Abstract
Axonal regeneration and fiber regrowth is limited in the adult central nervous system, but research over the last decades has revealed a high intrinsic capacity of brain and spinal cord circuits to adapt and reorganize after smaller injuries or denervation. Short-distance fiber growth and synaptic rewiring was found in cortex, brain stem and spinal cord and could be associated with restoration of sensorimotor functions that were impaired by the injury. Such processes of structural plasticity were initially observed in the corticospinal system following spinal cord injury or stroke, but recent studies showed an equally high potential for structural and functional reorganization in reticulospinal, rubrospinal or propriospinal projections. Here we review the lesion-induced plastic changes in the propriospinal pathways, and we argue that they represent a key mechanism triggering sensorimotor recovery upon incomplete spinal cord injury. The formation or strengthening of spinal detour pathways bypassing supraspinal commands around the lesion site to the denervated spinal cord were identified as prominent neural substrate inducing substantial motor recovery in different species from mice to primates. Indications for the existence of propriospinal bypasses were also found in humans after cortical stroke. It is mandatory for current research to dissect the biological mechanisms underlying spinal circuit remodeling and to investigate how these processes can be stimulated in an optimal way by therapeutic interventions (e.g., fiber-growth enhancing interventions, rehabilitation). This knowledge will clear the way for the development of novel strategies targeting the remarkable plastic potential of propriospinal circuits to maximize functional recovery after spinal cord injury.
Keywords: spinal cord injury, propriospinal system, neural plasticity, fiber sprouting, neural repair, compensation, regeneration, propriospinal detours
The central nervous system (CNS) of adult mammals has only limited capacities for axonal regrowth and regeneration upon injury. Long-distance regeneration of axotomized fibers is virtually absent and interventions aiming to enhance neural repair of large lesions were considered unachievable for many decades. Fortunately, this view has meanwhile changed by innovative research using cutting-edge technologies that have shed light on the basic mechanisms constraining CSN plasticity, in particular the inhibitory factors associated with CNS myelin, glial scars and perineuronal nets, and the low intrinsic capacity of adult neurons to reactivate efficient neurite growth programs. Besides the development of novel treatment strategies, recent research also disclosed the surprisingly high capacity of the CNS for short-distance rearrangements of fiber connections and synaptic rewiring to successfully repair or compensate for smaller lesions. Neural plasticity including molecular and structural changes at the synapse, sprouting of axons and dendrites up to representational map shifts can spontaneously occur after CNS injury. These plastic processes are a basic substrate mediating spontaneous or training-enhanced functional recovery after different kinds of CNS damage (Raineteau and Schwab, 2001; Isa and Nishimura, 2014; Ramer et al., 2014). The present perspective addresses the biological mechanisms driving spontaneous functional recovery following spinal cord injury, with the primary focus on neural plasticity of intrinsic spinal neurons and circuits.
Following a complete spinal cord transection, any behavioral adaptation must derive from remodeling of intraspinal circuits, possibly influenced by sensory afferents (see Figure 1). Classical experiments in cats revealed a remarkable plasticity of the lumbar spinal cord: Spinalized adult cats could recover weight-bearing hindlimb stepping closely resembling the normal feline walking pattern, but only after daily treadmill training for a few weeks. This data showed the importance of rehabilitative training to reactivate and reorganize the intraspinal locomotor circuits by sensory stimulation (Barbeau and Rossignol, 1987) and was in line with the concept of central pattern generators (CPGs) as intrinsic spinal networks able to generate stepping movements in the total absence of supraspinal inputs (Grillner and Wallen, 1985). The molecular and cellular mechanisms underlying the considerable functional recovery of spinal cats are not fully understood yet: Up-regulations of adrenergic and serotonergic receptors on motoneurons (and presumably interneurons) were shown to play essential roles for functional recovery after severe forms of spinal cord injury by re-establishing spinal excitability in the denervated cord (Giroux et al., 1999; Murray et al., 2010). Pharmacological stimulation of these receptors by monoaminergic neuromodulators, reversible inhibition of the GABAergic or glycinergic system, or electrical stimulations of the spinal cord in rodents, cats, monkeys and humans with complete or large subtotal lesions can induce a physiological state where isolated spinal networks are reactivated, integrate sensory information and produce well-coordinated motor outputs (Edgerton et al., 2001; Gerasimenko et al., 2008; Angeli et al., 2014). Fiber sprouting and network rearrangements of spinal interneurons or sensory fibers were observed caudal to total spinal cord transections (Krenz and Weaver, 1998; Kapitza et al., 2012; Beauparlant et al., 2013), but their specific roles in the development of neuronal dysfunctions (e.g., spasticity, autonomous dysreflexia) or functional restoration (e.g., by increased spinal excitability facilitating activity in sublesional neural networks) after complete spinal injury remain to be fully determined. Despite these remarkable structural and functional reorganizations of sublesional spinal circuits across species, spontaneous or training-induced recovery of walking in the absence of simultaneous extrinsic stimulation of the lumbar cord (e.g., by monoaminergic agonists or electric epidural stimulation or both) is absent in spinalized adult mammals with the exception of cats (Ichiyama et al., 2008; Kubasak et al., 2008; Courtine et al., 2009). Obviously, lumbar spinal networks require a minimal degree of unpatterned supraspinal drive enhancing spinal excitability in combination with highly specific descending connections controlling complex sensorimotor functions to perform locomotor function.
Figure 1.
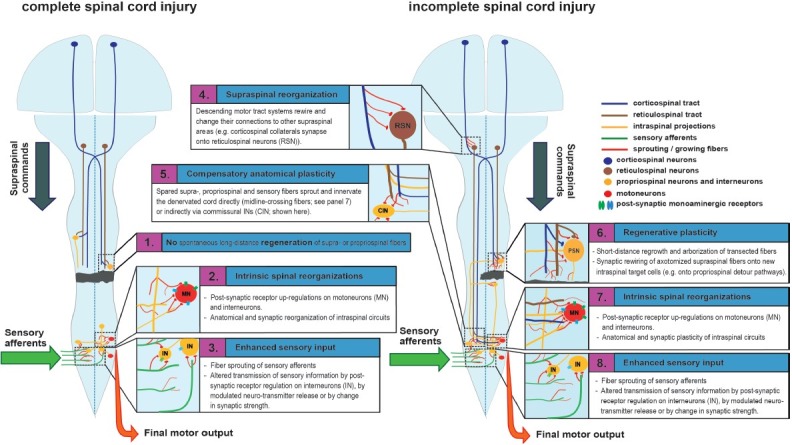
Central processes of neural plasticity underlying functional recovery in anatomically complete (left) and incomplete (right) spinal injury.
Left: After complete spinal cord injury, supraspinal control is completely and irreversibly abolished. (1) There is no long-distance regeneration of transected axons. (2) Structural and functional reorganizations of intrinsic spinal circuits can reestablish spinal excitability (which is severely reduced after the loss of supraspinal drive) and trigger functional recovery, but can also result in neuronal malfunctions including spastic symptoms and neuropathic pain. Receptor up-regulations (mainly serotonergic and adrenergic receptors) on motoneurons and possibly premotor interneurons facilitate the activation of spinal networks under the condition of reduced afferent input. Anatomical and synaptic rewiring of intraspinal circuits modifies spinal integration and processing of sensory inputs. (3) Sensory afferents sprout and reconnect. Up-regulated post-synaptic receptors on interneurons, changes in neurotransmitter release or processes of synaptic plasticity can enhance sensory excitation and activation of the sublesional spinal cord.
Right: After incomplete spinal cord injury, some spared descending supra- and propriospinal fibers reach the lower spinal cord. (4) Structural and functional remodeling on the supraspinal level helps to adjust the lesion-induced imbalance of descending motor systems, thereby optimizing the remaining descending transmission to spinal targets. (5) A main mechanism contributing to recovery after incomplete spinal cord injury is compensatory plasticity of unlesioned descending fibers at the spinal level. Spared supraspinal fibers sprout and arborize in the sublesional cord and strengthen pre-existing unlesioned connections by synaptic plasticity. (6) Lesioned fibers do not show long-distance regeneration but show regenerative sprouting over short distances, targeting e.g., propriospinal neurons, some of which bypass the lesion on remaining tissue bridges. These propriospinal detour pathways can enhance themselves their connections onto premotor and motor neurons below the lesion site, thereby re-establishing supraspinal drive to the denervated spinal cord. (7) Intrinsic spinal adaptations and (8) plasticity of sensory afferents also occur after partial spinal cord injury.
The majority of preclinical studies investigating neural plasticity use animals with anatomically incomplete spinal cord injury, which is the type of spinal injury most frequently observed in humans (McKinley et al., 2007). Spontaneous long-distance regeneration of CNS fibers is absent in higher adult vertebrates, and can only partially by induced by growth-enhancing treatments (e.g., growth factors, stimulation of intrinsic neuronal growth pathways, anti-Nogo-A antibodies, Chondroitinase ABC, etc.). Contrary to long-distance fiber regeneration, compensatory plasticity occurs in multiple descending motor systems (see Figure 1), probably acting as a driving mechanism by which spontaneous functional recovery is achieved (Filli and Schwab, 2012). In this process, spared neuronal fibers sprout and innervate denervated spinal targets, thereby compensating for either lost functions of the same tract system (Ballermann and Fouad, 2006; Rosenzweig et al., 2010; Takeoka et al., 2014; Zörner et al., 2014), or of another injured fiber system (Raineteau et al., 2001; Kanagal and Muir, 2009). A difficulty when studying incomplete spinal cord injury is the complexity of spared supra- and propriospinal projections, all being potential players mediating functional recovery by compensatory mechanisms. Re-lesions of the investigated tract system or novel technologies enabling selective and inducible silencing or excitation of neuronal pathways by genetic targeting or viral tracing will help to investigate the specific roles of the plastic rearrangements of a specific neural system (Esposito et al., 2014; Wahl et al., 2014).
After cervical unilateral hemisection of the spinal cord in rodents and primates, the spared corticospinal tract system shows substantial spontaneous compensatory sprouting over the spinal midline, targeting the denervated hemicord. This compensatory fiber growth induced significant restoration of skilled fine motor movements and locomotion (Rosenzweig et al., 2010). Compensatory mechanisms inducing substantial functional recovery upon incomplete spinal lesions have recently been demonstrated for descending rubrospinal (Belhaj-Saïf and Cheney, 2000) and reticulospinal projections (Takeoka et al., 2014; Zörner et al., 2014). The extensive compensatory plasticity of the reticulospinal tract system is particularly interesting, given the high relevance of this neural system to induce and control basic functions including locomotion and respiration. In parallel to supraspinal reorganizations, adaptive remodeling of intrinsic spinal networks is required for proper integration and processing of sensory and supraspinal afferents after incomplete spinal injury. This was shown in a dual lesion paradigm in adult cats, where an initial unilateral hemisection at spinal level T11 was followed by a complete spinal transection (level T13) a few weeks later. These cats could walk on a treadmill as early as some hours after the second, complete lesion (Barrière et al., 2008), which is significantly earlier than after a direct spinal transection at T11, where walking capacity only returns after a few weeks of intense treadmill training. This instantaneous stepping capacity after spinalization in the dual lesion paradigm points towards the important role of intrinsic spinal plasticity not only after complete spinal cord injury, but also after incomplete injury (Cohen-Adad et al., 2014).
Despite the requirement of supraspinal drive to exert specific motor functions in higher vertebrates (except for cats), restoration of walking function can occur in the total absence of direct supraspinal projections in rodents. Contrary to a complete thoracic transection of the cord, spatially and temporally separated subtotal lesions, and in particular the so-called “staggered hemilesions” which leave a tissue bridge across the contralateral thoracic lesion sites, are followed by considerable recovery of stereotyped walking in adult rodents. This motor restoration is achieved through the spontaneous formation of propriospinal detours that reroute interrupted supraspinal commands around the spinal lesions (Courtine et al., 2008). In this process, severed descending fiber tracts sprout and rewire onto propriospinal neurons located above the lower lesion in the tissue bridge and projecting their axons into the lower, denervated spinal cord. Pharmacological ablation of these relaying propriospinal neurons located between the hemilesions abolished the locomotor recovery, confirming the physiological relevance of the propriospinal bypass. Formation of lesion-induced detour pathways via propriospinal networks was initially shown for the corticospinal tract: thoracically axotomized corticospinal fibers sprouted in the cervical spinal cord, where they innervated propriospinal neurons. Initial contact was followed by refinement mechanisms (pruning) leading to the selective formation of synapses onto long-distance propriospinal neurons that bridged the lesion site. This propriospinal detour pathway led to significant motor restoration of hindlimb function, including the cortically dependent placing response (Bareyre et al., 2004). Use-dependent mechanisms leading to physiologically meaningful propriospinal relay connections were shown by van den Brand et al. (2012), where a challenging training paradigm led to a significant increase in cortico-propriospinal detours when compared to automated treadmill training in rats with staggered thoracic hemisections. Recently, it was demonstrated that intraspinal bridging of supraspinal input after partial spinal injury is not restricted to the corticospinal system: severed reticulospinal fibers originating from the mid-medullary region of the brainstem were shown to form glutamatergic contacts onto double-midline crossing C3–4 propriospinal neurons which crossed the lesion site on spared tissue bridges and recrossed to the denervated cervical hemicord below the injury. Reticulospinal fiber sprouting and innervation of propriospinal neurons, as well as propriospinal projections bypassing the cervical unilateral hemilesion were significantly enhanced 6 weeks after injury. This spontaneous reorganization of reticulo- and propriospinal fibers upon spinal hemisection was associated with substantial motor recovery seen in these adult rats (Filli et al., 2014). Propriospinal rerouting of interrupted brainstem commands is important, as the mid-medullary brainstem, including the nucleus reticularis gigantocellularis, is a phylogenetically preserved key unit in fish to man, feeding the spinal cord with essential motor drive. Besides its known impact on initiating and controlling locmotion and posture, the reticular gigantocellular nucleus is also involved in refined forelimb movements in monkeys and humans. The fundamental principle of propriospinal detours seems to apply also for primates including humans: After incomplete cervical spinal cord injury in primates, axotomized corticospinal fibers synapse onto C3–4 propriospinal neurons which innervate cervical motoneurons, resulting in significant recovery of reaching and digit movements (Alstermark et al., 2011). In patients with smaller cortical strokes, supraspinal transmission through the propriospinal relay is reinforced on the hemiplegic side, indicating a potential contribution of propriospinal bypasses to motor recovery also in humans (Pierrot-Deseilligny, 2002).
The molecular mechanisms which induce and direct the growth of new connections in the spinal cord below and above an injury are largely unknown. Interestingly, several observations point to a higher capacity of intraspinal neurons to sprout and reorganize upon spinal cord injury compared to supraspinal tract systems. Besides de novo formation and restructuration of entire intraspinal circuits after CNS injury, some commissural interneurons in the adult cat are even capable of fiber regeneration through a spinal midsagittal lesion forming new synaptic connections onto contralateral target cells (Fenrich and Rose, 2011). The reasons for the intriguing plastic capacity of propriospinal neurons are not fully understood. One potential key factor is the low distance of the cell body to the lesion site: Transcription of growth-associated proteins and of receptors for neurotrophic factors are dependent on the nucleus, and therefore from the distance of the soma to the lesion (Fernandes et al., 1999). Moreover, genetic profiles of propriospinal neurons after thoracic spinal lesions revealed significant up-regulations of growth-associated proteins (e.g., GAP-43), of receptors for neurotrophic factors (GDNF, LIF, CNTF), and of anti-inflammatory and neuroprotective proteins, which might account for the remarkable intrinsic capacity of propriospinal neurons for neural plasticity (Siebert et al., 2010). Furthermore, the unique position of the spinal cord receiving direct sensory afferents and supraspinal motor commands might facilitate lesion-induced plastic rearrangements within spinal networks. The vast majority of supraspinal commands does not directly signal to motoneurons, but to a complex network of interneurons. On this level, regrowth and rewiring of descending fibers is thought to be directed by activity-dependent mechanisms shaping functional reorganization of spinal circuits by sensory feedback (Craig and Boudin, 2001; Takeoka et al., 2014). The activity-dependent reorganization of fiber systems is based on simultaneous pre- and post-synaptic activity, a fundamental principle found in the CNS. By this mechanism, sprouting descending axons, propriospinal detour fibers and segmental interneurons can be integrated into spinal networks that exhibit identical temporal activity patterns and therefore are likely to be involved in the same functional tasks (Pettersson et al., 2007; Isa and Nishimura, 2014).
For a long time, spinal circuits were considered as hardwired and virtually unable to remodel after lesion. Research over the last two decades has changed this view by uncovering a high degree of molecular and cellular reorganizations occurring in the spinal cord below and above a lesion. These results indicate that combined plasticity of propriospinal, segmental and descending systems is a key mechanism for the often remarkable functional recovery seen after incomplete spinal lesions. However, we need more insights into the basic functional organization of spinal circuitries in order to understand the mechanisms of spinal plastic rearrangements after injury and their regulation by e.g., rehabilitative training. Cutting-edge technologies including genetic targeting or virus-based approaches permit a selective, inducible and reversible excitation or silencing of specific neuron types and circuits, thereby helping to dissect the functional anatomy of spinal circuits. Together with state-of-the-art imaging techniques visualizing the activity patterns of spinal circuits during specific tasks, these techniques are revolutionizing our basic knowledge on the physiology and plasticity of spinal networks in the coming years. The phylogenetically old brainstem-spinal systems and the plasticity of propriospinal and local spinal interneuron systems will have to receive particular attention. Future spinal cord injury research will have to target plasticity of propriospinal networks in addition to supraspinal tract systems to develop novel therapeutic approaches aiming to optimize spinal cord repair.
Acknowledgments
We would like to thank our colleague Anne Katrin Engmann for her support to design the figure.
References
- Alstermark B, Pettersson LG, Nishimura Y, Yoshino-Saito K, Tsuboi F, Takahashi M, Isa T. Motor command for precision grip in the macaque monkey can be mediated by spinal interneurons. J Neurophysiol. 2011;106:122–126. doi: 10.1152/jn.00089.2011. [DOI] [PubMed] [Google Scholar]
- Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain. 2014;137:1394–1409. doi: 10.1093/brain/awu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 1987;412:84–95. doi: 10.1016/0006-8993(87)91442-9. [DOI] [PubMed] [Google Scholar]
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- Barrière G, Leblond H, Provencher J, Rossignol S. Prominent role of the spinal central pattern generator in the recovery of locomotion after partial spinal cord injuries. J Neurosci. 2008;28:3976–3987. doi: 10.1523/JNEUROSCI.5692-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauparlant J, van den Brand R, Barraud Q, Friedli L, Musienko P, Dietz V, Courtine G. Undirected compensatory plasticity contributes to neuronal dysfunction after severe spinal cord injury. Brain. 2013;136:3347–3361. doi: 10.1093/brain/awt204. [DOI] [PubMed] [Google Scholar]
- Belhaj-Saïf A, Cheney PD. Plasticity in the distribution of the red nucleus output to forearm muscles after unilateral lesions of the pyramidal tract. J Neurophysiol. 2000;83:3147–3153. doi: 10.1152/jn.2000.83.5.3147. [DOI] [PubMed] [Google Scholar]
- Cohen-Adad J, Martinez M, Delivet-Mongrain H, Rossignol S. Recovery of locomotion after partial spinal cord lesions in cats: assessment using behavioral, electrophysiological and imaging techniques. Acta Neurobiol Exp (Wars) 2014;74:142–157. doi: 10.55782/ane-2014-1981. [DOI] [PubMed] [Google Scholar]
- Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, Qi J, Edgerton VR, Sofroniew MV. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, Song B, Ao Y, Ichiyama RM, Lavrov I, Roy RR, Sofroniew MV, Edgerton VR. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci. 2009;12:1333–1342. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AM, Boudin H. Molecular heterogeneity of central synapses: afferent and target regulation. Nat Neurosci. 2001;4:569–578. doi: 10.1038/88388. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Leon RD, Harkema SJ, Hodgson JA, London N, Reinkensmeyer DJ, Roy RR, Talmadge RJ, Tillakaratne NJ, Timoszyk W, Tobin A. Retraining the injured spinal cord. J Physiol. 2001;533:15–22. doi: 10.1111/j.1469-7793.2001.0015b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito MS, Capelli P, Arber S. Brainstem nucleus MdV mediates skilled forelimb motor tasks. Nature. 2014;508:351–356. doi: 10.1038/nature13023. [DOI] [PubMed] [Google Scholar]
- Fenrich KK, Rose PK. Axons with highly branched terminal regions successfully regenerate across spinal midline transections in the adult cat. J Comp Neurol. 2011;519:3240–3258. doi: 10.1002/cne.22686. [DOI] [PubMed] [Google Scholar]
- Fernandes KJ, Fan DP, Tsui BJ, Cassar SL, Tetzlaff W. Influence of the axotomy to cell body distance in rat rubrospinal and spinal motoneurons: differential regulation of GAP-43, tubulins, and neurofilament-M. J Comp Neurol. 1999;414:495–510. doi: 10.1002/(sici)1096-9861(19991129)414:4<495::aid-cne6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Filli L, Schwab ME. The rocky road to translation in spinal cord repair. Ann Neurol. 2012;72:491–501. doi: 10.1002/ana.23630. [DOI] [PubMed] [Google Scholar]
- Filli L, Engmann AK, Zörner B, Weinmann O, Moraitis T, Gullo M, Kasper H, Schneider R, Schwab ME. Bridging the gap: a reticulo-propriospinal detour bypassing an incomplete spinal cord injury. J Neurosci. 2014;34:13399–13410. doi: 10.1523/JNEUROSCI.0701-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko Y, Roy RR, Edgerton VR. Epidural stimulation: comparison of the spinal circuits that generate and control locomotion in rats, cats and humans. Exp Neurol. 2008;209:417–425. doi: 10.1016/j.expneurol.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroux N, Rossignol S, Reader TA. Autoradiographic study of alpha1-and alpha2-noradrenergic and serotonin 1A receptors in the spinal cord of normal and chronically transected cats. J Comp Neurol. 1999;406:402–414. [PubMed] [Google Scholar]
- Grillner S, Wallen P. Central pattern generators for locomotion, with special reference to vertebrates. Annu Rev Neurosci. 1985;8:233–261. doi: 10.1146/annurev.ne.08.030185.001313. [DOI] [PubMed] [Google Scholar]
- Ichiyama RM, Courtine G, Gerasimenko YP, Yang GJ, van den Brand R, Lavrov IA, Zhong H, Roy RR, Edgerton VR. Step training reinforces specific spinal locomotor circuitry in adult spinal rats. J Neurosci. 2008;28:7370–7375. doi: 10.1523/JNEUROSCI.1881-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isa T, Nishimura Y. Plasticity for recovery after partial spinal cord injury – hierarchical organization. Neurosci Res. 2014;78:3–8. doi: 10.1016/j.neures.2013.10.008. [DOI] [PubMed] [Google Scholar]
- Kanagal SG, Muir GD. Task-dependent compensation after pyramidal tract and dorsolateral spinal lesions in rats. Exp Neurol. 2009;216:193–206. doi: 10.1016/j.expneurol.2008.11.028. [DOI] [PubMed] [Google Scholar]
- Kapitza S, Zörner B, Weinmann O, Bolliger M, Filli L, Dietz V, Schwab ME. Tail spasms in rat spinal cord injury: changes in interneuronal connectivity. Exp Neurol. 2012;236:179–189. doi: 10.1016/j.expneurol.2012.04.023. [DOI] [PubMed] [Google Scholar]
- Krenz NR, Weaver LC. Sprouting of primary afferent fibers after spinal cord transection in the rat. Neuroscience. 1998;85:443–458. doi: 10.1016/s0306-4522(97)00622-2. [DOI] [PubMed] [Google Scholar]
- Kubasak MD, Jindrich DL, Zhong H, Takeoka A, McFarland KC, Muńoz-Quiles C, Roy RR, Edgerton VR, Ramón-Cueto A, Phelps PE. OEG implantation and step training enhance hindlimb-stepping ability in adult spinal transected rats. Brain. 2008;131:264–276. doi: 10.1093/brain/awm267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley W, Santos K, Meade M, Brooke K. Incidence and outcomes of spinal cord injury clinical syndromes. J Spinal Cord Med. 2007;30:215–224. doi: 10.1080/10790268.2007.11753929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KC, Nakae A, Stephens MJ, Rank M, D’Amico J, Harvey PJ, Li X, Harris RL, Ballou EW, Anelli R, Heckman CJ, Mashimo T, Vavrek R, Sanelli L, Gorassini MA, Bennett DJ, Fouad K. Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors. Nat Med. 2010;16:694–700. doi: 10.1038/nm.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson LG, Alstermark B, Blagovechtchenski E, Isa T, Sasaski S. Skilled digit movements in feline and primate--recovery after selective spinal cord lesions. Acta Physiol (Oxf) 2007;189:141–154. doi: 10.1111/j.1748-1716.2006.01650.x. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E. Propriospinal transmission of part of the corticospinal excitation in humans. Muscle Nerve. 2002;26:155–172. doi: 10.1002/mus.1240. [DOI] [PubMed] [Google Scholar]
- Raineteau O, Schwab ME. Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci. 2001;2:263–273. doi: 10.1038/35067570. [DOI] [PubMed] [Google Scholar]
- Raineteau O, Fouad K, Noth P, Thallmair M, Schwab ME. Functional switch between motor tracts in the presence of the mAb IN-1 in the adult rat. Proc Natl Acad Sci U S A. 2001;98:6929–6934. doi: 10.1073/pnas.111165498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramer LM, Ramer MS, Bradbury EJ. Restoring function after spinal cord injury: towards clinical translation of experimental strategies. Lancet Neurol. 2014;13:1241–1256. doi: 10.1016/S1474-4422(14)70144-9. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Courtine G, Jindrich DL, Brock JH, Ferguson AR, Strand SC, Nout YS, Roy RR, Miller DM, Beattie MS, Havton LA, Bresnahan JC, Edgerton VR, Tuszynski MH. Extensive spontaneous plasticity of corticospinal projections after primate spinal cord injury. Nat Neurosci. 2010;13:1505–1510. doi: 10.1038/nn.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert JR, Middelton FA, Stelzner DJ. Intrinsic response of thoracic propriospinal neurons to axotomy. BMC Neurosci. 2010;11:69. doi: 10.1186/1471-2202-11-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeoka A, Vollenweider I, Courtine G, Arber S. Muscle spindle feedback directs locomotor recovery and circuit reorganization after spinal cord injury. Cell. 2014;159:1626–1639. doi: 10.1016/j.cell.2014.11.019. [DOI] [PubMed] [Google Scholar]
- van den Brand R, Heutschi J, Barraud Q, DiGiovanna J, Bartholdi K, Huerlimann M, Friedli L, Vollenweider I, Moraud EM, Duis S, Dominici N, Micera S, Musienko P, Courtine G. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science. 2012;336:1182–1185. doi: 10.1126/science.1217416. [DOI] [PubMed] [Google Scholar]
- Wahl AS, Omlor W, Rubio JC, Chen JL, Zheng H, Schröter A, Gullo M, Weinmann O, Kobayashi K, Helmchen F, Ommer B, Schwab ME. Neuronal repair. Asynchronous therapy restores motor control by rewiring of the rat corticospinal tract after stroke. Science. 2014;344:1250–1255. doi: 10.1126/science.1253050. [DOI] [PubMed] [Google Scholar]
- Zörner B, Bachmann LC, Filli L, Kapitza S, Gullo M, Bolliger M, Starkey ML, Röthlisberger M, Gonzenbach RR, Schwab ME. Chasing CNS plasticity: the brainstem's contribution to locomotor recovery in spinal cord injured rats. Brain. 2014;137:1716–1732. doi: 10.1093/brain/awu078. [DOI] [PubMed] [Google Scholar]


