Abstract
Purpose:
To assess changes in aqueous humor levels of different interleukins (IL), tumor necrosis factor (TNF)-α and vascular endothelial growth factor (VEGF) in patients with uveitis treated with adalimumab.
Methods:
In this study, 24 aqueous humor samples including 12 pre- and post-treatment samples from 6 patients with uveitis treated with subcutaneous adalimumab and 12 samples from patients with cataracts (serving as controls) were evaluated. The levels of IL-1β, IL-2, IL-6, IL-10, TNF-α and VEGF were measured using a Luminex® 200™ flow cytometer (Merckmillipore, Merck KGaA, Darmstadt, Alemania) and a highly sensitive ELISA system.
Results:
The levels of IL-1β, IL-2, IL-6 and IL-10 in the aqueous humor before and after treatment with adalimumab did not show significant differences. Aqueous VEGF levels significantly reduced after treatment with adalimumab (P = 0.028). Aqueous TNF-α levels did not significantly change after treatment with adalimumab, however the post-treatment level was significantly higher in patients as compared to control subjects (P = 0.032). IL-2 showed significantly higher levels in uveitis patients before treatment as compared to controls (P = 0.024), while its post-treatment levels were almost normalized.
Conclusion:
Decrease in the aqueous humor levels of VEGF and IL-2 after treatment with systemic adalimumab indicates that anti-TNF-α therapy induces modifications of some inflammatory mediators involved in the pathogenesis of uveitis. Aqueous humor samples may be useful to assess the effect of adalimumab on intraocular inflammation through measurement of cytokines.
Keywords: Adalimumab, Aqueous Humor, Interleukins, Tumor Necrosis Factor Alpha, Uveitis, Vascular Endothelial Growth Factor
INTRODUCTION
Autoimmune uveitis comprises an extensive array of inflammatory eye diseases and often results in irreversible visual loss. Uveitis may be primarily restricted to the eye or occur in association with systemic inflammatory conditions. The disease is particularly prevalent in young people and can be difficult to manage due to frequent relapses and complications.[1,2] Uveitis represents the fifth leading cause of visual loss in Europe with a prevalence of approximately 35-80 per 100,000[3] and responsible for 5-20% of blindness.[3] The severe morbidity of uveitis provides a stimulus for research on its pathogenetic mechanisms and the development of effective therapies.
Although the dysregulation of the immune system in uveitis is not thoroughly characterized, experimental models of autoimmune uveitis revealed a pivotal role for antigen-specific CD4 + T-helper (Th) lymphocytes with activation of Th-1 and Th-2 mediated intraocular inflammatory pathways and overexpression of chemokines related to the Th1 and Th2 systems. High levels of interleukin (IL)-2, IL-6, IL-10, IL-13, IL-18, tumor necrosis factor (TNF)-α, interferon-γ and vascular endothelial growth factor (VEGF) were detected in the aqueous humor of patients with uveitis[4,5,6] and in endotoxin-induced models of uveitis.[7,8] It has been shown that TNF-α actively participates in the pathogenesis of clinical uveitis[5] and elevated serum levels of TNF-α seem to be associated with recurrent uveitis.[9]
Therapeutic options for non-infectious uveitis have largely improved with the introduction of immunosuppressant agents, such as cyclosporine, azathioprine, methotrexate and mycophenolate mofetil. However, despite successful immunosuppression of ocular inflammation, some cases of uveitis cannot be controlled. The availability of biologic agents has markedly improved the therapy of uveitis and considerably increased the possibility of long-term remissions, in particular, for patients with refractory and sight threatening inflammatory disorders.[10,11] Adalimumab, a recombinant IgG1 monoclonal antibody targeting TNF-α, showed efficacy in approved targeted indications for a number of systemic autoimmune disorders. Administrating a single 40 mg dose of adalimumab subcutaneously is absorbed and distributed slowly, reaching peak serum concentrations within almost 5 days.[12] After single intravenous doses ranging from 0.25-10 mg/kg, concentrations are dose-proportional. For a dose of 0.5 mg/kg (~40 mg), clearance range from 11 to 15 mL/hour, the distribution volume ranges from 5 to 6 litres and the mean terminal phase half-life is approximately 2 weeks.[12] Adalimumab concentrations in the synovial fluid in patients with rheumatoid arthritis range from 31 to 96% of those in the serum.[12] No data is available on the ocular penetration of adalimumab but clinical trials are currently being conducted for uveitis. However, adalimumab has been used off-label for ocular inflammatory diseases, such as uveitis, with satisfactory results.[13,14,15] Moreover, in a recent evidence-based interdisciplinary guideline for anti-inflammatory treatment of uveitis associated with juvenile idiopathic arthritis, adalimumab was recommended as the preferred TNF-α inhibitor agent.[16]
The present study was designed to assess changes in the aqueous humor levels of four different interleukins, TNF-α and VEGF in patients with uveitis before and after treatment with adalimumab and in patients with cataract as a control group.
METHODS
The study was conducted at the Uveitis Unit in the Ophthalmology Service at General University Hospital, Valencia, Spain in daily practice conditions. In this study, the levels of IL-1β, IL-2, IL-6, IL-10, TNF-α and VEGF in 24 aqueous humor samples including 12 pre-and post-treatment samples from 6 non-consecutive patients with chronic, non-infectious, refractory uveitis treated with subcutaneous adalimumab and 12 samples from patients with cataract were measured. Six eligible patients were selected for the study according to criteria proposed by the Standardization of Uveitis Nomenclature (SUN) Working Group.[17] The inclusion criteria comprised age ≥ 18 years, patients with bilateral, chronic, non-infectious, refractory uveitis in whom treatment with at least one additional immunosuppressant drug (cyclosporine, methotrexate, azathioprine or mycophenolate mofetil) as well as oral corticosteroids was ineffective, Inability to maintain the disease without relapse for more than one year before enrolment. The patients were treated with 40 mg adalimumab (Humira, Abbott Laboratories, Chicago, IL, USA) subcutaneously every other week for 6 months (12 episodes of treatment with a total dose of 480 mg adalimumab). Treatment was administered on a compassionate-use basis.
Aqueous humor samples were obtained before initiating adalimumab and at the day after administration of the last dose of adalimumab at month six. During this period, the underlying immunosuppressive therapy was not modified and if a patient required additional topical or systemic treatment owing to disease relapse, the subject was excluded. All patients underwent a protein-purified derivative skin test (PPD) and chest radiography before enrolment because of the risk of tuberculosis associated with TNF inhibition. The control group included non-selected patients of any age routinely scheduled for cataract surgery. All subjects underwent complete physical and ocular examination as well as a detailed medical history. Inclusion criteria for the control group were absence of systemic diseases, inflammatory diseases, infection, neoplasms, diabetes mellitus, hypertension, cardiovascular disease, osteoporosis or other rheumatic diseases, as well as eye diseases including age-related macular degeneration and diabetic retinopathy. Cases and controls were not matched for age.
The study protocol was approved by the Ethics Committee of the University General Hospital of Valencia, and written informed consent was obtained from all participants.
Samples of aqueous humor were obtained by anterior chamber paracentesis. Pre- and post-procedure antibiotic prophylaxis was used before and after sampling, the eyes were rinsed with 5% povidone-iodine solution. Anterior chamber paracentesis was performed at the slit lamp using a 30G needle in patients with uveitis, or using an operation microscope before starting cataract surgery in the control subjects. Samples of aqueous humor (0.1-0.2 mL) were collected in sterile tubes and immediately frozen to-80°C until further analysis, when samples were thawed just prior to the assays.
Measurement of IL-1β, IL-2, IL-6, IL-10 and TNF-α was performed employing a Luminex® 200™ flow cytometer (Merck Millipore, Merck KGaA, Darmstadt, Germany) multiple array assay system using Multiplex kits (Millipore) based on the Luminex xMAP technology designed to simultaneously measure multiple protein targets in a single sample according to the manufacturer's instructions.[18] The MILLIPLEX MAP Human Cytokine/Chemokine panel (EMD Millipore Corporation, Billerica, MA, USA) incubating 25 μL of the sample with antibodies at − 4°C was used. Values were expressed as pg/mL, standard curve range from 3.2-10,000 pg/mL. Aqueous humor levels of VEGF were measured with a high sensitive ELISA kit (RandD Systems, Minneapolis, MN, USA) using 25 μL of the sample and values were expressed as ng/mL.
Statistical Analysis
Results were expressed using median and range (10th-90th percentile). Data in the control group and pre- and post-treatment data in patients with uveitis were compared employing the Mann-Whitney U test. Pre- and post-treatment data in patients with uveitis were compared using the Wilcoxon signed-rank test. P values less than 0.05 were considered as statistically significant.
RESULTS
A total of 24 aqueous humor samples were analysed, comprised of 12 samples from 6 patients with bilateral uveitis including 2 male and 4 female subjects aged 33-68 years, and 12 samples from the control group. Out of six uveitis cases, two patients had idiopathic panuveitis, two were affected with Vogt-Koyanagi-Harada (VKH) syndrome, one subject had idiopathic vasculitis and the last one was affected with Behçet disease. Individual aqueous humor levels of cytokines in patients and controls are shown in Table 1.
Table 1.
Individual aqueous humor levels of cytokines in patients with uveitis and in control eyes

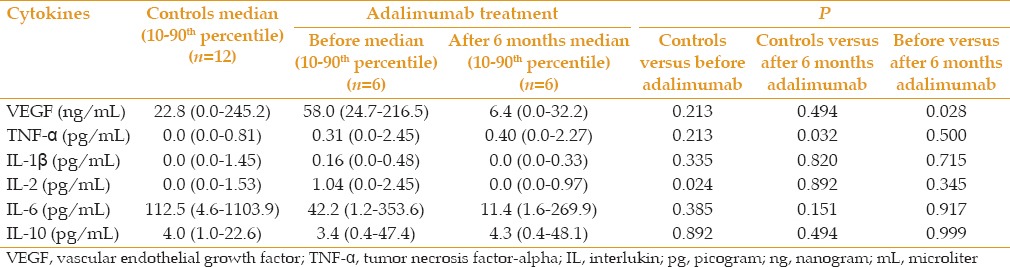
Aqueous humor levels of VEGF in patients with uveitis after 6 months of treatment with adalimumab were significantly decreased (median [10th-90th percentile], 6.4; range, 0.0-32.2 ng/mL) as compared to pre-treatment levels (mean, 58.0; range, 24.7-216.5 ng/mL) (P = 0.028). This reduction was correlated with the clinical response observed in all patients after 6 months of treatment with adalimumab. All patients remained without inflammatory activity both in the anterior and posterior segments of the eye and maintained stable visual acuity. Moreover, in one patient with Behçet disease and another one with idiopathic vasculitis, visual acuity was improved by 5 and 7 letters, respectively.
There was a non-significant reduction in the aqueous humor levels of IL-1β, IL-2, IL-6 and IL-10 after treatment. TNF-α level after treatment (mean, 0.40; range 0.0-2.27 mg/mL) remained comparable to pre-treatment levels (mean, 0.31; range 0.0-2.45 pg/mL).
Comparing uveitis patients with the control group revealed that levels of TNF-α significantly increased after treatment in patients as compared to controls (P value = 0.032). In addition, IL-2 showed significantly higher levels in uveitis patients before treatment (mean 1.04; range, 0.0-2.45 pg/mL) as compared to controls (P value = 0.024), while its post-treatment levels were almost normalized [Table 2]. With regard to IL-6, although there were no significant differences between the study groups, high levels were found in the uveitis group (mean, 42.2; range, 1.2-353.6 pg/mL) [Table 2]. All comparisons between control groups and patients with uveitis before and after 6 months of treatment with adalimumab are detailed in Table 2.
Table 2.
Comparison of aqueous humor levels of cytokines in the control group and uveitis patients before and after treatment with adalimumab
DISCUSSION
Adalimumab is a first fully human (100% human peptide sequences) anti-TNF-α monoclonal antibody which also inhibits the production of some pro-inflammatory cytokines, such as IL-1 and IL-6.[19] Secondary effects reported in association with the use of adalimumab include reactivation of tuberculosis, a higher incidence of infections (severe infections, sepsis and other opportunistic infections) and deterioration of demyelinating neurological diseases. Therefore, patients should be closely monitored to minimize the risk of adverse reactions.
TNF-α is a very potent pro-inflammatory cytokine playing a key role in the regulation of ocular inflammation,[20] therefore agents inhibiting TNF-α might be effective in the control of ocular inflammatory processes.[13,21,22] The mechanisms of action of cytokines and TNF-α in intraocular inflammation have been studied in experimental autoimmune uveitis,[23] however, the type of effector and regulatory responses to one or more retinal antigens have remained obscure.[24]
In the present study, the effects of TNF-α blockade on intraocular inflammation were investigated via measurement of cytokines in the aqueous humor. Lower levels of IL-1β, IL-2 and IL-6 in eyes group treated with adalimumab were observed but differences were not significant. However, significantly lower VEGF levels were found in adalimumab-treated patients. VEGF is a glycoprotein which can induce angiogenesis and enhance microvascular permeability, and there are increasing experimental and clinical data regarding its role in inflammation and neovascularization. TNF-α is known to modulate angiogenesis resulting in the promotion of angiogenesis,[25] with an increase in the expression of angiogenic factor VEGF. Thus, biologic therapy targeting inflammatory cytokines such as TNF-α is able to significantly reduce VEGF levels as shown in current study by measurement of aqueous humor levels of VEGF before and after treatment with adalimumab. On the other hand, aqueous humor levels of TNF-α did not show any modification.
Aqueous humor levels of IL-6 were higher than expected both in patients with uveitis and in the controls, consistent with other studies.[26] It seems that IL-6 is an acute-phase reactant increasing in patients with uveitis and also in other inflammatory processes. The high level of IL-6 in the control subjects may be related to older age as compared with patients with uveitis. An increase in IL-6 due to other diseases (e.g., osteoporosis, arthritis, type 2 diabetes, cancers, etc.) seems to be unlikely given our inclusion criteria for selection of control subjects. Studies on other biologic drugs such as tocilizumab, a new humanized monoclonal antibody against the IL-6 receptor, have confirmed the efficacy of this agent in refractory uveitis,[27] supporting the important role of IL-6 in intraocular inflammation.
The use of an anti-TNF-α agent (adalimumab) is a novel aspect of the study as compared to previous reports on the measurement of cytokines in the aqueous humor. Although our patients were previously treated with immunosuppressant agents, we attempted to analyze changes following the administrating adalimumab. However, the level of cytokines already altered due to previous immunosuppressive therapy, and thus the altered immune response, is an important limitation in the current study. Ideally, naïve patients for immunosuppressive therapy should be selected but in routine daily practice, this is hardly applicable because patients with uveitis usually have been previously treated with corticosteroids and other immunosuppressive agents.
On the other hand, in order to increase the statistical power of the study, a larger sample size was necessary but recruitment of more patients was difficult due to the selection criteria set for patients with uveitis treated with adalimumab. Patients with uveitis and control subjects were not matched by age given that the control group consisted of patients undergoing cataract surgery, which are usually older people, and aqueous humor samples were obtained at the time of surgery. The selection of younger healthy patients was difficult considering the interventional nature of aqueous humor extraction.
In summary, modifications of some inflammatory cytokines involved in the pathogenesis of uveitis after TNF-α blocking treatment, herein adalimumab, were observed in the current series. Aqueous humor samples may be useful to assess the immune response to biologic therapies[28,29,30] but further studies with large sample size are needed. Immune responses in the eye have complex mechanisms and many interacting antigen-presenting cells, T and B lymphocytes, antibodies and other inflammatory mediators are still unclear. More homogeneous studies with regard to uveitis subtypes with a higher number of patients may clarify differences in the response to immunosuppressant agents among patients with certain types of uveitis, and may be beneficial to define appropriate therapy for those with refractory or relapsing chronic uveitis.
ACKNOWLEDGMENTS
The authors thank Marta Pulido, MD, for editing the manuscript and editorial assistance.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Holland GN, Denove CS, Yu F. Chronic anterior uveitis in children: Clinical characteristics and complications. Am J Ophthalmol. 2009;147:667–678.e5. doi: 10.1016/j.ajo.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Thorne JE, Woreta F, Kedhar SR, Dunn JP, Jabs DA. Juvenile idiopathic arthritis-associated uveitis: Incidence of ocular complications and visual acuity loss. Am J Ophthalmol. 2007;143:840–846. doi: 10.1016/j.ajo.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 3.Durrani OM, Meads CA, Murray PI. Uveitis: A potentially blinding disease. Ophthalmologica. 2004;218:223–236. doi: 10.1159/000078612. [DOI] [PubMed] [Google Scholar]
- 4.Paroli MP, Teodori C, D’Alessandro M, Mariani P, Iannucci G, Paroli M. Increased vascular endothelial growth factor levels in aqueous humor and serum of patients with quiescent uveitis. Eur J Ophthalmol. 2007;17:938–942. doi: 10.1177/112067210701700611. [DOI] [PubMed] [Google Scholar]
- 5.Sijssens KM, Rijkers GT, Rothova A, Stilma JS, Schellekens PA, de Boer JH. Cytokines, chemokines and soluble adhesion molecules in aqueous humor of children with uveitis. Exp Eye Res. 2007;85:443–449. doi: 10.1016/j.exer.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Abu El-Asrar AM, Struyf S, Descamps FJ, Al-Obeidan SA, Proost P, Van Damme J, et al. Chemokines and gelatinases in the aqueous humor of patients with active uveitis. Am J Ophthalmol. 2004;138:401–411. doi: 10.1016/j.ajo.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 7.Caspi RR. Understanding autoimmune uveitis through animal models. The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 2011;52:1872–1879. doi: 10.1167/iovs.10-6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trinh L, Brignole-Baudouin F, Pauly A, Liang H, Houssier M, Baudouin C. Th1- and Th2-related chemokine and chemokine receptor expression on the ocular surface in endotoxin-induced uveitis. Mol Vis. 2008;14:2428–2434. [PMC free article] [PubMed] [Google Scholar]
- 9.Santos Lacomba M, Marcos Martín C, Gallardo Galera JM, Gómez Vidal MA, Collantes Estévez E, Ramírez Chamond R, et al. Aqueous humor and serum tumor necrosis factor-alpha in clinical uveitis. Ophthalmic Res. 2001;33:251–255. doi: 10.1159/000055677. [DOI] [PubMed] [Google Scholar]
- 10.Posarelli C, Arapi I, Figus M, Neri P. Biologic agents in inflammatory eye disease. J Ophthalmic Vis Res. 2011;6:309–316. [PMC free article] [PubMed] [Google Scholar]
- 11.Imrie FR, Dick AD. Biologics in the treatment of uveitis. Curr Opin Ophthalmol. 2007;18:481–486. doi: 10.1097/ICU.0b013e3282f03d42. [DOI] [PubMed] [Google Scholar]
- 12.Humira®. Summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000481/WC500050870.pdf .
- 13.Díaz-Llopis M, Salom D, Garcia-de-Vicuña C, Cordero-Coma M, Ortega G, Ortego N, et al. Treatment of refractory uveitis with adalimumab: A prospective multicenter study of 131 patients. Ophthalmology. 2012;119:1575–1581. doi: 10.1016/j.ophtha.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 14.Martel JN, Esterberg E, Nagpal A, Acharya NR. Infliximab and adalimumab for uveitis. Ocul Immunol Inflamm. 2012;20:18–26. doi: 10.3109/09273948.2011.633205. [DOI] [PubMed] [Google Scholar]
- 15.Biester S, Deuter C, Michels H, Haefner R, Kuemmerle-Deschner J, Doycheva D, et al. Adalimumab in the therapy of uveitis in childhood. Br J Ophthalmol. 2007;91:319–324. doi: 10.1136/bjo.2006.103721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heiligenhaus A, Michels H, Schumacher C, Kopp I, Neudorf U, Niehues T, et al. Evidence-based, interdisciplinary guidelines for anti-inflammatory treatment of uveitis associated with juvenile idiopathic arthritis. Rheumatol Int. 2012;32:1121–1133. doi: 10.1007/s00296-011-2126-1. [DOI] [PubMed] [Google Scholar]
- 17.Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Funding M, Hansen TK, Gjedsted J, Ehlers N. Simultaneous quantification of 17 immune mediators in aqueous humour from patients with corneal rejection. Acta Ophthalmol Scand. 2006;84:759–765. doi: 10.1111/j.1600-0420.2006.00755.x. [DOI] [PubMed] [Google Scholar]
- 19.Efthimiou P, Markenson JA. Role of biological agents in immune-mediated inflammatory diseases. South Med J. 2005;98:192–204. doi: 10.1097/01.SMJ.0000153119.37032.8B. [DOI] [PubMed] [Google Scholar]
- 20.Hale S, Lightman S. Anti-TNF therapies in the management of acute and chronic uveitis. Cytokine. 2006;33:231–237. doi: 10.1016/j.cyto.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Petropoulos IK, Vaudaux JD, Guex-Crosier Y. Anti-TNF-alpha therapy in patients with chronic non-infectious uveitis: The experience of Jules Gonin Eye Hospital. Klin Monbl Augenheilkd. 2008;225:457–461. doi: 10.1055/s-2008-1027361. [DOI] [PubMed] [Google Scholar]
- 22.Mushtaq B, Saeed T, Situnayake RD, Murray PI. Adalimumab for sight-threatening uveitis in Behçet's disease. Eye (Lond) 2007;21:824–825. doi: 10.1038/sj.eye.6702352. [DOI] [PubMed] [Google Scholar]
- 23.Dick AD, Forrester JV, Liversidge J, Cope AP. The role of tumour necrosis factor (TNF-alpha) in experimental autoimmune uveoretinitis (EAU) Prog Retin Eye Res. 2004;23:617–637. doi: 10.1016/j.preteyeres.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Caspi R. Autoimmunity in the immune privileged eye: Pathogenic and regulatory T cells. Immunol Res. 2008;42:41–50. doi: 10.1007/s12026-008-8031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naldini A, Carraro F. Role of inflammatory mediators in angiogenesis. Curr Drug Targets Inflamm Allergy. 2005;4:3–8. doi: 10.2174/1568010053622830. [DOI] [PubMed] [Google Scholar]
- 26.Perez VL, Papaliodis GN, Chu D, Anzaar F, Christen W, Foster CS. Elevated levels of interleukin 6 in the vitreous fluid of patients with pars planitis and posterior uveitis: The Massachusetts eye and ear experience and review of previous studies. Ocul Immunol Inflamm. 2004;12:193–201. doi: 10.1080/092739490500282. [DOI] [PubMed] [Google Scholar]
- 27.Muselier A, Bielefeld P, Bidot S, Vinit J, Besancenot JF, Bron A. Efficacy of tocilizumab in two patients with anti-TNF-alpha refractory uveitis. Ocul Immunol Inflamm. 2011;19:382–383. doi: 10.3109/09273948.2011.606593. [DOI] [PubMed] [Google Scholar]
- 28.Campochiaro PA, Choy DF, Do DV, Hafiz G, Shah SM, Nguyen QD, et al. Monitoring ocular drug therapy by analysis of aqueous samples. Ophthalmology. 2009;116:2158–2164. doi: 10.1016/j.ophtha.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 29.van Kooij B, Rothova A, Rijkers GT, de Groot-Mijnes JD. Distinct cytokine and chemokine profiles in the aqueous of patients with uveitis and cystoid macular edema. Am J Ophthalmol. 2006;142:192–194. doi: 10.1016/j.ajo.2006.02.052. [DOI] [PubMed] [Google Scholar]
- 30.El-Asrar AM, Struyf S, Kangave D, Al-Obeidan SS, Opdenakker G, Geboes K, et al. Cytokine profiles in aqueous humor of patients with different clinical entities of endogenous uveitis. Clin Immunol. 2011;139:177–184. doi: 10.1016/j.clim.2011.01.014. [DOI] [PubMed] [Google Scholar]


