Progesterone: The sexual hormone progesterone is a member of the steroid hormone family, and is the most important representative of the gestagenes sub-group. It plays an elementary role in the female menstruation cycle and is essential for the establishment and the maintenance of a pregnancy, however gestagenes like progesterone are also abundant in males. In 1990, the existence of steroids was described in different cells of the central nervous system (CNS) (Baulieu and Robel, 1990). Up until this point, the effect of sexual hormones on neural cells was rather unknown, other than in the well known regulatory centers of the hypothalamus. Since then the essential enzymes of steroid synthesis, cytochrome P450 side chain cleavage enzyme (P450scc) and 3 β-hydroxysteroid-dehydrogenase (3 β-HSD), have been detected in the central (Mellon et al., 1993) as well as in the peripheral nervous system (Schaeffer et al., 2010). Within the cerebellum Purkinje cells were identified as major sites for neurosteroid formation in the mammalian brain, synthesizing progesterone as well as estradiol (Tsutsui et al., 2011). Traditionally, the effects of progesterone are mediated by genomic mechanisms of classical progesterone receptors which act as transcription factors. Basically, two relevant isoforms, the N-terminal shortened A-form (PR-A, 86 kDa) and the native B-form (PR-B, 110 kDa) are known. Nevertheless, in addition to the genomic signaling pathway, other, non-genomic pathways have been described. The most important member of this non-genomic receptor family seems to be the “progesterone receptor membrane component 1” (PGRMC1). Neural expression of PR-A, PR-B and PGRMC1 could already be proven in different components of the CNS and the peripheral nervous system (PNS) e.g., the hypothalamus, the cerebellum and the dorsal root ganglia (Wessel et al., 2014b).
Clinical relevance of progesterone: The effects of neurosteroids like progesterone on neuronal tissue in the CNS and PNS are of enormous therapeutic interest. The clinical relevance of progesterone has already been proven in many studies in different neural glial cells (De Nicola et al., 2013). Indeed, numerous preclinical studies verified the neuroprotective effects of progesterone after cranial traumatic brain and cerebral injuries. Furthermore, experimental data from various animal models emphasize the benefit of progesterone treatment on other neurological disorders like traumatic brain injury, peripheral nerve injury, amyotrophic lateral sclerosis and cerebral ischemia (Wessel et al., 2014b). Progesterone seems to have neuroprotective and anti-inflammatory influences on neuronal cells. For instance, post ischemic treatment with progesterone leads to a reduction of the necrotic area. Based on the versatile application of progesterone, and the outlined positive effects on poor prognosis neurological disorders of the CNS and PNS, the neurosteroids seem to be a very potent group for new therapeutic strategies.
But the question is still whether progesterone serves as a universal stimulus for neuronal cells, or if there are therapeutical limitations to a progesterone treatment approach. Is it possible to treat children as well as adults with progesterone after injuries of the CNS or PNS? As there is no answer to this question yet, further basic research is mandatory. Recent studies reviewed the impact of progesterone on neonatal, juvenile and matured cells in the CNS and PNS. In the cerebellum, specifically in rat Purkinje cells, the expression of progesterone with high endogenous concentrations during the neonatal and juvenile periods have been shown (Wessel et al., 2014a). Here, progesterone induces denrito-, spino-, and somatogenesis (Tsutsui et al., 2011; Wessel et al., 2014a). In this study, an age-dependent increase in intracellular progesterone concentrations during the maturation of Purkinje cells and other neurons of the cerebellar cortex, along with an increased receptor expression in juvenile cells suggest that progesterone plays an important role during the physiological development of the cerebellar cortex (Figure 1a). Although Wessel et al. (2014a) demonstrated the expression of the classical progesterone receptors at all developmental stages in rats, the stimulation of matured cells with progesterone had no positive effects concerning neuroplasticity (Figure 1b). Interestingly, at the same time points, the positive impact of progesterone could be verified in the PNS. In primary cultures from chicken dorsal root ganglia (DRG) treated with progesterone, a significant enhancement of neuritic outgrowth was evident (Figure 1a). Blocking of progesterone receptors with mifepristone leads to the extinction of this effect (Olbrich et al., 2013). These results give a strong hint that the use of neurosteroids can be a strategy in pediatric neonatology and traumatology, but at this time point it seems to be limited to juvenile stages and is not applicable in adults. Therefore, we have to investigate and to understand the expression and regulation of the different progesterone receptors in the nervous system, especially in adults. Beside these data progesterone often acts in concert with estrogen. In Purkinje cells, estrogen also promotes dendritic growth, spino- and synaptogenesis during neonatal life (Tsutsui et al., 2011).
Figure 1.
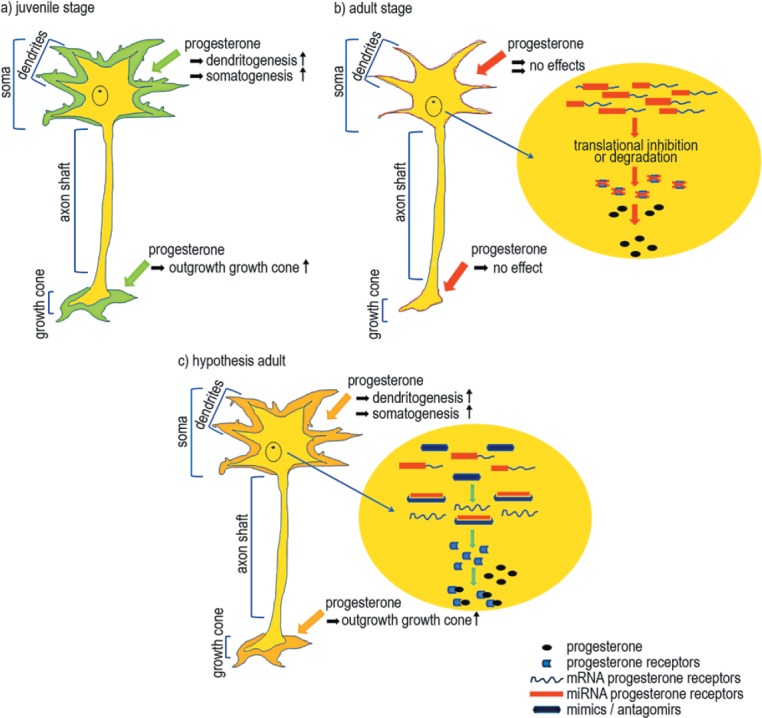
Impact of progesterone on neuronal cells
a) Progesterone stimulated juvenile neuronal cells in the central nervous system show an increase in dendritogenesis, somatogenesis and spinogenesis. Additionally, cells in the peripheral nervous system show an enlargement in the growth cone after progesterone incubation.
b) Adult neurons show no effects after progesterone treatment, neither in the central nervous system nor in the peripheral nervous system. We assume that miRNAs inhibit or degrade the mRNA of progesterone receptors, so that the cell loses its sensitivity to progesterone.
c) The regulation of these miRNAs by local or systematic inhibition might increase the number of functional progesterone receptor mRNA molecules. This may result in an increased sensitivity to progesterone in adult stages, possibly leading to effects comparable to those seen in juvenile neuronal cells.
MicroRNAs and their relevance in neurology and neurodegenerative diseases: Many studies have been carried out to analyze the function, and subsequently confirm the relevance of microRNAs (miRNA) in the CNS. It has been shown that the biogenesis of miRNAs is crucial for the development and the functionality of neuronal structures. In different independent studies of the cerebral cortex and the cerebellum of mice, it became apparent that inhibition or a complete loss of Dicer leads to different manifestations of neurodegeneration (Hong et al., 2013).
MiRNAs are short (21–23 nucleotide, nt), highly conserved, non-coding RNAs. They play a crucial role in posttranscriptional gene regulation e.g., neuroplasticity-related processes (Hommers et al., 2015). The biogenesis of miRNAs, a multistage process, is an important procedure to ensure their functional efficiency. In the first step, the primary transcript of miRNAs (pri-miRNA) is generated in the nucleus. Pri-miRNA has a length of 500–3,000 nucleotides and carries a poly-A-tail at its 3′-end, as well as a 7-methylguanosine cap. Subsequently, the primary transcript is converted into a hairpin structure and cleaved into an approximately 70 nt precursor form (pre-miRNA) by RNase III (Drosha). Pre-miRNA is then transported from the nucleus into the cytoplasm by two proteins, Exportin 5 and Ras-related nuclear protein. In the cytoplasm the RNaseIII, Dicer, and its co-factor Tar RNA-binding protein (TRBP) process the pre-miRNA by cleaving the loop structure and the pre-miRNA into 21–23 nt miRNAs. The single, mature miRNA strands are loaded onto the Argonaute homologue protein (Ago2) in order to form the RNA-induced silencing complex (RISC). In this conformation the miRNA binds to its target mRNA. MiRNAs bind to the 3′ untranslated regions (3′UTR) of their target mRNA and can affect it in two different ways depending on the complementarity to its binding sequence. The transcription can either be inhibited or the mRNA can be degraded completely. A partial complementarity leads to inhibition whereas a perfect base matching causes degradation of the mRNA.
Hong et al. (2013) showed that disruption of miRNA biogenesis results in microcephaly in differentiated neurons of the cerebral cortex in Dicer-knockout mice. In comparison to control mice without disruption in pre-miRNA procession, brains of knockout mice were significantly smaller. Total loss of miRNA function leads to a reduced cell soma size of mature neurons and a reduced neurite growth. In a second study it became clear that a knockout of the Dicer enzyme in Purkinje cells is accompanied by dramatic consequences. In contrast to the results of Hong et al., the Dicer-knockout led to cellular death and cerebellar degeneration, and at least induced ataxia (Schaefer et al., 2007). Disruptions due to a knockout of the Dicer enzyme show explicit similarities to different mouse models of neurodegenerative diseases. Apart from the universal step of correct processing of the miRNA, several miRNAs are known to disturb the neuronal development, like the loss of miR-592. Also the involvement of miRNAs in neurodegenerative disease development should not be underestimated. Therefore the emphasis in the investigation of neurodegenerative disorders over the last decade has been concentrated on the involvement of miRNAs. Several miRNAs show a negative effect in the pathogenesis of Parkinson's disease, Alzheimer's disease, Huntington's disease, epilepticus and multiple-system atrophy. For instance in multiple-system atrophy, one single miRNA called miR-202 is the key factor.
Effect of miRNAs on progesterone and its receptors: The existence of numerous mRNA targets implicate that miRNAs are capable to regulate thousands of genes. This is why miRNAs are essential in various development stages, tissues and diseases. In terms of progesterone, several studies revealed the effect of miRNAs on progesterone and its receptors. Most of these studies deal with the investigation of miRNAs in breast tumors and their significance in the establishment and maintenance of pregnancy. In both cases the amount of progesterone and its receptors are regulated by different miRNAs. MiR-200a is one key mediator in the decline of progesterone receptor function leading to term and preterm labor (Williams et al., 2012). Progesterone metabolism is up-regulated and the sensitivity of the receptors for progesterone is down-regulated. Apart from these data, progesterone and its receptors could have a strong impact in the nervous system.
We know that the classical progesterone receptors are most abundant and sensitive in the early stages of neuronal development. Clinical research in brain injuries, animal models, or even traumata in childhood show promising results when treated with progesterone. The present challenge is to understand the post-transcriptional mechanisms in neuronal cells and to expand the positive effects of progesterone in adulthood. miRNAs, the most important post-transcriptional regulators, are implicated in brain development and in the formation of neurological disorders. Complete comprehension of miRNA function in the neuroscientific field could help to reveal the versatile molecular consequences of miRNA interaction. The understanding of these mechanisms is supposed to be the key to designing new therapeutic tools for the treatment of neuronal damage, in which miRNAs could be used as target molecules for drugs. One promising approach could be the possibility to regulate specific miRNAs by the systematic or local use of miRNA inhibitors, known as antagomirs, and stimulators, known as mimics which are artificial RNA molecules (Figure 1c). An investigation into the gene encoding progesterone resulted in the detection of several binding sites for miRNAs at the 3’ UTR which appear to regulate its expression. This opens up new possibilities to interfere with the functionality of the miRNAs that target these sites. One example for a promising miRNA mimic is miR-193b-mimic, a down-regulator of progesterone receptors in a breast cancer cell line (Younger and Corey 2011). This knowledge strongly encourages us to investigate progesterone receptor-regulating miRNAs.
Founded on the state of knowledge about the interference of miRNAs in neuronal structures, the main goals are: (1) to reveal regulation mechanisms concerning the classical progesterone receptors, (2) to synthesize mimics and/or antagomirs to replicate the positive impact of progesterone in mature neuronal cells (Figure 1c).
We would like to acknowledge D. Terheyden-Keighley for the critical reading of this article. We gratefully thank FoRUM (RUB) for financial support (F812-2014).
References
- Baulieu EE, Robel P. Neurosteroids: a new brain function? J Steroid Biochem Mol Biol. 1990;37:395–403. doi: 10.1016/0960-0760(90)90490-c. [DOI] [PubMed] [Google Scholar]
- De Nicola AF, Coronel F, Garay LI, Gargiulo-Monachelli G, Deniselle MC, Hommers LG, Domschke K, Deckert J. Heterogeneity and individuality: microRNAs in mental disorders. J Neural Transm. 2015;122:79–97. doi: 10.1007/s00702-014-1338-4. [DOI] [PubMed] [Google Scholar]
- Hong J, Zhang H, Kawase-Koga Y, Sun T. MiRNA function is required for neurite outgrowth of mature neurons in the mouse postnatal cerebral cortex. Front Cell Neurosci. 2013;7:151. doi: 10.3389/fncel.2013.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon SH, Deschepper CF. Neurosteroid biosynthesis: genes for adrenal steroidogenic enzymes are expressed in the brain. Brain Res. 1993;629:283–292. doi: 10.1016/0006-8993(93)91332-m. [DOI] [PubMed] [Google Scholar]
- Olbrich L, Wessel L, Balakrishnan-Renuka A, Böing M, Brand-Saberi B, Theiss C. Rapid impact of progesterone on the neuronal growth cone. Endocrinology. 2013;154:3784–3795. doi: 10.1210/en.2013-1175. [DOI] [PubMed] [Google Scholar]
- Schaefer A, O’Carroll D, Tan CL, Hillman D, Sugimori M, Llinas R, Greengard P. Cerebellar neurodegeneration in the absence of miRNAs. J Exp Med. 2007;204:1553–1558. doi: 10.1084/jem.20070823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer V, Meyer L, Patte-Mensah C, Mensah-Nyagan AG. Progress in dorsal root ganglion neurosteroidogenic activity: basic evidence and pathophysiological correlation. Prog Neurobiol. 2010;92:33–41. doi: 10.1016/j.pneurobio.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Ukena K, Sakamoto H, Okuyama S, Haraguchi S. Biosynthesis mode of action, and functional significance of neurosteroids in the purkinje cell. Front Endocrinol (Lausanne) 2011;2:61. doi: 10.3389/fendo.2011.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel L, Balakrishnan-Renuka A, Henkel C, Meyer HE, Meller K, Brand-Saberi B, Theiss C. Long-term incubation with mifepristone (MLTI) increases the spine density in developing Purkinje cells: new insights into progesterone receptor mechanisms. Cell Mol Life Sci. 2014a;71:1723–1740. doi: 10.1007/s00018-013-1448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel L, Olbrich L, Brand-Saberi B, Theiss C. New aspects of progesterone interactions with the actin cytoskeleton and neurosteroidogenesis in the cerebellum and the neuronal growth cone. J Histochem Cytochem. 2014b;62:835–845. doi: 10.1369/0022155414550691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KC, Renthal NE, Condon JC, Gerard RD, Mendelson CR. MicroRNA-200a serves a key role in the decline of progesterone receptor function leading to term and preterm labor. Proc Natl Acad Sci U S A. 2012;109:7529–7534. doi: 10.1073/pnas.1200650109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younger ST, Corey DR. Transcriptional regulation by miRNA mimics that target sequences downstream of gene termini. Mol Biosyst. 2011;7:2383–2388. doi: 10.1039/c1mb05090g. [DOI] [PMC free article] [PubMed] [Google Scholar]


