Stroke is considered the most common and severely disabling neurological disease. It is one of the leading causes of death after heart disease and cancer, causing 10% deaths worldwide and involving risk factors such as smoking, obesity and nutritional disbalance. Disability affects 75% of stroke survivors through severe mental and/or physical impairment depending on the affected brain area (Go et al., 2014).
Stroke treatment is limited to thrombolysis and antiplatelet therapy with a tissue plasminogen activator (tPA), which is only useful if administered within 3 hours of the appearance of early symptoms. This therapy is only used in 1–2% stroke patients and has, indeed, limited clinical success, as it does not protect neurons from the hypoxic insult. Therefore, the development and evaluation of new drugs to reduce the life-threatening effects of stroke and hypoxia might prove extremely fruitful.
Stroke is produced by a decrease in blood supply due to different types of alterations in the brain blood vessels, which cause cerebral hypoxia/ischemia. In turn, the hypoxic damage produces several changes in gene expression patterns in brain tissue cells (neurons and glial cells). Rapid thrombolysis is effective in protecting the injured ischemic core, although preventing the pathological features that produce neuronal death requires the development of new treatments and the implementation of a dual therapy combining the disruption of the clot and the preservation of neuronal function. In this respect, many preclinical studies have been successful in finding neuroprotective agents, but subsequent clinical trials have rendered disappointing results.
Inflammation is one of the distinctive events in stroke, while microglial cells are the predominant inflammatory effectors in brain. Reactive astrocytosis and the formation of a glial scar in the boundary zone of the ischemic core are also critical events which can produce both positive and negative consequences.
Diet and fatty acid consumption are risk factors associated with the development of obesity, cardiovascular disease and cancer. In Mediterranean countries, where olive oil is the principal source of fat, the incidence of these pathologies is lower than in the United States (Lopez-Miranda et al., 2010). Although these benefits of the Mediterranean diet have been attributed to its high content of monounsaturated fatty acids, a wide variety of minor components, such as triterpenes and phenolic compounds, are under evaluation.
For centuries, natural triterpenoids have been used as antioxidant and anti-inflammatory agents. 3b-hydroxyolean-12-en-28-oic acid, known as oleanolic acid (OA), is a well characterized triterpenoid present in medicinal plants, fruits, herbs and olive oil such as Olea europaea, Calendula officinalis, and Viscum album L (Pollier and Goossens, 2012). Traditional Chinese medicine claims that OA helps to balance the “yin” fluids of the body, while regulating the “chi”.
Modern medicine also recognizes the therapeutic value of OA. Abundant research has shown the therapeutic effects of OA in multiple pathologies, from neurodegenerative diseases to infections and cancer. Regarding neurodegenerative diseases, animal models of amyotrophic lateral sclerosis, multiple sclerosis, Parkinson's disease, Huntington's disease, Alzheimer's disease and cerebral ischemia, among others, have been used to test the protective activities of OA and other synthetic triterpenoids, improving motor performance and slowing disease progression (Martin et al., 2010, 2012; Zhang et al., 2012).
Recent data suggest that OA and its derivatives are also useful in preventing and treating type 2 diabetes and related complications (fatty liver disease, nephropathy, retinopathy and atherosclerosis). The molecular mechanisms proposed for these effects are the improvement of insulin signaling and the reduction of hyperglycemia, the up-regulation of anti-oxidants and the reduction of inflammatory processes (Camer et al., 2014). Also, anti-tumor activities of OA analogs have been described in animal cancer models and in human breast cancer cells lines, showing a reduction in angiogenesis and cell proliferation and inducing apoptosis (Shanmugam et al., 2014).
The protective role of triterpenoids, especially OA, has been studied using different in vitro and in vivo models of cerebral ischemia such as oxygen-glucose deprivation in neuronal primary cultures and global or focal ischemia in rodents. In 2012, Zhang and collaborators demonstrated that the intraperitoneal administration of triterpenoids reduced mortality, neurological deterioration and infarct brain area by inducing the expression of heme oxygenase-1 (Zhang et al., 2012).
As a hypoxic consequence, free radicals are generated which damage DNA and proteins and induce cellular damage and subsequent death. One of these free radicals is nitric oxide, produced by nervous tissue cells including neurons. Although OA is not a free radical scavenger, it reduces cellular oxidative stress response. The mechanism of action proposed for OA involves the up-regulation of nuclear factor (erythroid-derived 2)-like 2 (Nrf2), a transcription factor that increases the expression of antioxidant enzymes and the synthesis of glutathione and inhibits the expression of nuclear factor κB (NF-κB). Nrf2 is normally inactivated by Keap1 in cytoplasm. However, in the presence of triterpenoids such as OA, Nrf2 is phosphorylated by different protein kinase pathways and is liberated from Keap1 and traslocated to the nucleus to up-regulate the expression of antioxidant processes (Loboda et al., 2012).
Our studies have focused on the role of neuroprotective agents in experimental models of hypoxia. In particular, we developed a model of focal chemical hypoxia in rats which mimics the effects of cerebral ischemia by inducing the expression and stabilization of the hypoxia-inducible factor 1α (hif-1α), neuronal damage and glial reaction. By using a low OA dose (6 mg/kg per day) administered before and after the hypoxic injury, we observed a decrease in neuronal degeneration and glial reaction (Caltana et al., 2014).
These results could be related to a reduction (Figure 1) in the activity of neuronal nitric oxide synthase (nNOS) and inducible nitric oxide synthase (iNOS) in microglial cells, both of which decreased with OA exposure in hypoxic animals. Therefore, acting through the activation of Nrf2, OA reduces (Figure 2) the expression of nNOS and iNOS in the hypoxic brain, protecting neurons against the oxidative damage triggered by microglial cells. We also showed an astrocytic reaction involving high levels of protein S100β which could stimulate the uptake of excitotoxic levels of glutamate and prevent neuronal damage, while also inducing the recovery of cytoskeletal protein assembly and thus stabilize cell morphology and function.
Figure 1.
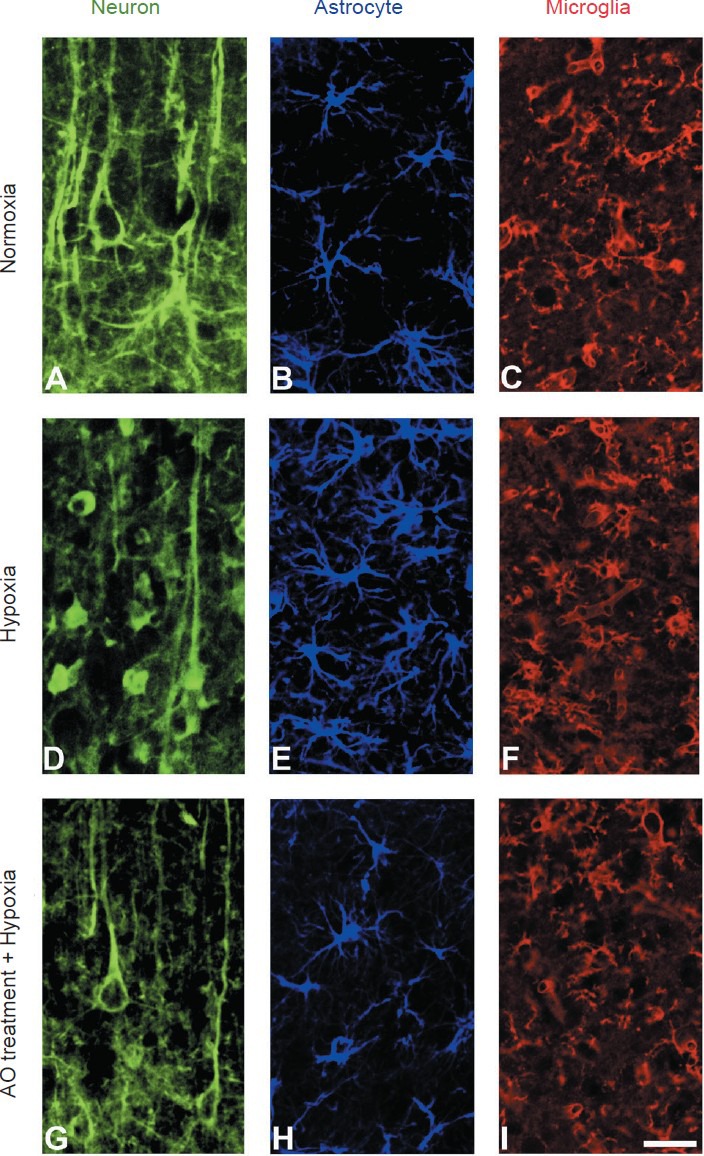
Photomicrographs showing neurons (A, D, G), astrocytes (B, E, H) and microglial cells (C, F, I) in different experimental conditions.
Hypoxia induces neuronal degeneration with loss of dendritic trees (D) and glial reaction – astrocytosis (E) and microglial activation (F). The pretreatment with oleanolic acid (OA) decreases neuronal damage (G), astrocytic reaction (H) and microglial activation (I). (A–C) Normoxic conditions. (D–F) Hypoxic coditions. (G–I) Pretreatment with OA and hypoxic conditions. Immunofluorescence for 200 kDa neurofilaments in A, D, and G, and for gliofribrillary acidic protein in B, E and H. Histochemical staining for microglial cells (C, F and I) was made with tomato lectin. Scale bar: 15 μm.
Figure 2.
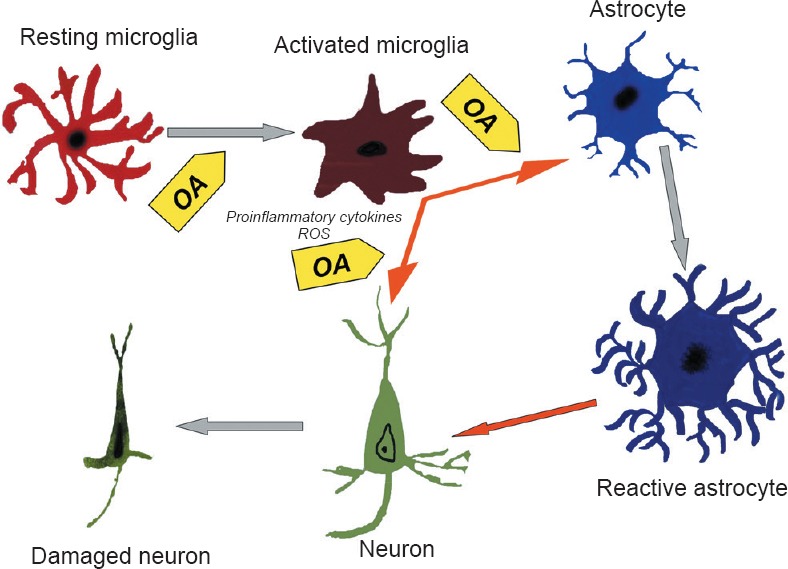
Schematic representation of cellular processes occurring in the hypoxic brain and the effects of oleanolic acid (OA) pretreatment.
Hypoxia induces the activation of microglial cells, which suffer morphological transformations and become ameboid-shaped. The activated microglial cells release proinflammatory cytokines and reactive oxygen species (ROS) such as nitric oxide, which induce astroglial reaction and neuronal damage. OA reduces microglial reaction and then decreases astroglial reaction, thus protecting neuron morphology and function. Gray arrows indicate the effects of hypoxia on neurons, astrocytes and microglial cells. Orange arrows indicate inflammatory effects on nervous tissue cells. Yellow arrows indicate OA preventive effects.
Although experimental data is still required, growing evidence demonstrates the beneficial consequences of OA in the prevention and treatment of different neuropathologies. The enrichment of food and diet with this compound could prove a useful tool in health and quality of life improvement and decrease the incidence of frequent pathologies such as vascular diseases.
This work was supported by UBACYT 20020130100258BA (A.B.), PIP CONICET 00269 (A.B.) and UBACYT 20020130300033BA (L.C.).
References
- Caltana L, Rutolo D, Nieto ML, Brusco A. Further evidence for the neuroprotective role of oleanolic acid in a model of focal brain hypoxia in rats. Neurochem Int. 2014;79:79–87. doi: 10.1016/j.neuint.2014.09.011. [DOI] [PubMed] [Google Scholar]
- Camer D, Yu Y, Szabo A, Huang XF. The molecular mechanisms underpinning the therapeutic properties of oleanolic acid, its isomer and derivatives for type 2 diabetes and associated complications. Mol Nutr Food Res. 2014;58:1750–1759. doi: 10.1002/mnfr.201300861. [DOI] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loboda A, Rojczyk-Golebiewska E, Bednarczyk-Cwynar B, Lucjusz Z, Jozkowicz A, Dulak J. Targeting nrf2-mediated gene transcription by triterpenoids and their derivatives. Biomol Ther. 2012;20:499–505. doi: 10.4062/biomolther.2012.20.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Miranda J, Pérez-Jiménez F, Ros E, De Caterina R, Badimón L, Covas MI, Escrich E, Ordovαs JM, Soriguer F, Abiα R, de la Lastra CA, Battino M, Corella D, Chamorro-Quirós J, Delgado-Lista J, Giugliano D, Esposito K, Estruch R, Fernandez-Real JM, Gaforio JJ, et al. Olive oil and health: summary of the II international conference on olive oil and health consensus report, Jaén and Córdoba (Spain) 2008. Nutr Metab Cardiovasc Dis. 2010;20:284–294. doi: 10.1016/j.numecd.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Martín R, Carvalho-Tavares J, Hernαndez M, Arnés M, Ruiz-Gutiérrez V, Nieto ML. Beneficial actions of oleanolic acid in an experimental model of multiple sclerosis: a potential therapeutic role. Biochem Pharmacol. 2010;79:198–208. doi: 10.1016/j.bcp.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Martín R, Hernαndez M, Córdova C, Nieto ML. Natural triterpenes modulate immune-inflammatory markers of experimental autoimmune encephalomyelitis: therapeutic implications for multiple sclerosis. Br J Pharmacol. 2012;166:1708–1723. doi: 10.1111/j.1476-5381.2012.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollier J, Goossens A. Oleanolic acid. Phytochemistry. 2012;77:10–15. doi: 10.1016/j.phytochem.2011.12.022. [DOI] [PubMed] [Google Scholar]
- Shanmugam MK, Dai X, Kumar AP, Tan BK, Sethi G, Bishayee A. Oleanolic acid and its synthetic derivatives for the prevention and therapy of cancer: preclinical and clinical evidence. Cancer Lett. 2014;346:206–216. doi: 10.1016/j.canlet.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang S, Zhang M, Weng Z, Li P, Gan Y, Zhang L, Cao G, Gao Y, Leak RK, Sporn MB, Chen J. Pharmacological induction of heme oxygenase-1 by a triterpenoid protects neurons against ischemic injury. Stroke. 2012;43:1390–1397. doi: 10.1161/STROKEAHA.111.647420. [DOI] [PMC free article] [PubMed] [Google Scholar]


