MA, Perez VL, Wang J, Ide T, Jiao S, Chen Q, et al. Use of ultra-high-resolution optical coherence tomography to detect in vivo characteristics of Descemet's membrane in Fuchs’ dystrophy. Ophthalmology. 2010;117:1220–1227. doi: 10.1016/j.ophtha.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shousha MA, Karp CL, Perez VL, Hoffmann R, Ventura R, Chang V, et al. Diagnosis and management of conjunctival and corneal intraepithelial neoplasia using ultra high-resolution optical coherence tomography. Ophthalmology. 2011;118:1531–1537. doi: 10.1016/j.ophtha.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Jiao S, Ruggeri M, Shousha MA, Chen Q. In situ visualization of tears on contact lens using ultra high resolution optical coherence tomography. Eye Contact Lens. 2009;35:44–49. doi: 10.1097/ICL.0b013e31819579f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yaghouti F, Nouri M, Abad JC, Power WJ, Doane MG, Dohlman CH. Keratoprosthesis: Preoperative prognostic categories. Cornea. 2001;20:19–23. doi: 10.1097/00003226-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Ciolino JB, Dohlman CH. Biologic keratoprosthesis materials. Int Ophthalmol Clin. 2009;49:1–9. doi: 10.1097/IIO.0b013e3181924904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tao A, Wang J, Chen Q, Shen M, Lu F, Dubovy SR, et al. Topographic thickness of Bowman's layer determined by ultra-high resolution spectral domain-optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:3901–3907. doi: 10.1167/iovs.09-4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vajzovic LM, Karp CL, Haft P, Shousha MA, Dubovy SR, Hurmeric V, et al. Ultra high-resolution anterior segment optical coherence tomography in the evaluation of anterior corneal dystrophies and degenerations. Ophthalmology. 2011;118:1291–1296. doi: 10.1016/j.ophtha.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Khalifa YM, Davis D, Mamalis N, Moshirfar M. Epithelial growth over the optic surface of the type 1 Boston Keratoprosthesis: Histopathology and implications for biointegration. Clin Ophthalmol. 2010;4:1069–1071. doi: 10.2147/OPTH.S12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doane MG, Dohlman CH, Bearse G. Fabrication of a keratoprosthesis. Cornea. 1996;15:179–184. doi: 10.1097/00003226-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Garcia JP, Jr, Ritterband DC, Buxton DF, De la Cruz J. Evaluation of the stability of Boston type I keratoprosthesis-donor cornea interface using anterior segment optical coherence tomography. Cornea. 2010;29:1031–1035. doi: 10.1097/ICO.0b013e3181ca2ea5. [DOI] [PubMed] [Google Scholar]
- 14.Garcia JP, Jr, de la Cruz J, Rosen RB, Buxton DF. Imaging implanted keratoprostheses with anterior-segment optical coherence tomography and ultrasound biomicroscopy. Cornea. 2008;27:180–188. doi: 10.1097/ICO.0b013e318159bc7d. [DOI] [PubMed] [Google Scholar]


