Dendrites are exquisitely specialized cellular compartments that critically influence how neurons receive and process information. Most synaptic inputs are received onto dendritic shafts, or small protuberances known as dendritic spines, and are then integrated and transmitted to the cell soma. During nervous system development, dendrites are dynamic, expanding and retracting rapidly, in response to intrinsic and environmental cues. Selective elimination of dendritic processes, also known as developmental pruning, ensures precise connectivity and refines neuronal networks. In contrast, dendrites in adult neurons are extremely stable and do not change over long periods of time which, in humans, might span decades (Koleske, 2013). Emerging evidence reveals that dendritic arbor stability is crucial for adult brain function. Loss of stability leads to defects in dendrite structure and connectivity that contributes to the pathology of psychiatric and neurodegenerative disorders. Dendritic abnormalities and loss of synapses have been reported in schizophrenia and depression, as well as in neurodegenerative conditions including Alzheimer's disease (AD), amyotrophic lateral sclerosis, stroke and glaucoma.
Much of what is known about dendrite pathology and neurodegeneration comes from extensive analysis of dendritic abnormalities in human subjects with AD. Human neuropathology data reveal that dendritic defects in AD, which include dystrophic neurites, reduced arbor complexity and loss of spines, are widespread and occur early in the disease (Cochran et al., 2014). Computational modeling predicts that these structural changes can substantially disrupt signal integration and firing of action potentials, which might directly contribute to neural system failure and dementia. The “synaptic hypothesis” of AD is based on the premise that a reduction in dendritic spines, the primary sites of synaptic input, leads to early neuronal dysfunction (Selkoe, 2002). Therefore, the pathological outcome of many neurodegenerative conditions might not be primarily determined by cell loss, but by subtle changes in dendrites and spines/synapses that limit neuronal functionality within a circuit. Yet, little is known about the mechanisms that promote dendrite pathology and synapse loss during neurodegeneration.
The mammalian target of rapamycin (mTOR) kinase, which is well known for its function in the growth and proliferation of non-neuronal cells, has attracted substantial attention due to emerging roles in the developing nervous system (Jaworski and Sheng, 2006). mTOR is a key signal integrator for a variety of extracellular stimuli including growth factors, neurotransmitters, energy and stress. mTOR is stimulated by the availability of nutrients, while it is inhibited by cellular stressors such as hypoxia, inflammation or low ATP levels. The canonical mTOR pathway starts with receptor-mediated activation of phosphoinositide-3’ kinase (PI3K) and its downstream target Akt. Active Akt leads to phosphorylation and inhibition of the tuberous sclerosis complex (TSC1/2), which results in increased GTP-bound Rheb protein and mTOR activation. Many of the signals that impinge upon mTOR activity act through the TSC1/2 complex, a negative regulator of mTOR function. For example, hypoxia activates TSC1/2 through the regulated in development and DNA damage response (REDD) proteins (Corradetti et al., 2005) leading to loss of mTOR function. In contrast, Akt and the extracellular-signal regulated kinases 1/2 (ERK1/2), inactivate TSC1/2 leading to mTOR simulation. mTOR interacts with several intracellular partners to form the rapamycin-sensitive mTORC1 complex that controls multiple cellular processes of which the best characterized is protein synthesis. mTORC1 phosphorylates the p70 ribosomal S6 kinase (p70S6K) leading to phosphorylation of the ribosomal protein S6, which stimulates mRNA translation rate. In addition, mTORC1 phosphorylates 4E-BP1 thereby inhibiting its repression of eIF4E, which leads to enhanced initiation of translation. mTOR can also form the mTORC2 complex, which is rapamycin insensitive. mTORC2 phosphorylates Akt on serine 473 (S473), commonly used as a readout of mTORC2 activity, and its main role is the regulation of actin polymerization.
Recent studies have identified mTOR as a critical component of dendrite development. For example, a substantial reduction in the number of dendritic branches and arbor shrinkage were observed in developing hippocampal neurons when mTOR was inhibited (Jaworski et al., 2005). mTOR has been implicated in the regulation of dendritic spine morphology, synaptogenesis and synaptic plasticity (Hoeffer and Klann, 2010). More recently, excessive mTORC1 activation and autophagy were linked to increased dendritic spine density and aberrant behavior in mouse models of autistic spectrum disorder (Tang et al., 2014). The emerging role of mTOR in the regulation of dendrite structure and spine dynamics during development led us to postulate that dysregulation of mTOR function might contribute to dendritic pathology in neurodegenerative diseases.
To investigate this hypothesis, we focused on the retinal ganglion cells (RGCs), a population of inner retinal neurons that convey visual information from the retina to the brain. The selective death of RGCs is a cardinal feature of glaucoma, and shrinkage of RGC dendritic arbors has been observed in primate, cat and rodent models of this disease as well as in human glaucomatous retinas. We used an in vivo model of acute optic nerve lesion (axotomy) to determine whether axonal damage had a direct effect on RGC dendrite morphology and, if so, to identify the molecular pathways that regulate this injury-induced response. We carried out a detailed analysis of dendritic structure in transgenic mice that selectively express yellow fluorescent protein (YFP) in RGCs under control of the RGC-specific Thy1 promoter. In this strain (B6.Cg.Tg[Thy1-YFPH]2Jrs/J) less than 1% of RGCs are YFP-labeled allowing visualization of individual dendritic trees without interference from overlapping dendrites in neighboring neurons. YFP fills the entire RGC dendritic tree providing a Golgi-like stain suitable for acquisition of high resolution images by confocal microscopy and three-dimensional reconstruction with imaging software.
Using this approach, we demonstrated that axonal injury triggered rapid shrinkage of RGC dendrites accompanied by marked reduction of arbor field area and complexity. Retinal immunohistochemistry using antibodies that recognize phosphorylated S6(Ser240/244), a widely accepted functional readout of mTORC1 activity, demonstrated that selective damage to RGC axons led to downregulation of mTORC1 function in these neurons but not in other retinal cells (Morquette et al., 2015). This observation is consistent with a previous study showing phospho-S6 downregulation in axotomized RGCs (Park et al., 2008). Importantly, we showed that optic nerve lesion led to selective upregulation of REDD2, a potent mTORC1 inhibitor, which coincided with loss of mTORC1 activity. REDD2 knockdown using targeted siRNA restored mTORC1 function in injured neurons and fully rescued their dendritic arbors, increasing dendritic length, field area and branch complexity. REDD2 depletion also abrogated pathological RGC hyperexcitability, restoring light-triggered responses, and extended cell survival after axotomy (Morquette et al., 2015). Rapamycin administration obliterated the effect of REDD2 knockdown confirming that dendritic rescue was mediated by mTORC1. In summary, our study demonstrates that damage to RGC axons leads to a cell-specific increase in REDD2, mTORC1 inhibition, and dendritic retraction. These findings provide evidence that REDD2-dependent loss of mTORC1 function can underlie RGC dendrite pathology, and that mTORC1 activity is required for the stability of adult RGC dendrites and cell survival.
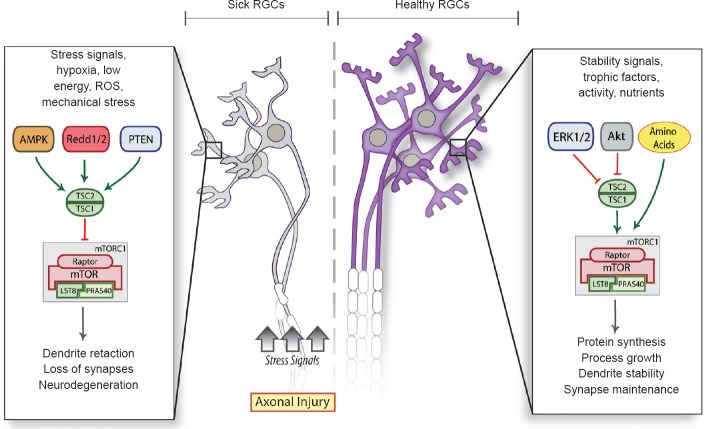
Based on these observations, we propose a working model in which several key pathways such as Akt, Erk1/2, and nutrients, converge in healthy RGCs to maintain endogenous levels of mTOR that drive protein synthesis, dendrite stability and synaptic integrity. In sick neurons afflicted by axonal injury, which are more vulnerable to stressors including hypoxia, low energy, reactive oxygen species and mechanical stress, mTOR inhibitors are upregulated leading to dendrite degeneration and synapse loss (Figure 1). REDD2 levels increase with hypoxia, via the hypoxia-inducible factor-1 alpha (HIF-1α), as well as oxidative and osmotic stress. Optic nerve damage increases HIF-1α and reactive oxygen species in RGCs (Kanamori et al., 2010), which may account for the axotomy-induced upregulation of REDD2. REDD2 has also been shown to promote human monocyte cell death through a reduction in thioredoxin-1 expression (Imen et al., 2009), thus we cannot rule out that REDD2 knockdown leading to downregulation of this pathway contributes to RGC dendrite protection. Although our study focused on REDD2, it is possible that other stress response proteins, which also inhibit mTOR, play a role in the context of different injury modalities or neurodegenerative diseases (Figure 1). Given that mTOR is a versatile, central regulator that responds to a variety of stressors, it is tempting to speculate that stress signals thought to contribute to RGC damage in glaucoma, including hypoxia, nutrient deprivation and reactive oxygen species, might do so by inhibiting mTOR function in these neurons, thereby triggering early dendritic retraction, synaptic disconnection and neurodegeneration.
Figure 1.
A model of dendrite retraction triggered by injury-related stressors.
In sick neurons afflicted by axonopathy and more vulnerable to hypoxia, low energy, reactive oxygen species (ROS) and mechanical stress, mTOR inhibitors are upregulated leading to dendrite retraction, loss of synapses and neurodegeneration. Healthy neurons, on the other hand, maintain constitutive levels of mTOR that drive protein synthesis, dendrite stability and synaptic integrity.
mTOR: Mammalian target of rapamycin; RGCs: retinal ganglion cells; AMPK: AMP-activated protein kinase; Redd1/2: regulated in development and DNA damage response 1/2; PTEN: phosphatase and tensin homolog; TSC: tuberous sclerosis complex; ERK1/2: extracellular signal-regulated kinases 1/2; mTORC1: mTOR complex 1; LST8: lethal with SEC13 protein 8; PRAS40: proline rich Akt substrate 40 kDa.
Further research is required to identify mTOR-specific targets that contribute to RGC dendrite stability. It is possible that mTOR mediates the translation of structural or signaling proteins in dendrites to ensure arbor maintenance and synaptic integrity. Two well characterized mTOR targets, p70S6K and 4E-BP1, play crucial roles in dendritic arborization during hippocampal neuron development (Jaworski et al., 2005). It is of future interest to assess whether these and other molecules involved in dendrite stability in developing neurons, such as the microtubule associated proteins (MAP) 1A and 2, integrin α3β1, calcium/calmodulin-dependent protein kinase II, nuclear Dbf2-related kinases and the guanine deaminase cypin, are abnormally regulated in the context of glaucoma or other neurodegenerative diseases (Koleske, 2013). mTOR has also been shown to enhance the interaction between microtubules and actin-binding proteins required for proper dendritic arbor morphology (Swiech et al., 2011), suggesting a role beyond that of protein translation. The identification of pathways that contribute to RGC dendritic arbor maintenance, including those upstream and downstream of mTOR, will be essential to understand the molecular basis of pathological changes in glaucoma and to identify therapeutic targets potentially applicable to other neurodegenerative diseases.
This work was supported by grants from the Canadian Institutes of Health Research to ADP and the Fonds de recherche du Québec-Santé (FRQS). ADP holds a National Chercheur Boursier award from FRQS. The author thanks Dr. Timothy E Kennedy for helpful comments on the manuscript, and Mr. James Correia for assistance with the preparation of the figure. Due to space limitations, the author regrets the omission of many important studies and their corresponding references.
References
- Cochran JN, Hall AM, Roberson ED. The dendritic hypothesis for Alzheimer's disease pathophysiology. Brain Res Bull. 2014;103:18–28. doi: 10.1016/j.brainresbull.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradetti MN, Inoki K, Guan KL. The stress-inducted proteins RTP801 and RTP801L are negative regulators of the mammalian target of rapamycin pathway. J Biol Chem. 2005;280:9769–9772. doi: 10.1074/jbc.C400557200. [DOI] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imen JS, Billiet L, Cuaz-Pérolin C, Michaud N, Rouis M. The regulated in development and DNA damage response 2 (REDD2) gene mediates human monocyte cell death through a reduction in thioredoxin-1 expression. Free Radic Biol Med. 2009;46:1404–1410. doi: 10.1016/j.freeradbiomed.2009.02.020. [DOI] [PubMed] [Google Scholar]
- Jaworski J, Sheng M. The growing role of mTOR in neuronal development and plasticity. Mol Neurobiol. 2006;34:205–219. doi: 10.1385/MN:34:3:205. [DOI] [PubMed] [Google Scholar]
- Jaworski J, Spangler S, Seeburg DP, Hoogenraad CC, Sheng M. Control of dendritic arborization by the phosphoinositide-3’-Kinase– Akt–mammalian target of rapamycin pathway. J Neurosci. 2005;25:11300–11312. doi: 10.1523/JNEUROSCI.2270-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori A, Catrinescu MM, Kanamori N, Mears KA, Beaubien R, Levin LA. Superoxide is an associated signal for apoptosis in axonal injury. Brain. 2010;133:2612–2625. doi: 10.1093/brain/awq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koleske AJ. Molecular mechanisms of dendrite stability. Nat Rev Neurosci. 2013;14:536–550. doi: 10.1038/nrn3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morquette B, Morquette P, Agostinone J, Feinstein E, McKinney RA, Kolta A, Di Polo A. REDD2-mediated inhibition of mTOR promotes dendrite retraction induced by axonal injury. Cell Death Differ. 2015;22:612–625. doi: 10.1038/cdd.2014.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Swiech L, Blazejczyk M, Urbanska M, Pietruszka P, Dortland BR, Malik AR, Wulf PS, Hoogenraad CC, Jaworski J. CLIP-170 and IQGAP1 cooperatively regulate dendrite morphology. J Neurosci. 2011;31:4555–4568. doi: 10.1523/JNEUROSCI.6582-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, Gudsnuk K, Kuo SH, Cotrina Marisa L, Rosoklija G, Sosunov A, Sonders Mark S, Kanter E, Castagna C, Yamamoto A, Yue Z, Arancio O, Peterson Bradley S, Champagne F, Dwork Andrew J, Goldman J, Sulzer D. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron. 2014;83:1131–1143. doi: 10.1016/j.neuron.2014.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]