Abstract
Both matrix biochemistry and neurotrophic factors are known to modulate neurite outgrowth and pathfinding; however, the interplay between these two factors is less studied. While previous work has shown that the biochemical identity of the matrix can alter the outgrowth of neurites in response to neurotrophins, the importance of the concentration of cell-adhesive ligands is unknown. Using engineered elastin-like protein matrices, we recently demonstrated a synergistic effect between matrix-bound cell-adhesive ligand density and soluble nerve growth factor treatment on neurite outgrowth from dorsal root ganglia. This synergism was mediated by Schwann cell-neurite contact through L1CAM. Cell-adhesive ligand density was also shown to alter the pathfinding behavior of dorsal root ganglion neurites in response to a gradient of nerve growth factor. While more cell-adhesive matrices promoted neurite outgrowth, less cell-adhesive matrices promoted more faithful neurite pathfinding. These studies emphasize the importance of considering both matrix biochemistry and neurotrophic factors when designing biomaterials for peripheral nerve regeneration.
Keywords: neurotrophic factors, cell-adhesive ligands, dorsal root ganglia, L1CAM, nerve growth factor, biomaterials, elastin-like proteins
Most studies to date have investigated the isolated effects of either neurotrophic factors or matrix composition on neurite extension and pathfinding. However, it is well known that regeneration in the peripheral nervous system relies on the complex interplay of neurotrophic factors and the extracellular matrix (ECM) with both neurons and Schwann cells. After the injury site is cleared of debris, Schwann cells from the distal nerve stump proliferate and migrate into the vacated endoneurial tubes. These Schwann cells guide regenerating axons by secreting neurotrophic factors, depositing new extracellular matrix, and directly contacting the regenerating axons (Son and Thompson, 1995; Fu and Gordon, 1997). Neurotrophins secreted by Schwann cells, such as nerve growth factor (NGF), brain derived neurotrophic factor (BDNF), and neurotrophin-4/5 (NT-4/5), are known to promote neuronal survival and enhance cell-cell contact between regenerating axons and Schwann cells (Fu and Gordon, 1997). Schwann cells also secrete and maintain the ECM in the basal lamina and endoneurium, which consists of molecules known to promote regeneration, including laminin, fibronectin, tenascin, heparin sulfate, and collagen IV and V (Fu and Gordon, 1997).
While the large majority of studies have investigated neurotrophic action without considering matrix composition, a handful of studies have revealed that the biochemical identity of the substrate on which neurons are cultured can drastically alter patterns of neurite outgrowth in response to neurotrophins. For instance, netrin-1 commonly serves as a chemoattractant for extending neurites. However, when Xenopus retinal neurons were cultured on substrates coated with laminin-111, axonal growth cones were found to turn away from, as opposed to toward, the source of netrin-1 (Hopker et al., 1999). Later studies have demonstrated that neurite outgrowth behavior in response to various neurotrophins is context dependent, varying based on both the identity of the neurotophins present and the substrate on which the neurons are cultured. When dorsal root ganglion (DRG) explants were cultured within 3D collagen gels, stimulation with NT-3 encouraged extension of neurites between adjacent DRGs, while NGF treatment resulted in repulsion (Hari et al., 2004). Upon repeating this experiment with DRGs cultured on laminin, fibronectin, and poly-L-lysine (PLL) coated surfaces, the authors observed extension and intermingling of neurites following treatment with NGF on fibronectin and PLL, but repulsion on laminin (Hari et al., 2004). In other instances, laminin has been found to favor neurite extension. Masuda et al. (2009) demonstrated enhanced neurite outgrowth from DRGs toward soluble factors secreted from spinal cord explants on surfaces treated with either laminin-111 or the YIGSR peptide derived from laminin. It is important to note that these studies used the laminin-111 isoform, isolated from murine sarcomas, whereas laminin-211 and laminin-411 are the most common isoforms in peripheral nerves (Wallquist et al., 2002). Masuda et al. (2009) also used the YIGSR peptide, which is derived from the laminin β1 chain and is thus present in all three of these isoforms, suggesting that similar effects on neurite outgrowth may be observed using the more commonly expressed -211 and -411 isoforms. The identity of the neurotrophic factor can also modulate the outgrowth response on a given substrate. Hanamura et al. (2003) illustrated this point using BDNF and NT-3 to stimulate thalamic axonal growth on collagen-coated membranes or fixed cortical sections. Axonal growth on collagen-coated membranes was accelerated by BDNF, but not NT-3, while the reverse was true for neurons cultured on fixed cortical sections.
Recent work in our laboratory has revealed an interplay between matrix adhesion ligand density and NGF treatment regarding neurite outgrowth from chick DRGs. We developed a synthetic ECM material through the use of recombinant protein engineering that permits decoupled control of the mechanical and biochemical properties of the matrix (Straley and Heilshorn, 2009; Romano et al., 2011). This material (an elastin-like protein, or ELP) is composed of two alternating domains: an elastin-like structural domain that endows the material with its mechanical properties, and a bioactive domain, which contains either the Arg-Gly-Asp (RGD) cell-adhesive sequence derived from the ECM protein fibronectin or a non-adhesive, scrambled Arg-Asp-Gly (RDG) sequence (Figure 1A). By blending the RGD and RDG variants, matrices with varying concentrations of cell adhesive ligand can be produced (Figure 1B). We have used these materials to vary the presentation of RGD ligands to DRG explants with both uniform and gradient presentation of soluble NGF. DRGs were used for these studies, as they are a common model for peripheral nerve regeneration. However, as in all of the studies utilizing DRGs reviewed in this article, the measured neurite outgrowth does not distinguish between the different subpopulations of sensory neurons present in DRGs (Sommer et al., 1985), which in turn may impact the successful translation of in vitro results to in vivo regenerative applications.
Figure 1.
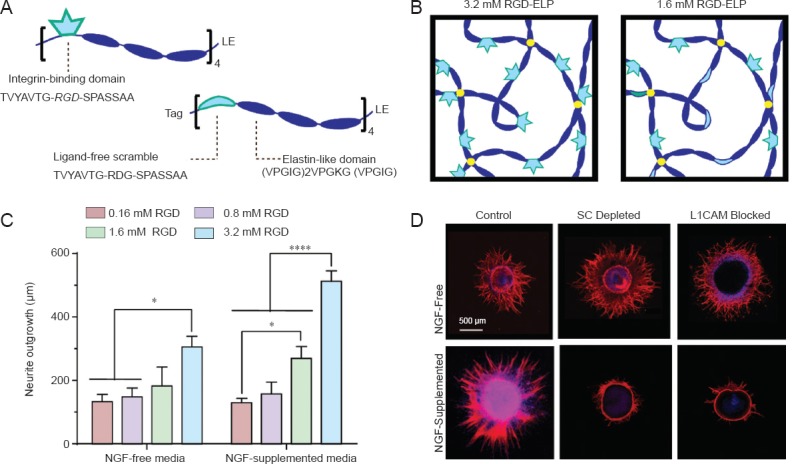
Matrix adhesive ligand density and nerve growth factor (NGF) treatment synergistically enhance neurite outgrowth.
(A) Schematic of engineered elastin-like protein (ELP) containing either the cell-adhesive (RGD) sequence or the non-adhesive, scrambled RDG sequence. (B) Schematic of ELP hydrogels used as (ECM) materials. Adhesive ligand concentration is tuned by blending RGD-ELP with non-adhesive RDG-ELP. (C) Neurite outgrowth from DRGs cultured on ELP matrices increased with increasing RGD concentration and was further enhanced by supplementation with NGF. *P < 0.05, ****P < 0.0001. Error bars represent standard error of the mean (SEM). (D) Depletion of Schwann cells (SC) from DRG explants or blocking L1CAM-mediated cell-cell contact drastically reduced neurite outgrowth from DRGs treated with NGF, but not from NGF-free DRGs. Blue: DAPI (nuclei). Red: β-Tubulin III (neurites). (Figures A and B were reproduced with permission from Romano et al., Small, 2015, and figures C and D were reproduced with permission from Romano et al., Acta Biomater., 2015.); DRG: dorsal root ganglion.
When whole DRG explants were cultured on RGD-presenting ELP hydrogels, neurite outgrowth increased with increasing RGD concentration in both NGF-free and NGF-supplemented conditions (Romano et al., Acta Biomater., 2015). This dependence on adhesion ligand concentration is consistent with our previous work (Lampe et al., 2013) and that of others in the field (Shepard et al., 2012). However, our recent work demonstrated that NGF treatment and RGD density exhibited a synergistic effect on neurite outgrowth, as outgrowth on matrices presenting a high concentration of RGD (3.2 mM) was further enhanced by treatment with NGF (Figure 1C) (Romano et al., Acta Biomater., 2015). Interestingly, the interplay of NGF treatment and RGD density was also shown to impact the migration of Schwann cells from the DRG explants. NGF supplementation resulted in a ligand concentration-dependent increase in Schwann cell migration distance, while in the absence of NGF, Schwann cell migration did not show a strong dependence on ligand density. Furthermore, NGF supplementation caused neurite outgrowth and Schwann cell migration to track closely, regardless of ligand density, implying increased association between neurites and Schwann cells in response to NGF.
To investigate the dependence of neurite outgrowth on the observed association with Schwann cells, cultures were treated with cytosine arabinoside (AraC), a chemotherapeutic agent that selectively eliminates proliferative (i.e., non-neuronal) cells from the DRG explants. AraC interferes with DNA synthesis, and thus is commonly used to specifically eliminate actively dividing cells, such as Schwann cells, from post-mitotic neurons without significant adverse effects on the neuronal cell population. Surprisingly, Schwann cell depletion dramatically reduced neurite outgrowth on high RGD density matrices in cultures supplemented with NGF, while neurite outgrowth in NGF-free samples was unaffected (Figure 1D). Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis revealed that NGF supplementation downregulated expression of RGD-binding integrins and upregulated expression of the cell adhesion molecules L1CAM and NCAM, which are known to play a role in Schwann cell-neurite interactions following peripheral nervous system injury (Fu and Gordon, 1997). These data imply that NGF treatment favors cell-cell interactions at the expense of cell-matrix interactions. The role of specific cell-cell adhesion was confirmed by blocking L1CAM binding in cultures with and without NGF treatment. NGF-treated cultures exhibited reduced neurite outgrowth in response to L1CAM blocking, while outgrowth in NGF-free cultures was unaffected (Figure 1D). Taken together, these results demonstrate that NGF treatment favors neurite-Schwann cell interactions over neurite-matrix interactions, thereby modulating the response of neurite outgrowth to RGD adhesion ligand concentration.
In a related study, we investigated the effects of RGD adhesion ligand concentration on neurite outgrowth in response to gradients of soluble NGF. Spheroids consisting of sensory neurons and Schwann cells from dissociated chick DRGs were encapsulated in 3D ELP hydrogels within microfluidic gradient generating devices (Figure 2A) (Romano et al., Small, 2015). As above, the RGD ligand concentration within the hydrogels was varied by blending ELP variants containing either the RGD ligand or non-adhesive RDG. When a gradient of NGF was generated across the spheroid-containing gels, a greater number of neurites were initiated in 3.2 mM RGD matrices compared to 1.6 mM matrices (Figure 2B). This result is consistent with our previously reported results demonstrating enhanced neurite outgrowth in response to higher RGD ligand concentrations (Lampe et al., 2013). In a surprising contrast, neurite directionality and pathfinding were found to be enhanced in the lower 1.6 mM RGD matrices compared to the 3.2 mM matrices. In particular, the neurite outgrowth ratio (i.e., the ratio of neurites growing toward versus away from the NGF source) was enhanced in 1.6 mM RGD matrices compared to the 3.2 mM matrices (Figure 2C). Neurite pathfinding within a neurotrophic gradient can be divided into two phases: neurite initiation and neurite turning. We observed that NGF induced more pronounced directional bias during both the initiation and turning phases for neurites growing in 1.6 mM compared to 3.2 mM RGD matrices. Thus, while neurite outgrowth length is enhanced at higher matrix ligand densities, this occurs at the expense of NGF-induced neurite directionality. This study indicates that modulating the density of adhesive ligands can adjust the balance of cell-matrix interactions to favor either growth cone guidance or neurite extension in response to gradients of NGF.
Figure 2.

Matrix adhesive ligand density modulates neurite outgrowth and pathfinding in nerve growth factor (NGF) gradients.
(A) Dorsal root ganglion (DRG) spheroid grown in an ELP hydrogel with applied NGF gradient. Blue: DAPI (nuclei). Red: β-Tubulin III (neurites). Green: S100 (Schwann cells). (B) The number of neurites initiated per spheroid was greater in the 3.2 mM RGD matrices. Initiation was biased toward the NGF source in both matrices.*P < 0.05. Error bars represent SEM. (C) Neurite outgrowth ratio was significantly enhanced in the 1.6 mM matrices compared to the 3.2 mM matrices. ****P < 0.0001. Error bars represent SEM. (Figures were reproduced with permission from Romano et al., Small, 2015.)
The studies reviewed in this article have focused on the interplay between matrix composition, specifically the biochemical identity of natural and engineered proteins and synthetic polymers, and neurotrophin-mediated neurite outgrowth and pathfinding. In addition to adhesive ligands, other substrate properties such as stiffness and topography are known to regulate neurite outgrowth behavior (Schmidt and Leach, 2003). We have previously demonstrated enhanced neurite outgrowth from DRGs in more compliant ELP hydrogels (Lampe et al., 2013) and Man et al. (2011) have reported similar findings using fibrin gels. Furthermore, Cai et al. (2012) demonstrated an interplay between substrate stiffness and surface chemistry, as the most extensive neurite outgrowth from neuronal-like PC12 cells was observed on poly(ethylene glycol) gels with low shear moduli grafted with PLL. Neurite branching has also been shown to vary with substrate stiffness (Flanagan et al., 2002). Therefore, in addition to matrix biochemistry and soluble factors, other substrate parameters can mediate neurite outgrowth behavior.
As a whole, the results of the studies presented above emphasize the importance of optimizing the design parameters for biomaterials to be used in neurite guidance channels for peripheral nerve regeneration. While increased adhesion ligand density has been shown to increase total neurite outgrowth (Shepard et al., 2012; Lampe et al., 2013; Romano et al., Acta Biomater., 2015), the directional control of neurite outgrowth exerted by gradients of neurotrophic factors is optimized at lower adhesion ligand densities (Romano et al., Small, 2015). Therefore, when designing engineered matrices for peripheral nerve regeneration, the concentration of adhesive ligand should be chosen to balance total neurite outgrowth with neurotrophin-mediated pathfinding. Furthermore, neurite outgrowth in response to neurotrophins is modulated not only by matrix biochemistry, but also by interactions with supporting cells. Neurotrophins have long been known to enhance cell-cell contact between neurites and Schwann cells (Fu and Gordon, 1997), and our results demonstrate that L1CAM engagement between neurites and Schwann cells is a critical requirement for neurite outgrowth on RGD-presenting engineered matrices in the presence of NGF (Romano et al., Acta Biomater., 2015). Thus, biomaterial design strategies that incorporate delivery of exogenous neurotrophic factors must take into consideration not only neurite-matrix interactions, but also Schwann cell-matrix and neurite-Schwann cell interactions, to create an environment conducive to the coordinated efforts of neurites and Schwann cells in peripheral nerve regeneration. Biomaterials for peripheral nerve guidance channels must be designed such that the goal of enhancing neurite extension is balanced with the need for directional guidance and the complex interactions among the various cell types involved in regeneration.
Footnotes
Funding: This work was supported by the National Institutes of Health (1DP2-OD006477, R01-DK085720, R21-AR062359-01), the National Science Foundation (DMR-0846363).
References
- Cai L, Lu J, Sheen V, Wang S. Promoting nerve cell functions on hydrogels grafted with poly(L-lysine) Biomacromolecules. 2012;13:342–349. doi: 10.1021/bm201763n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan LA, Ju YE, Marg B, Osterfield M, Janmey PA. Neurite branching on deformable substrates. Neuroreport. 2002;13:2411–2415. doi: 10.1097/01.wnr.0000048003.96487.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997;14:67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- Hanamura K, Harada A, Katoh-Semba R, Murakami F, Yamamoto N. BDNF and NT-3 promote thalamocortical axon growth with distinct substrate and temporal dependency. Eur J Neurosci. 2003;19:1485–1493. doi: 10.1111/j.1460-9568.2004.03228.x. [DOI] [PubMed] [Google Scholar]
- Hari A, Djohar B, Skutella T, Montazeri S. Neurotrophins and extracellular matrix molecules modulate sensory axon outgrowth. Int J Dev Neurosci. 2004;22:113–117. doi: 10.1016/j.ijdevneu.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Hopker VH, Shewan D, Tessier-Lavigne M, Poo M, Holt C. Growth-cone attraction to netrin-1 is converted to repulsion by laminin-1. Nature. 1999;402:69–73. doi: 10.1038/43441. [DOI] [PubMed] [Google Scholar]
- Lampe KJ, Antaris AL, Heilshorn SC. Design of three-dimensional engineered protein hydrogels for tailored control of neurite growth. Acta Biomater. 2013;9:5590–5599. doi: 10.1016/j.actbio.2012.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man AJ, Davis HE, Itoh A, Leach JK, Bannerman P. Neurite outgrowth in fibrin gels is regulated by substrate stiffness. Tissue Eng Part A. 2011;17:2931–2942. doi: 10.1089/ten.tea.2011.0030. [DOI] [PubMed] [Google Scholar]
- Masuda T, Sakuma C, Kobayashi K, Kikuchi K, Soda E, Shiga T, Kobayashi K, Yaginuma H. Laminin peptide YIGSR and its receptor regulate sensory axonal response to the chemoattractive guidance cue in the chick embryo. J Neurosci Res. 2009;87:353–359. doi: 10.1002/jnr.21868. [DOI] [PubMed] [Google Scholar]
- Romano NH, Lampe KJ, Xu H, Ferreira MM, Heilshorn SC. Microfluidic gradients reveal enhanced neurite outgrowth but impaired guidance within 3D matrices with high integrin ligand densities. Small. 2015;11:722–730. doi: 10.1002/smll.201401574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano NH, Madl CM, Heilshorn SC. Matrix RGD ligand density and L1CAM-mediated Schwann cell interactions synergistically enhance neurite outgrowth. Acta Biomater. 2015;11:48–57. doi: 10.1016/j.actbio.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano NH, Sengupta D, Chung C, Heilshorn SC. Protein-engineered biomaterials: Nanoscale mimics of the extracellular matrix. Biochim Biophys Acta. 2011;1810:339–349. doi: 10.1016/j.bbagen.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CE, Leach JB. Neural tissue engineering: strategies for repair and regeneration. Annu Rev Biomed Eng. 2003;5:293–347. doi: 10.1146/annurev.bioeng.5.011303.120731. [DOI] [PubMed] [Google Scholar]
- Shepard JA, Stevans AC, Holland S, Wang CE, Shikanov A, Shea LD. Hydrogel design for supporting neurite outgrowth and promoting gene delivery to maximize neurite extension. Biotechnol Bioeng. 2012;109:830–839. doi: 10.1002/bit.24355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer EW, Kazimierczak J, Droz B. Neuronal subpopulations in the dorsal root ganglion of the mouse as characterized by combination of ultrastructural and cytochemical features. Brain Res. 1985;346:310–326. doi: 10.1016/0006-8993(85)90865-0. [DOI] [PubMed] [Google Scholar]
- Son YJ, Thompson WJ. Schwann cell processes guide regeneration of peripheral axons. Neuron. 1995;14:125–132. doi: 10.1016/0896-6273(95)90246-5. [DOI] [PubMed] [Google Scholar]
- Straley KS, Heilshorn SC. Independent tuning of multiple biomaterial properties using protein engineering. Soft Matter. 2009;5:114–124. [Google Scholar]
- Wallquist W, Patarroyo M, Thams S, Carlstedt T, Stark B, Cullheim S, Hammarberg H. Laminin chains in rat and human peripheral nerve: distribution and regulation during development and after axonal injury. J Comp Neurol. 2002;454:284–293. doi: 10.1002/cne.10434. [DOI] [PubMed] [Google Scholar]


